The importance of streptococci in ovine and caprine mastitis has not been studied as extensively as that of staphylococci, probably because staphylococci cause more than 70% of the cases of mastitis in small ruminants (Gelasakis et al., Reference Gelasakis, Mavrogianni, Petridis, Vasileiou and Fthenakis2015). Various studies (mainly in Italy) have indicated that streptococci could be of greater importance for small ruminant mastitis than previously believed (Addis et al., Reference Addis, Pisanu, Marogna, Cubeddu, Pagnozzi, Cacciotto, Campesi, Schianchi, Rocca and Uzzau2013; Pisanu et al., Reference Pisanu, Cubeddu, Pagnozzi, Rocca, Cacciotto, Alberti, Marogna, Uzzau and Addis2015; Albenzio et al., Reference Albenzio, Santillo, Caroprese, Ciliberti, Marino and Sevi2016). The objectives of this work were (a) to determine the presence of streptococci in samples from small ruminant dairy farms, specifically, in bulk-tank milk and on milking machine teatcups, (b) to investigate the potential adverse effects of streptococci on milk quality and (c) to evaluate the importance of some management practices on the presence of streptococci.
Materials and methods
Farms
In total, 51 sheep and 23 goat dairy farms were studied. Dairy farms (milking was performed by machine or by hand) were selected by collaborating veterinarians on willingness of farmers to accept a visit by University personnel for sample collection and visited once each. Three investigators (DTL, CKM and GCF) accompanied by an assisting investigator (NGCV, APP, NGK or KSI) visited all farms for sample collection. At the start of each visit, the farmer was interviewed to obtain information on udder health management practices in use (online Supplementary Material 1).
All farms visited were for dairy production. Lambs or kids born were weaned at 45 to 50 or 60 to 90 d of age, respectively. After weaning of their offspring, ewes or nannies were milked twice or thrice daily for up to 8 months.
Sampling
At each farm, sampling took place after milking and cleaning of the parlour by following the usual farm routine. Initially, two 20 mL samples were collected using aseptic techniques from the milk tank.
If machine milking was used, three teatcups were swabbed in parlours with up to 12 milking units (n = 28 sheep and 6 goat farms), six teatcups in parlours with 13 to 24 units (n = 16 and 4) and nine teatcups in parlours with 25 to 36 units (n = 3 and 1). In total, 207 teatcups were sampled on sheep farms and 51 on goat farms. The specific teatcups to be sampled were predetermined using an electronic random number generator. Two swab samples were taken from each teatcup, one from the upper (approx. 1–1.5 cm deep) and one from the lower (approx. 10–12 cm deep) part of the teatcup; swabbing included the entire circumference of the inner wall of the teatcup in a circular manner.
Transportation of samples to the laboratory in Karditsa was performed by car, by the investigators. Samples were stored at 0.0 to 4.0 °C using ice packs in portable refrigerators.
Laboratory examination
Bacteriological examinations started within 12 h of collection of samples. Milk samples (10 μl) and swabs were cultured in duplicate on streptococcus selective media (Streptococcus selective agar; BioPrepare Microbiology, Athens, Greece). Plates were incubated aerobically at 37 °C for 48 h. If there was no growth, plates were re-incubated for another 24 h. Bacterial isolation and initial identification were performed using standards methods (Barrow and Feltham, Reference Barrow and Feltham1993; Euzeby, Reference Euzeby1997). Detection of at least three confirmed streptococcal colonies on at least one agar plate of those inoculated with milk samples or swabs from each farm, was considered to indicate presence of the organism in the bulk-tank milk or the teatcup, respectively. Identification to species level was performed using the Microgen® Strep-ID system (Microgen Strep-ID; Microgen Bioproducts Ltd, Camberley, United Kingdom).
After identification, all streptococcal isolates were cultured on Congo Red agar plates (BioPrepare Microbiology, Athens, Greece) for evaluation of biofilm formation. The plates were incubated aerobically at 37 °C for 24 h (Freeman et al., Reference Freeman, Falkiner and Keane1989). Slime-producing strains (‘ + ’) were indicated by black colonies with dry crystalline consistency. Dark-coloured colonies with no dry crystalline culture appearance were assigned an intermediate result (‘ ± ’), whilst colonies that remained pink, were considered to reflect non slime-producing strains (‘−’) (Freeman et al., Reference Freeman, Falkiner and Keane1989; Schönborn et al., Reference Schönborn, Wente, Paduch and Krömker2017).
Somatic cell counting (Lactoscan SCC; Milkotronic Ltd, Nova Zagora, Bulgaria) and milk composition measurement (Lactoscan Farm Eco; Milkotronic Ltd) were performed in duplicate; the two results were averaged, then the two means again averaged for the final result from each bulk-tank milk.
Data management and analysis
Data were entered into Microsoft Excel. Descriptive analysis was performed initially. The following outcomes were considered: ‘isolation of streptococci from bulk-tank milk’ and ‘isolation of streptococci from the milking machine teatcups’. The importance of predictors was assessed by using cross-tabulation with the Fisher exact test or the one-way analysis of variance, as appropriate, and with simple logistic regression. Moreover, the frequency of identification of Streptococcus uberis and the frequency of slime-producing streptococci among relevant isolates were also calculated. Statistical significance was defined at P < 0.05.
Results
Isolation of streptococci
Data are shown in Table 1. Streptococci were isolated from bulk-tank milk of 16/51 sheep (31.4%) and 4/23 goat (17.4%) farms (the difference between farm type was not significant, P > 0.05).
Table 1. Results of isolation of streptococci from bulk-tank milk samples and milking machine teatcups in sheep or goat farms

Streptococci were isolated from 10/207 teatcups in 5/44 sheep farms (4.8% and 11.4%, respectively) and 3/51 teatcups in 2/11 goat farms (5.9% and 18.2%) (once again, the difference between farm type was not significant, P > 0.05); in total, 18 isolates were obtained, 14 (10 from the upper and 4 from the lower part of the teatcups) on sheep and 4 (3 and 1) on goat farms. The more frequent isolation of streptococci from the upper part of the teatcups, 5.0% (13/258) vs. 1.9% (5/258), was significantly different (P < 0.05), whilst in five teatcups (4 in sheep farms and 1 in a goat farm) isolations from both sites were recorded.
Most isolates (57.9%) were identified as S. uberis; Streptococcus acidominimus was also identified (5.3% of all isolates), whilst 36.8% of the isolates could not be speciated. There was no difference in the frequency of isolation of S. uberis from sheep (18/30) or goat (4/8) farms (P > 0.05) and from swabs (12/18) or milk samples (10/20) (P > 0.05: Table 1).
Most isolates (26/38, 68.4%) were slime-producing. Slime-production was numerically more frequent among isolates obtained from swabs from teatcups (15/18, 83.3%) than among isolates from milk (11/20, 55.0%), although this difference was not statistically significant (P = 0.065). There was no difference in the frequency of slime-production according to species: 68.2% of S. uberis isolates and 68.8% of the other isolates were slime-producing (P > 0.05: Table 1). As shown in Fig. 1, the concurrent isolation of slime-producing streptococci from milk and teatcups within the same farm occurred more frequently than that of non-slime producing streptococci (n = 6 vs. n = 1 respectively), although this difference was not statistically significant. This was not seen for isolation of such strains only from milk or teatcups within the same farm (n = 6, vs. n = 9 for non-slime-producing isolates: Fig. 1).
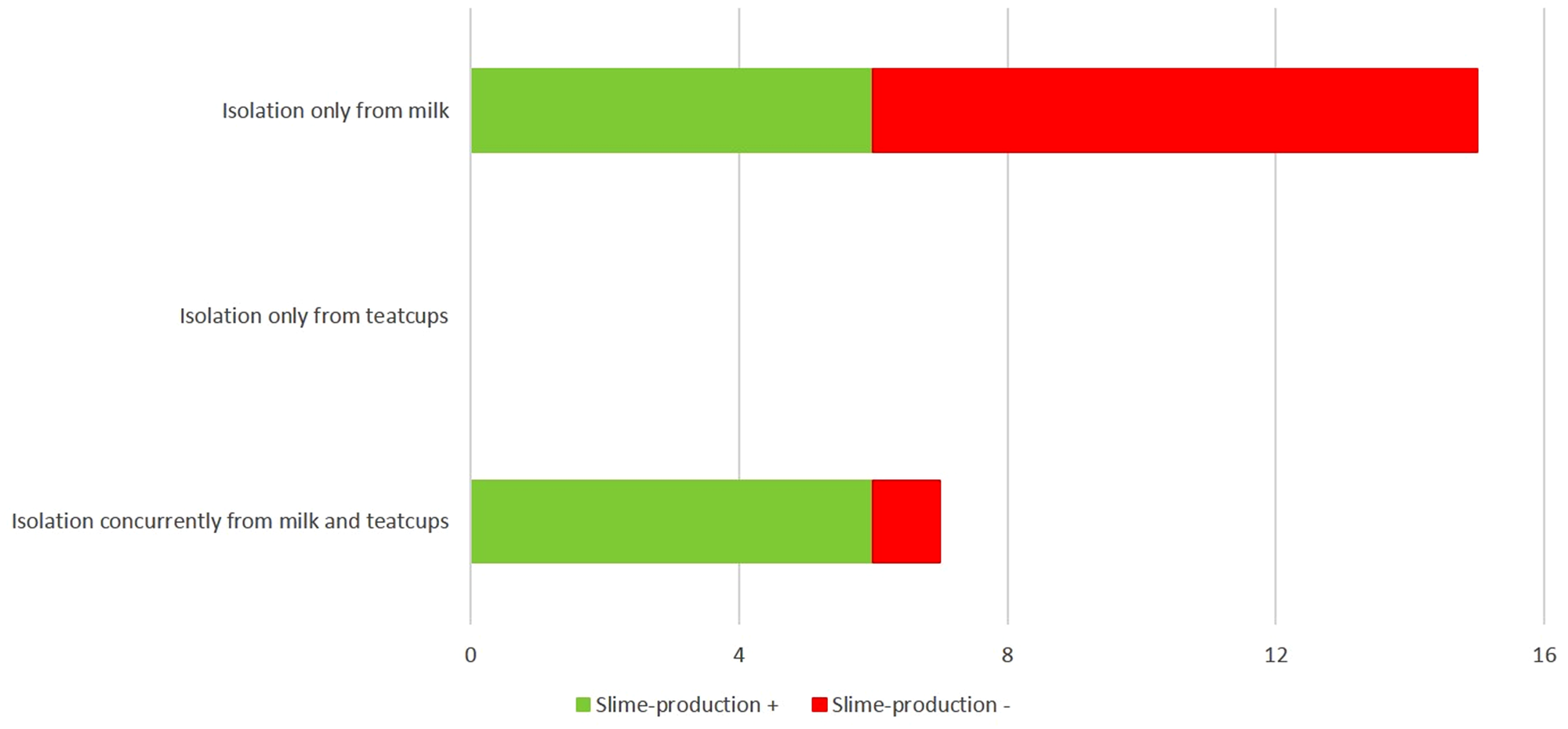
Fig. 1. Cases of isolation of slime- or non-slime-producing streptococci from the milk or the milking machine teatcups alone or concurrently, from dairy sheep or goat farms in Greece.
Milk somatic cell counts and composition
The data are in Table 2. There were no significant differences in somatic cell counts between farms from the bulk-tank milk samples of which streptococci were or were not isolated (P > 0.05 for both sheep and goats). Also, there were no significant differences in milk composition between farms from the bulk-tank milk samples of which streptococci were or were not isolated (P > 0.05 for sheep and goats: Table 2).
Table 2. Milk somatic cell counts and composition in relation to isolation of streptococci from the bulk-tank milk in sheep or goat farms

P > 0.22 between samples from farms in milk samples from which streptococci were or were not isolated.
Potential risk factors for isolation of streptococci
Data are in Table 3. Only machine-milking was associated with the isolation of streptococci from bulk-tank milk samples. This effect was significant for goat farms (P < 0.05) but not for sheep farms (P = 0.058). For the other variables evaluated, no such associations were evident (P > 0.21). On farms with sheep during the initial stage of the milking period (first two months), isolation of streptococci from teatcups was more frequent than from other farms (P < 0.01). For the other variables, no such associations were evident (P > 0.2: Table 3, online Supplementary Material 2).
Table 3. Findings regarding factors associated with isolation of streptococci from the bulk-tank milk or the milking machine teatcups in sheep or goat farms

Only factors with P ≤ 0.2 are presented.
Discussion
Streptococci are little-studied mastitis pathogens in sheep and goats, with most mastitis-related literature in these species referring to staphylococcal infections (Vasileiou et al., Reference Vasileiou, Chatzopoulos, Sarrou, Fragkou, Katsafadou, Mavrogianni, Petinaki and Fthenakis2019). Nevertheless, the importance of streptococci as potential pathogens for mastitis in small ruminants has recently been highlighted. Various species, including S. acidominimus, S. agalactiae, S. bovis, S. dysgalactiae, S. equi, S. pluranimalium, S. mitis, S. porcinus, S. suis, S. uberis, have been implicated as aetiological agents of the infection in both sheep and goats (Devriese et al., Reference Devriese, Vandamme, Collins, Alvarez, Pot, Hommez, Butaye and Haesebrouck1999; Fernandez et al., Reference Fernandez, Blume, Garrido, Collins, Mateos, Dominguez and Fernandez-Garayzabal2004; Marogna et al., Reference Marogna, Pilo, Vidili, Tola, Schianchi and Leori2012; Queiroga, Reference Queiroga2017).
Previously, we reported that the prevalence of subclinical streptococcal mastitis in ewes in Greece was approximately 5% (Vasileiou et al., Reference Vasileiou, Cripps, Ioannidi, Chatzopoulos, Gougoulis, Sarrou, Orfanou, Politis, Calvo Gonzalez-Valerio, Argyros, Mavrogianni, Petinaki and Fthenakis2018). Here, we took a different approach and examined samples from the bulk-tank and the milking machine teatcups, to obtain information from the entire flock or herd, which might be a potential indicator of the presence of streptococci in the animals. The results indicate a high frequency of streptococci in the bulk-tank milk. The two species identified, S. acidominimus and S. uberis, are both mammary gland pathogens, hence, this is likely to indicate intramammary infections.
It is also noteworthy that S. uberis is an environmental pathogen, present in the bedding in animal houses and on various sites of the animals (eg intestine), as described by Bramley (Reference Bramley1982) and Kruze and Bramley (Reference Kruze and Bramley1982). These authors isolated S. uberis in high numbers from straw samples in cattle farms, as well as from body sites (rectum, vulva). The present results did not indicate an association of S. uberis recovery with the use of straw as bedding in the farms studied.
The only factor associated with isolation of streptococci from bulk-tank milk was the application of milking machine in the farm. It is interesting that in the extensive countrywide study of Vasileiou et al. (Reference Vasileiou, Cripps, Ioannidi, Chatzopoulos, Gougoulis, Sarrou, Orfanou, Politis, Calvo Gonzalez-Valerio, Argyros, Mavrogianni, Petinaki and Fthenakis2018), machine-milking was also found to be the factor associated with subclinical mastitis of streptococcal aetiology. Machine-milking encompasses a variety of factors (e.g. milking conditions, cleaning practices), which, to a varying extent, may potentially contribute to either the development of mastitis or its effective control. Although on their own these factors were not associated with the isolation of streptococci, their combination can possibly contribute to the development of streptococcal mastitis, as indicated by the present results allied to those of Vasileiou et al. (Reference Vasileiou, Cripps, Ioannidi, Chatzopoulos, Gougoulis, Sarrou, Orfanou, Politis, Calvo Gonzalez-Valerio, Argyros, Mavrogianni, Petinaki and Fthenakis2018). One may thus postulate that with the expansion of mechanical milking of sheep and goats, the significance of streptococci as aetiological agents of mastitis might also increase.
Slime-production and biofilm formation has been shown to occur frequently in streptococcal isolates (including S. uberis) from mastitis in cows, with a frequency as high as 85% among evaluated collections of strains (Kaczorek et al., Reference Kaczorek, Małaczewska, Wójcik and Siwicki2017; Schönborn et al., Reference Schönborn, Wente, Paduch and Krömker2017; Dieser et al., Reference Dieser, Fessia, Zanotti, Raspanti and Odierno2019). The current results present for the first time the slime-production and biofilm-formation from streptococcal isolates from small ruminants and are comparable to those reported in strains from cattle.
The current study also reports for the first time the isolation of streptococci from milking machine teatcups in small ruminant farms. In cattle, Santos et al. (Reference Santos, Pires, Behaine, Araújo, de Andrade and de Carvalho2013) reported the identification of S. agalactiae from teatcups and suggested their recovery as an indicator of incorrect cleaning procedures in the farms. In this context, it is noteworthy that S. uberis is an environmental organism and can be found in farm bedding. One may postulate that the organism contaminates the skin of the udder and the teats possibly when animals would be lying on straw, particularly in wet and muddy conditions. When the animals are brought into the milking parlour, S. uberis present on the teat-end may possibly colonise the milking machine teatcups, especially slime-producing strains, which attach easily onto these. The bacteria may then possibly colonise the teat-end(s) of the animals milked subsequently, which can potentially lead to an increase in intramammary infections. In a recent challenge study, Hillerton (Reference Hillerton2020) reported that streptococci could cause clinical mastitis more frequently if infused immediately after milking than at other times in relation to milking. This may possibly be allied to a potential transmission of the bacteria from the teatcups to ewes occurring during milking.
The increased frequency of slime-producing isolates recovered from the teatcups, especially from their upper part, indicates that these bacteria could attach and multiply on these, thus increasing potential of intramammary infections. Moreover, presence of slime-production in streptococcal isolates also raises concerns regarding treatment of these pathogens in cases of intramammary infections, as it increases the antimicrobial resistance of biofilm-forming bacteria (Hickey et al., Reference Hickey, Wong, Khazandi, Ogunniyi, Petrovski, Garg, Page, O'Handley and Trott2018; Dieser et al., Reference Dieser, Fessia, Zanotti, Raspanti and Odierno2019).
In conclusion, the study found frequent recoveries of streptococci from bulk-tank and milking teatcups in sheep and goat dairy farms. S. uberis predominated among isolates. Machine-milking was associated with the isolation of streptococci from bulk-tank milk samples; on sheep farms, isolation of streptococci from teatcups was more frequent during the initial stage of the milking period. Whilst the results do not prove origin of these bacteria from intramammary infections, they nevertheless indicate a potential to create streptococcal intramammary infections during milking of the animals.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029920000734.






