Many bioactive peptides, such as opioid peptides, are produced after digestion of milk protein casein by digestive enzymes (Clare & Swaisgood, Reference Clare and Swaisgood2000; Shah, Reference Shah2000; Silva & Malcata, Reference Silva and Malcata2005; Phelan et al. Reference Phelan, Aherne, FitzGerald and O'Brien2009). Amongst these, four major casein phosphopeptides (CPPs) have been isolated from αs1-, αs2-, and β-casein tryptic hydrolysates. All of these CPPs contain the phosphoseryl cluster sequence Ser(P)-Ser(P)-Ser(P)-Glu-Glu. These motifs are known to interact with cations such as Ca++, Fe++, and Zn++ and increase their bioavailability (Tsuchita et al. Reference Tsuchita, Goto, Shimizu, Yonehara and Kuwata1995; Pérès et al. Reference Oudshoorn, Hiemstra, Hessing, Rutten, Visser and Simons1998; Saito et al. Reference Saito, Lee and Kimura1998; Scholz-Ahrens & Schrezenmeir, Reference Scholz-Ahrens and Schrezenmeir2000; Bouhallab et al. Reference Bouhallab, Ginga, Ait-Okuhara, Bureau, Neuville, Arhan, Naubois and Bougle2002; Bouhallab & Bougle, Reference Bouhallab and Bougle2004). As to bioavailability of Ca++, however, the results are controversial. Some studies reported negative effects of CPPs on Ca++ absorption (Narva et al. Reference Narva, Kärkkäinen, Poussa, Lamberg-Allardt and Korpela2003; López-Huertas et al. Reference López-Huertas, Teucher, Boza, Martínez-Férez, Majsak-Newman, Baró, Carrero, González-Santiago, Fonollá and Fairweather-Tait2006; Teucher et al. Reference Teucher, Majsak-Newman, Dainty, McDonagh, FitzGerald and Fairweather-Tait2006). Several factors were suggested to influence the effects of CPPs, such as species, the food matrix, and the ratio of CPP to calcium (Erba et al. Reference Erba, Ciappellano and Testolin2002), (López-Huertas et al. Reference López-Huertas, Teucher, Boza, Martínez-Férez, Majsak-Newman, Baró, Carrero, González-Santiago, Fonollá and Fairweather-Tait2006). Furthermore, CPP stabilises nanoclusters of amorphous calcium phosphate (ACP) and the CPP-amorphous calcium phosphate (CPP-ACP) formed has been demonstrated to enhance remineralization in vitro and in vivo (Reynolds et al. Reference Reynolds, Cain, Webber, Black, Riley, Johnson and Perich1995; Reynolds, Reference Reynolds1997, Reference Reynolds1998). CPP-ACP in chewing gum, mouth rinse, and dairy products has been shown to remineralise subsurface enamel lesions in human in situ experiments (Reynolds et al. Reference Reynolds, Cai, Shen and Walker2003; Walker et al. Reference Walker, Cai, Shen, Reynolds, Ward, Fone, Honda, Koganei, Oda and Reynolds2006, Reference Walker, Cai, Shen, Bailey, Yuan, Cochrane, Reynolds and Reynolds2009; Morgan et al. Reference Morgan, Adams, Bailey, Tsao and Reynolds2008).
CPPs obtained after proteolytic digestion can be selectively enriched through interaction with polyvalent ions either by precipitation (Manson & Annan, Reference Manson and Annan1971; Reynolds et al. Reference Reynolds, Riley and Adamson1994) or by affinity chromatography (Lund & Ardö, Reference Lund and Ardö2004; Negroni et al. Reference Negroni, Claverol, Rosenbaum, Chevet, Bonneu and Schmitter2012). Gaucheron et al. (Reference Gaucheron, Mollé, Léonil and Maubois1995) reported a useful procedure for the selective determination of β-CPP (1–25) by adding Fe (ΙΙ) to tryptic hydrolysates of β-casein. The combination of a selective procedure for CPP enrichment and mass spectrometry after anion exchange fast protein liquid chromatography (FPLC) and/or reversed phase high-performance liquid chromatography (HPLC) has been successfully employed to identify CPP components of dairy products (Adamson & Reynolds, Reference Adamson and Reynolds1997; Ferranti et al. Reference Ferranti, Barone, Chianese, Addeo, Scaloni, Pellegrino and Resmini1997; Miquel et al. Reference Miquel, Alegria, Barbera and Farre2005). Additionally, Adamson et al. (Reference Adamson, Riley and Reynolds1993) showed that capillary zone electrophoresis was applicable to quantitative analysis of CPP. These methods each have individual merits but are relatively costly, time consuming and require extensive professional training to operate the sophisticated equipment involved and to identify individual peaks. Furthermore, the identification and quantitation of CPP in dairy products produced by processes such as fermentation or exogenous addition is difficult because of the existence of closely related endogenous molecules in the target analytes. Moreover, to quantify the overall amount of CPP complexes such as CPP-ACP is not straightforward because CPP forms various phases with calcium and phosphate at differing pH and concentrations (Reynolds, Reference Reynolds1997). Aoki et al. (Reference Aoki, Nakano, Iwashita, Sugimoto, Ibrahim, Toba, Aoe and Nakajima1998) reported the separation of CPP and the phosphopeptide complex formed in casein micelles by ion exchange HPLC. However, this method is not suitable as a routine analytical tool because of limitations in specificity and ease of handling.
In contrast, immunoassays are generally rapid, sensitive, specific, and cost-effective. In addition, they facilitate the routine handling of large sample numbers. Methods using polyclonal antibodies reported to date include the quantitation of β-casein derived phosphopeptides during cheese ripening (Pizzano et al. Reference Pizzano, Nicolai, Padovano, Ferranti, Barone and Addeo2000) and the detection of CPPs in the distal ileostomy fluid of human subjects fed with milk or CPP (Meisel et al. Reference Meisel, Bernard, Fairweather-Tait, FitzGerald, Hartmann, Lane, McDonagh, Teucher and Wal2003). Generally, it would be more convincing to characterise biological markers using authentic molecules through the use of monoclonal antibodies (mAbs) directed to a single, biospecific target.
We describe in this communication, the specific and simple measurement of β-CPP in dairy foods using a combination of sample pretreatment designed for CPP enrichment and a specific mAb-based competitive enzyme–linked immunosorbent assay (ELISA).
This method is applicable to the detection and quantification of β-CPP in CPP or CPP complexes naturally present, added exogenously, or produced during fermentation of dairy products.
Material and Methods
Monoclonal antibody
A mouse monoclonal antibody 1A5 (mAb 1A5) was produced against HPLC-purified β-casein derived tryptic peptide f(1–25) (Kaneko et al. Reference Kaneko, Kurisaki, Mizumachi and Ishiguro1995). The antibody was a member of the IgG2b subclass and purified by Protein A Sepharose (HiTrap Protein A HP Column, GE Healthcare Ltd, Uppsala, Sweden) according to the supplier's instructions.
Peroxidase-conjugated, affinity purified, rabbit anti-mouse IgG F(c) was purchased from Rockland (Gilbertsville, PA, USA).
Peptide purification and chemical synthesis
To address the specificity of mAb 1A5, casein phosphopeptides were isolated from tryptic digests of α-casein or β-casein using selective precipitation (Reynolds et al. Reference Reynolds, Riley and Adamson1994) and purification by preparative and analytical reversed phase (RP) HPLC combinations. A Waters HPLC system, 600ES System Controller/996 Photodiode Array Detector (Nihon Waters K.K. Tokyo, Japan), was used in this study. As a first step, the tryptic peptides were run through a preparative column (TSK gel ODS-80Ts, 20×250 mm, Tosoh Co., Ltd, Tokyo, Japan). Solvent A was 0·1% (v/v) trifluoroacetic acid (TFA) in HPLC-grade water, while solvent B consisted of 0·15% (v/v) TFA in HPLC-grade acetonitrile (Wako Pure Chemicals Co. Ltd, Osaka, Japan). The column was initially equilibrated with 5% solvent B. Flow was set at 5 ml/min and detection was at 214 nm. For preparation of α-casein-derived phosphopeptides (α-CPPs), elution was performed at room temperature with a linear gradient of solvent B from 5 to 15% over 5 min, followed by increases to 25 and 100% over 30 and 5 min, respectively. For preparation of β-CPP the gradient of solvent B was increased from 5 to 25% over 5 min, then after 30 min it was increased to 100% over 5 min at room temperature.
The desired fractions recovered from preparative RP-HPLC were further purified using an analytical column. The peptide fractions derived from α-CPP were run through analytical columns (TSK gel ODS-80Ts, 4·6×250 mm, Tosoh, or Inertsil ODS-3, 4·6×250 mm, GL Sciences, Inc. Tokyo, Japan). Elution was performed at room temperature by a linear gradient of solvent B from 5 to 15% over 5 min, followed by increases to 25 and 100% over 30 and 5 min, respectively. For further purification of β-CPP the fractions recovered were loaded onto a column (Inertsil ODS-3, 4·6×250 mm, GL Sciences) and elution was performed at room temperature using a linear gradient of solvent B from 5 to 25% over 5 min, followed after 35 min by an increase to 100% over 5 min. In both cases, the flow rate was set at 1 ml/min and detection was at 214 nm.
The structures of the target CPPs were confirmed by five N-terminal amino acid sequences performed in a Procise 494 HT protein sequencing system (Applied Biosystems, Foster City, CA, USA) and molecular masses by a Bruker Biflex matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometer (Bruker-Franzen Analytik, Bremen, Germany) at the APRO Life Science Institute, Inc. (Tokushima, Japan).
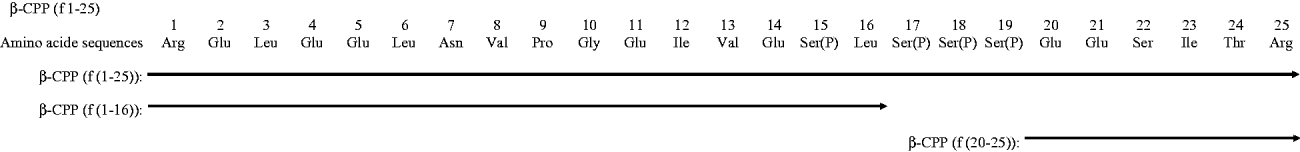
In addition, two peptide components of β-CPP, f(1–16) and f(20–25), were chemically synthesised (Genemed Synthesis, Inc., San Francisco, CA, USA).
Alkaline dephosphorylation of phosphopeptide
CPP-ACP (Cadbury Schweppes, Melbourne, Victoria, Australia) was used as test phosphopeptide material. CPP-ACP (0·5 mg/ml) was treated with increasing concentrations (0–0·6 mol/l) of sodium hydroxide for 3 h at 37 °C, following the procedure of Jiang & Mine (Reference Jiang and Mine2000). After treatment, the pH was neutralised by the addition of 1 mol/l HCl and partially dephosphorylated CPP (dep CPP) was recovered by solid-phase extraction, as described below. Controls were treated in the same way except they were neutralised beforehand to suppress alkaline dephosphorylation. Total phosphorus content of dep CPP was determined by the method of Ames (Reference Ames1966). The reactivity of dep CPP to mAb 1A5 was evaluated by the competitive ELISA, as described below.
Preparation of milk samples containing CPP-ACP for immunological analyses
CPP-ACP amount in dairy products
Reference milk samples were prepared by dissolving CPP-ACP in milk at concentrations of 0, 1, 3, or 4 mg/ml. CPP-ACP-supplemented commercial dairy products were used as test samples. Pretreatment for deproteination and peptide purification was conducted before the competitive ELISA. Briefly, to 0·3 ml of the reference or test samples in plastic tubes, 1·2 ml 15% trichloroacetic acid (TCA) and 1·5 ml 10 mm phosphate pH 7·3 containing 145 mm NaCl (PBS) were sequentially added, dropwise while vortexing. Acid-denatured high molecular weight proteins (e.g., casein and whey) were removed by centrifugation at 17 000 g for 6 min at 4 °C. The supernatant (1·2 ml) recovered was mixed with PBS (1·2 ml) and the aliquot (2 ml) was then applied to the solid-phase extraction cartridge (Waters OASIS™ HLB1cc, 30 mg, Waters Corporation, Milford, MA, USA) equilibrated with HPLC grade water (0·1%TFA). The unabsorbed substances and TCA were removed by sequential washing with 2 ml water (0·1% (v/v) TFA) and 5% methanol (0·1% (v/v) TFA). The CPP-enriched fractions were eluted with 2 ml of 50% acetonitrile (0·1% TFA), and dried in a centrifugal concentrator. The peptide extracts were reconstituted in PBS and the CPP-ACP amount was estimated using the competitive ELISA.
β-CPP in CPP-ACP
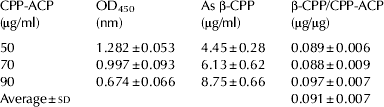
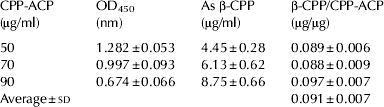
Reference samples were prepared by dissolving purified β-CPP in PBS at concentrations of 0, 2, 4, 6, 8, or 10 μg/ml. As test samples, CPP-ACP was dissolved in PBS at concentrations of 50, 70 and 90 μg/ml (all within the standard curve range) and used directly in the competitive ELISA.
Competitive ELISA procedure
Immunological quantitation was performed by a competitive ELISA according to a modified method of Hornbeck (Reference Hornbeck, Coligan, Kruisbeek, Margulies, Shevach and Strober1991).
Ninety-six-well plates (Nunc MaxiSorp, Thermo Fisher Scientific Inc., Roskilde, Denmark) were coated with 100 μl of 50 μg/ml CPPIII (Meiji Food Material Co., Ltd, Tokyo, Japan) in PBS for a minimum of 2 h at 37 °C. About 1·5 h after the coating of wells with CPP III, the mAb 1A5 was diluted to 2 mg/ml with PBS containing 2% (w/v) BSA, mixed with the same volume of appropriately diluted analyte solutions (pretreated dairy products, CPP-ACP, or purified α- and β-CPP) in PBS, and incubated in U-shaped 96-well plates (FALCON #351177, Becton Dickinson and Company, Franklin, NJ, USA) for 1·5 h at 37 °C. CPPIII solution for coating was then removed and the modules were blocked by adding 200 μl PBS containing 1% (w/v) BSA for 1 h at 37 °C. The blocking BSA was removed and an aliquot (50 μl) of each mixture of antibody and competing analyte samples were added to each well and incubated for 1 h at 37 °C. The modules were washed three times with PBS containing 0·05% (v/v) Tween 20 (T) and 100 μl peroxidase-conjugated secondary antibody diluted to 1/6000 in PBS containing 1% (w/v) BSA was added per well. After incubation for 1 h at 37 °C, the modules were washed three times with PBST and 100 μl of ready-to-use substrate-chromogen (DAKO™ TMB+ Substrate-Chromogen, Dako Japan Inc., Tokyo, Japan) was added per well. The enzyme reaction was stopped after 5 min incubation at room temperature (in the dark) by the addition of 100 μl 0·5 mol/l H2SO4 (Wako Pure Chemicals Co. Ltd) per well. The reaction product formed per well was measured spectrophotometrically at 450 nm with a Bio-Rad Model 3550 Microplate Reader (Bio-Rad Laboratories Ltd, Hercules, CA, USA). The standard and test samples were processed in the same plate. A standard curve was constructed by plotting the concentrations (x) of standards against the mean absorbance (y) of standards at 450 nm. An exponential regression was used to fit the standards and calculate the sample concentrations.
Results
Epitope region and specificity of mAb 1A5
Chemically synthesised (f(1–16) and f(20–25)) and purified (f(1–25)) peptide sequences encompassing β-CPP are shown in Fig. 1. The 1–25 fragment of β-casein inhibited antibodies binding to CPP III (as assessed by competitive ELISA), while the 1–16 and 20–25 fragments did not. This suggests that the epitope could be mapped around the region f(17–19) containing the phosphoseryl cluster sequence (Fig. 2).
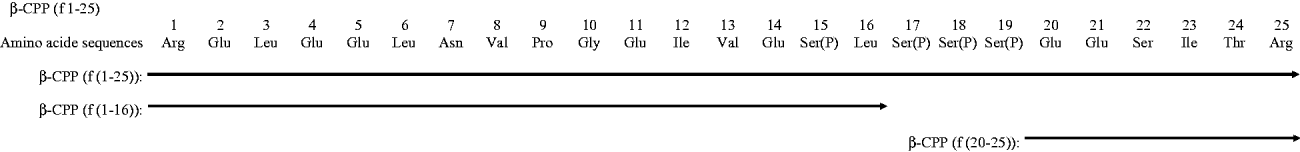
Fig. 1. Amino acid sequences of β-CPP Both β-CPP (f(1–25)) and the two chemically synthesised peptides (f(1–16), f(20–25)) are shown.

Fig. 2. Reactivity of mAb 1A5 against different regions of β-CPP: ○, purified peptide f(1–25); ●, chemically synthesised peptide f(1–16); △ chemically synthesised peptide f(20–25). Each point represents the mean±sd for triplicate measurements.
The importance of the phosphoseryl cluster sequence for the recognition of mAb 1A5 was further explored using dep CPPs. Dep CPPs were prepared by treating CPP-ACP with an increasing concentration of sodium hydroxide. A gradual loss of total phosphate content and antigenicity was observed with increasing sodium hydroxide concentration (Table 1). These results show that the phosphorylated residues in serine segments are critical for antibody binding to CPP.
Table 1. Decrease in total phosphorus contents and binding activity with mAb 1A5 after NaOH treatment Incubation of CPP-ACP (0·5 mg/ml) with NaOH (0–0·6 mol/l) was carried out at 37 °C for 3 h. Dephosphorylated CPPs were recovered by solid-phase extraction prior to the measurement of total phosphorus content and the competitive ELISA. Peptides retaining binding activity with mAb 1A5 were expressed as authentic CPP-ACP concentrations. Each value is the mean±sd for triplicate measurements

It has been shown that digestion of bovine casein with trypsin releases four major phosphopeptides (Reynolds, Reference Reynolds1998). To clarify the specificity, these four peptides were purified by HPLC and N-terminal amino acid sequences and mass spectrometry of the peptides was carried out. The elution profile of tryptic α-CPP with the TSK gel ODS-80Ts preparative column is shown in Fig. 3a. Peptides were collected in five fractions (A to E), and fractions A, B, and D were subjected to further purification. Fractions A, B, and D were further fractionated into four (A1 to A4), one (B1), and two (D1, D2) samples, respectively, by analytical columns (Fig. 3b, d, f). These fractions were further purified to a single peak (Fig. 3c, e, g), and fractions A3, B1, and D1 were confirmed to be αs1-casein (f(59–79)), αs2-caseins (f(46–70)), and (f(1–21)), respectively, by N-terminal amino acid sequencing and mass spectrometry (data not shown). Figure 4a shows the elution profile of tryptic β-CPP with the TSK gel ODS-80Ts preparative column. Fraction F was sequentially purified into two fractions (F1, F2) and a single fraction (F1) (Fig. 4b, c). The results of N-terminal amino acid sequencing and mass spectrometry showed that fraction F1 corresponded to β-casein (f(1–25)) (data not shown).

Fig. 3. HPLC chromatograms of the preparative separation of phosphoryl tryptic peptides from α-casein (a), further purification of fraction A (b, c), fraction B (d, e), and fraction D (f, g). Chromatographic conditions were as follows. (a): Column, TSK gel ODS-80Ts (20×250 mm); solvent A, 0·1% trifluoroacetic acid in deionised water; solvent B, 0·1% trifluoroacetic acid in acetonitrile; gradient, 5–15% B over 5 min, 15–25% B over 30 min, and 25–100% B over 5 min; flow, 5 ml/min; absorbance, 214 nm; column temperature, room temperature. (b, c, d, e): Chromatographic conditions were the same as in (a) except for the column, TSK gel ODS-80Ts (4·6×250 mm); flow, 1 ml/min. (f, g): Chromatographic conditions were the same as in (a) except for the column, Inertsil ODS-3 (4·6×250 mm); flow, 1 ml/min.

Fig. 4. HPLC chromatograms of preparative separation of phosphoryl tryptic peptides from β-casein (a) and further purification of fraction F (b, c). Chromatographic conditions were as follows. (a): Column, TSK gel ODS-80Ts (20×250 mm); solvent A, 0·1% trifluoroacetic acid in deionised water; solvent B, 0·1% trifluoroacetic acid in acetonitrile; gradient, 5–25% B over 5 min, 25% B for 30 min, and 25–100% B over 5 min; flow, 5 ml/min; absorbance, 214 nm; column temperature, room temperature. (b, c): Chromatographic conditions were the same as in (a) except for the column, Inertsil ODS-3 (4·6×250 mm); gradient, 5–25% B over 5 min, 25% B for 35 min, and 25–100% B over 5 min; flow, 1 ml/min.
The reactivity of each peptide to mAb 1A5 was assessed by competitive ELISA (Fig. 5). The immunological response could be quantified by its capacity to inhibit the binding of mAb 1A5 to the antigen (CPPIII) adsorbed on the solid phase. The ELISA value decreased noticeably when mAb 1A5 was first incubated with β-CPP (f(1–25)) but not when incubated with the other major tryptic bovine CPP, i.e. αs1-CPP (f(59–79)) and αs2-CPPs (f(1–21), f(46–70)), indicating that β-CPP had the highest antibody-binding activity.

Fig. 5. Specificity of mAb 1A5 for β-CPP The mAb 1A5 reactivity against ○, β-CPP f(1–25); ●, αS1-CPP f(59–70); △, αS2-CPP f(1–21); ▲, αS2-CPP f(46–70). Serial dilutions (0·78–100 μg/ml) of each peptide were pre-incubated with mAb 1A5 for 1·5 h at 37 °C. Specificity of the mAb 1A5 was evaluated by the residual antibody titre using the competitive ELISA. Each point represents the mean±sd for triplicate measurements.
Overall, these results suggest that the epitope recognised by mAb 1A5 is located in the region of β-CPP (f(1–25)) containing the common motif Ser(P)-Ser(P)-Ser(P)-Glu-Glu and its surrounding residues.
Measurement of CPP-ACP added into dairy products
Using the new competitive ELISA, we measured the actual amount of CPP-ACP added into dairy products. A standard curve for CPP-ACP was constructed by plotting absorbance values at 450 nm on the y-axis, and known amounts of CPP-ACP (0–4 mg/ml) along the x-axis. The exponential regression was given by y=2·7396e−0·4456x. To calculate unknown CPP-ACP values, absorbance readings, y, were measured in test samples and the equation was transformed to x=(ln(y/2·7396))/−0·4456. The absorbance of each test sample was substituted into the equation, and the CPP-ACP content was calculated (Table 2).
Table 2. CPP-ACP amount in test samples The abundance of CPP-ACP in the test samples was determined by the competitive ELISA. Each value is the mean±sd for triplicate measurements

Measurement of β-CPP in CPP-ACP as a dairy ingredient
To describe β-CPP as a representative index of CPP, we measured the β-CPP content in CPP-ACP used for commercial dairy products. The standard curve for β-CPP was constructed by plotting absorbance values at 450 nm on the y-axis, and known amounts of purified β-CPP (0–10 μg/ml) along the x-axis. The exponential regression was given by y=2·4896e−0·1494x. To calculate unknown β-CPP values, the absorbance, y, was measured in test samples and the equation was transformed to x=(Ln(y/2·4896))/−0·1494. The absorbance of each known amount of CPP-ACP (50, 70, 90 μg/ml) was substituted into the equation, and the β-CPP contents per unit amount of CPP-ACP were calculated (Table 3). This strategy made it possible to calculate β-CPP as a representative key functional component in dairy products supplemented with CPP-ACP.
Table 3. β-CPP amount per unit CPP-ACP The β-CPP content in the test samples was determined by the competitive ELISA. Each value is the mean±sd for triplicate measurements
Discussion
We have described an immunological method that is accurate, highly specific, economical, and easy to handle during food analysis, especially for dairy products. Using competitive ELISA, we demonstrated mAb 1A5 raised against β-CPP specifically recognises β-CPP (f(1–25)) amongst four major tryptic casein phosphopeptides. Our method is thus a promising tool for detecting β-CPP without cross-detection of endogenous related milk proteins and peptides. From the results of the response to chemically synthesised peptides, dep CPPs, and four major purified CPPs, the epitope of mAb 1A5 could be located in the region containing the common motif Ser(P)-Ser(P)-Ser(P)-Glu-Glu and surrounding residues. Epitopes of β-casein including f(1–25) have been reported in the literature. Otani et al. (Reference Otani, Higashiyama and Tokita1984, Reference Otani, Mine and Hosono1987) identified the antigenic regions of β-casein that were recognised by rabbits residues as f(1–25), f(26–60), f(61–93), f(94–102), f(103–109), f(110–144), and f(157–185). Senocq et al. (Reference Senocq, Dupont, Rolet-Répécaud and Levieux2001) raised 21 monoclonal antibodies against bovine β-casein and identified six discrete antigenic determinants, i.e. f(4–8), f(14–24), f(33–48), f(49–91), f(178–183), and f(184–209). These determinants were furthermore assigned 14 (respectively 1, 4, 1, 2, 1, and 5) distinct epitopes combining reactivity of the monoclonal antibodies with alien (ovine, caprine, human, equine) whole casein. Neither group, however, characterised specific recognition between α- and β- casein phosphopeptides. In this regard, using a rabbit anti-casein antibody-based competitive ELISA, Perich et al. (Reference Perich, Black, Huq and Reynolds1999) demonstrated that the phosphorylated residues in the cluster sequence –Ser(p)66-Ser(p)-Ser(p)68 and –Pro73-Asn-Ser(p)-Val-Glu77 in αs1-casein were critical for antibody recognition. Furthermore, they showed that after incubation, rabbit anti-casein antibody binding to αs1-casein (59–79) competed with β-casein (f(1–25)), indicating that αs1-casein (f(59–79)) and β-casein (f(1–25)) were immunologically indistinguishable in their assay system. Our results coincided with the above-mentioned reports in that the N-terminal epitope of β-casein was located in f(1–25) containing the phosphorylated serine cluster and further clarified the specific recognition of the tryptic phosphopeptide derived from β-casein but not from α-casein. In milk, casein molecules are associated into a supramolecular structure, the micelle. Johansson et al. (Reference Johansson, Lugand, Rolet-Répécaud, Mollé, Delage, Peltre, Marchesseau, Léonil and Dupont2009) produced a panel of monoclonal antibodies against κ-, β-, αs1-, and αs2-casein as structured within casein micelles to determine the casein immuno-dominant epitopes in assembled supramolecular structures. They obtained only one clone that recognised β-CPP (f(1–25)) amongst 25 monoclonal antibodies for β-casein. Although the epitope region of the monoclonal antibody seems to be close to our mAb1A5, the binding affinity appears to be different as a result of the immunogens used. Namely, Johansson et al. (Reference Johansson, Lugand, Rolet-Répécaud, Mollé, Delage, Peltre, Marchesseau, Léonil and Dupont2009) immunised mice with defatted raw milk, in which the corresponding region was suggested to be hidden in the micelle structure, causing low binding affinity. In addition, several other mAbs raised against β-casein have been isolated to date but they differ from mAb 1A5 in the epitope region (Nagaune et al. Reference Nagaune, Kaminogawa, Enomoto, Kobayashi, Kurisaki and Yamaguch1988; Oudshoorn et al. Reference Oudshoorn, Hiemstra, Hessing, Rutten, Visser and Simons1994; Gaiaschi et al. Reference Gaiaschi, Beretta, Poiesi, Conti, Giuffrida, Galli and Restani2001), and avidity with dephosphorylated determinants (Kuzmanoff et al. Reference Kuzmanoff, Andersen and Beattie1991). Anguita et al. (Reference Anguita, Martín, Gracía, Morales, Haza, González, Sanz and Hernández1995) also reported MAb AH4 against bovine β-casein but the epitope region was not identified.
Most of the above studies used purified β-casein as an immunogen to study quantitative detection of bovine components in ovine and caprine milk and cheese samples (Anguita et al. Reference Anguita, Martín, Gracía, Morales, Haza, González, Sanz and Hernández1995), immunological monitoring of β-casein proteolysis in cheese during ripening (Gaiaschi et al. Reference Gaiaschi, Beretta, Poiesi, Conti, Giuffrida, Galli and Restani2001), and identification and characterisation of multi epitopic profiles in β-casein (Nagaune et al. Reference Nagaune, Kaminogawa, Enomoto, Kobayashi, Kurisaki and Yamaguch1988; Kuzmanoff et al. Reference Kuzmanoff, Andersen and Beattie1991; Oudshoorn et al. Reference Oudshoorn, Hiemstra, Hessing, Rutten, Visser and Simons1994). In contrast, we immunised mice with purified β-CPP (f(1–25)) to develop an immunological tool to detect and quantify β-CPP, with high specificity and frequency, amongst coexisting, related phosphopeptides. In this way, we could effectively obtain a monoclonal antibody suitable for our purpose.
In our assay system, to measure the amounts of CPP adducts such as CPP-ACP added into dairy products, TCA treatment and subsequent solid-phase extraction is required, to remove endogenous native proteins, TCA, and recover CPPs. Although this set of procedures is essential, the influence of each step on the recovery of CPPs should be kept in mind. To resolve this potential technical problem, we successfully obtained reliable results from standard samples of CPP-ACP prepared in a similar solvating media (commercial whole milk) using the same sample preparation procedures as those used for test samples, in parallel.
The competitive ELISA method developed in this paper is promising for the detection and quantitation of β-CPP in dairy foods such as raw and processed milk, cheese, yogurt, or ice cream, containing structurally related peptides. This technology meets a pressing need for a sensitive and reliable method for the quantitative identification of key functional components, required for applications to the government for approval of foods for specified health uses (FOSHU).