‘Torta del Casar’ is a Spanish soft-ripened cheese produced under the Registry of the Protected Designation of Origin (PDO) ‘Torta del Casar’ (Casar de Cáceres, Cáceres, Spain) in accordance with Regulation (EC 1491/2003) of the European Commission. It is made from raw ewes’ milk from the Merino and Entrefino type breeds, using vegetable rennet made from Cynara cardunculus flower heads as clotting agent. Its microbiological and physicochemical characteristics have already been described (Mas et al. Reference Mas, González and Nieto1991; Poullet et al. Reference Poullet, Huertas, Sánchez, Cáceres and Larriba1991, Reference Poullet, Huertas, Sánchez, Cáceres and Larriba1993; Cáceres et al. Reference Cáceres, Castillo and Pizarro1997; Ordiales et al. Reference Ordiales, Benito, Martín, Casquete, Serradilla and Córdoba2013). One of the most distinctive characteristic of Torta del Casar cheese is its soft to spreadable texture due to the high proteolytic activity in curd (Delgado et al. Reference Delgado, Rodríguez, González, Ramírez and Roa2010). According to PDO Torta del Casar regulations, wet or dry salting is accepted but NaCl may not exceed 3%. Salt uptake during wet-salting has been extensively studied for many cheese varieties (Geurts et al. Reference Geurts, Walstra and Mulder1980; Guinee & Fox, Reference Guinee and Fox1983, Morris et al. Reference Morris, Guinee and Fox1985; González-Martínez et al. Reference González-Martínez, Chafer, Fito and Chiralt2002; Melilli et al. Reference Melilli, Barbano, Licitra, Tumino, Farina and Carpino2003, Reference Melilli, Barbano, Caccamo, Calvo, Schembari and Licitra2004, 2006), nevertheless there is no information for cheeses like Torta del Casar in this matter.
During ripening, salt level has a major effect on microbial growth, enzymatic activities and biochemical changes, which determine flavour, aroma and texture (Guinee & Fox, Reference Guinee, Fox, Fox, McSweeney, Cogan and Guinee2004; Ardö et al. Reference Ardö, Skeie and Guinee2014). As a result, salt influences the overall quality of cheese. Many factors affect salt uptake and diffusion in cheese. Parameters like cheese composition and structure play a crucial role. In addition, technological factors of brine salting, such as salt levels in brine, salting time, brining temperature, curd moisture, pH of cheese prior to brining, etc. must be taken into account (Guinee & Fox, Reference Guinee, Fox, Fox, McSweeney, Cogan and Guinee2004). Consequently, the precise control of these factors is an essential part of the cheesemaking process to ensure consistent and optimum quality (Guinee, Reference Guinee2004). Together with temperature, pH, water activity (aw), redox potential and microorganisms, salt assists in cheese conservation by minimising spoilage and preventing the growth of pathogens (Labrie et al. Reference Labrie, Bisig and Jordan2014). These factors interact with each other, and in many situations the distinction between them is not clear-cut.
Surface microbiota is essential not only for cheese ripening, but also for rind appearance, and therefore, cheese acceptance. Rind discolorations seem to be a frequent defect in many cheeses, frequently caused by the unbalanced growth of surface microbiota. Brevibacterium and Pseudomonas were identified as responsible for brown-violet colouration in Brie cheese (Asperger, Reference Asperger1986), and Yarrowia lipolytica in semi-soft ewes’ cheeses (Carreira et al. Reference Carreira, Paloma and Loureiro1998). In order to avoid these alterations, controlling growth of smear microorganisms is necessary.
The aim of this study was (i) to determine the influence of different factors (salt brine concentration, temperature and salting time) on salt uptake and its diffusion during cheese ripening and (ii) to investigate the influence of salt concentration on Torta del Casar physicochemical parameters and rind microbiota.
Materials and methods
Milk and cheese making
The milk was obtained by mixing evening and morning milkings from Merino and Entrefino breeds. 2500 l refrigerated raw milk were stirred and tempered in a stainless steel vat at 30 °C. Cheese was manufactured according to the PDO Torta del Casar regulation (OJEU, 2002). The curds were placed in cylindrical moulds and subjected to pressure. Resulting cheese was about 6 cm height, 12 cm diameter and 0·650 kg weight. Cheese ripening was carried out at 6–8 °C and 85–90% relative humidity for 60 d.
Cheese brining and sampling
Different brining methods were applied to Torta del Casar cheeses from the same batch. Three brine solutions at different concentrations (14, 18 and 24°Bé) were prepared at room temperature. Salt concentration was adjusted using a Baumé hydrometer (1 to 30°Bé) (Brannan & Sons Ltd, Cumbria, UK). Calcium level was set at 0·5% (w/w) with CaCl2. Tanks containing about 50 l brine were placed in temperature-controlled rooms set at 5 and 10 °C. Salt brines were stirred and checked for pH (adjusted to 6·0 with lactic acid and NaOH), salt concentration and temperature before salting. Three batches of ten cheeses each were kept submerged in brine and removed after 60, 120 and 180 min.
Cheeses were sampled at 0 time (12 h after brining), 7, 15, and 30 d. Each sample was divided in three portions: the external portion or rind (up to 1·5 mm from the cheese surface), the outer core (1·5 cm thick, after removing the rind) and the inner core (the remaining portion). The different cheese portions were chopped and homogenised.
Physicochemical and microbiological analysis
Cheeses portions were analysed for total solid (TS %), salt and fat by standard procedures (ISO, 2004, 2006, 2008). The pH was measured according to Shakeel-Ur-Rehman & Fox (Reference Rehman and Fox2002).
For the microbiological analyses, representative aliquots of cheese rind (10 g) were homogenised 1 : 10 in 2% trisodium citrate for 2 min using a Stomacher 400 (Seward Medical, London, UK). Serial dilutions were prepared by adding l to 9 ml sterile quarter-strength Ringer's solution. Samples were plated on the following media: plate count agar (PCA, Merck) for total aerobic mesophilic bacteria (72 h at 30 °C); PCA (Panreac) plus 5% NaCl for halotolerant bacteria (72 h at 30 °C); cetrimide agar (Merck) containing 1% (v/v) glycerol for Pseudomonas (72 h at 25 °C); yeast extract glucose chloramphenicol agar. (YGC agar, Cultimed) for moulds and yeast (5 d at 25 °C). All determinations were carried out in duplicate.
Statistical analysis
PASW Statistics, version 17.0 (SPSS, Chicago, IL, USA) was used for the statistical analysis. Two-way analysis of covariance (ANCOVA) was done to establish the presence or absence of significant differences (P ≤ 0·05) in salt content of the cheeses. Stepwise model (with forward selection procedure) was used to remove the non-significant covariates from the analysis.
One-way ANCOVA without interaction was applied to model salt uptake in Torta del Casar cheese and to establish the presence or absence of significant differences (P ≤ 0·05) in pH values from rind, outer and inner core.
Piecewise regression analysis was performed to determine the breakpoint at which pH response to NaCl concentration changes.
One-way analysis of variance (ANOVA) was used to determine the presence or absence of significant differences (P ≤ 0·05) in TS and Fat-in-TS (F/TS). When differences were significant, a post hoc Tukey's test was applied to compare mean values.
Results
Salt uptake and diffusion in Torta del Casar cheese
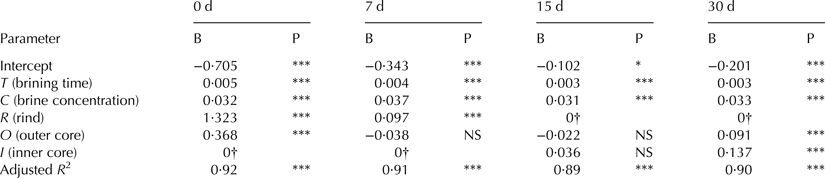
NaCl was determined in 144 cheese portions in order to establish differences in salt content depending on the brine treatment. Two-way analysis of covariance (ANCOVA) was performed according to the factors Cheese Portion and Ripening Time, using the Brine Concentration, Brining Time and Brine Temperature as covariates. Selected factors and covariates significantly influenced on salt content (P < 0·001). This ANCOVA model explained 92% of the variability found in test scores (adjusted R 2 = 0·921). Statistical analysis also showed a significant interaction between Cheese Portion and Ripening Time.
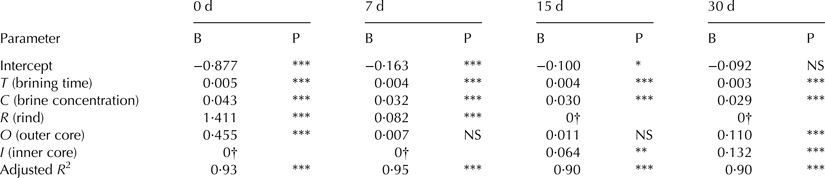
Mathematical equations were developed to model salt uptake and to predict salt concentration in cheese during the first month of ripening. Data from cheese brined at 5 and 10 °C were analysed independently by one-way ANCOVA without interaction for the factor Cheese Portion, using Brining Time and Brine Concentration as covariate on the salt concentration. To consider salt diffusion during cheese ripening, data from different sampling days were analysed separately. All regressions within the ANCOVA were highly significant (adjusted R 2 = 0·89 to 0·95).
The prediction equations derived from the ANCOVA model, represented by E(Y), allow the estimation of the salt concentration (% NaCl w/w) at different ripening days depending on the β values showed in Tables 1 and 2. The explanatory variables are Brining Time (T), Brine Concentration (C) and Cheese Portion (rind, R; Inner core, I; Outer core O).
Table 1. Parameter estimates of the one-way ANCOVA without interaction of the cheeses brined at 5 °C for different ripening days. B, β value; P, significance level

***P ≤ 0·001; **P ≤ 0·01; *P ≤ 0·05; NS, not significant
† This parameter is set to zero because it is redundant
Table 2. Parameter estimates of the one-way ANCOVA without interaction of the cheeses brined at 10 °C for different ripening days. B, β value; P, significance level

***P ≤ 0·001; **P ≤ 0·01; *P ≤ 0·05; NS, not significant
† This parameter is set to zero because it is redundant
The simplified model equations can be written as:
This mathematical approach suggests different parallel plane equations depending on the cheese portion, as R, O and I assume fixed values ( = 0 or = 1). Thus, the independent term is equal to the sum of β0 (intercept) and βR, βO or βI, for rind, outer or inner core respectively:
For a given ripening time, the different equations only vary for the independent term. The parallel plane equations imply that brining time (T) and brine concentration (C) influenced salt concentration to the same extent, regardless the cheese portion sampled.
Considering β values and their significance level for cheese brined at 5 °C (Table 1), it can be assumed that the salt concentration decreased with distance from the cheese surface during the first days of ripening. However, this gradient disappeared with time. Thus, inner and outer core showed the same NaCl content at 7 d, and no significant differences were found among the cheese portions sampled at 15 d. At 30 d ripening, this gradient was reversed, so NaCl values in rind were lower than those registered in inner and outer cheese core at 30 d ripening. Cheese salt absorption at 10 °C brining was higher than at 5 °C; nevertheless salt diffusion into cheese was similar, slightly faster though. The inversion of the salt content, as showed in Table 2, is observed at 15 d.
Brine salting and physicochemical changes in cheese
The different brining treatments applied resulted in a broad range of salt content in cheese. To determine significant differences in pH values from rind, outer and inner core samples were analysed independently by one-way ANCOVA without interaction. Significant variables were chosen by the forward selection procedure, considering Ripening Time as factor and Salt Concentration as covariate. Brining Time, Brine Concentration and Brine Temperature were considered confounding variables, because they all correlated with Salt Concentration. Statistical analysis showed that pH in outer and inner core was influenced by ripening time and salt concentration (P < 0·001; R 2 > 0·94); however, rind pH was only affected by ripening time (P < 0·001). In this case, only 64% of the variability in test scores was explained by ANCOVA model (R 2 = 0·63).
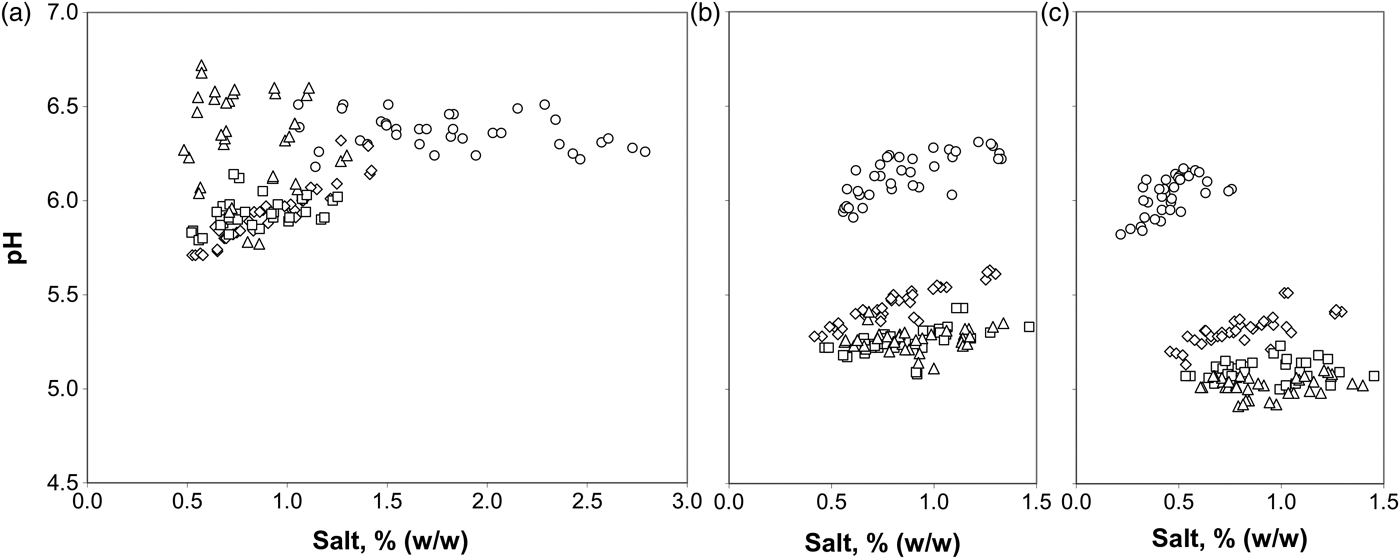
Several considerations arise from the graphic representation of the effect of salt concentration on pH at different sampling times illustrated in Fig. 1. A pH decrease can be seen in cheese core (inner and outer) throughout the first 15 d ripening, although pH drop was lower over time. Conversely, in cheese rind, the pH fell only during the first 7 d, rising after 15 d of ripening. This figure also shows how pH values increased with salt concentration, especially during the first 7 d cheese ripening, except in rind.

Fig. 1. Effect of salting on cheese pH in rind (a), outer core (b) and inner core (c) at 0 (○), 7(◊), 15 (□) and 30 d of ripening (▵).
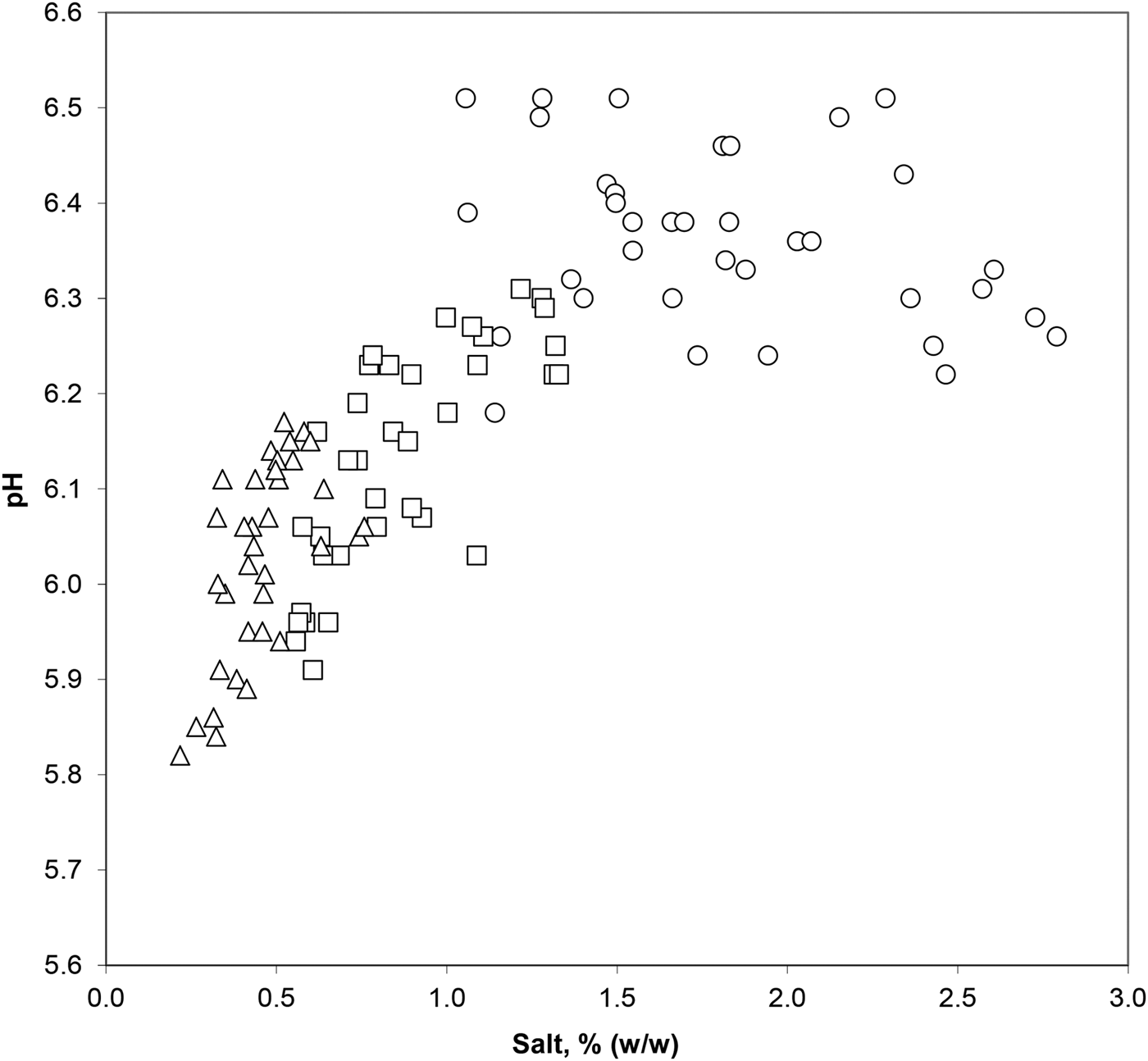
Considering data from the different cheese portions 12 h after brining, a threshold value in NaCl% can be deduced from the abrupt change in pH response towards salt concentration (Fig. 2). Piecewise regression analysis showed that salt levels below 1·49% (95% confidence interval, 1·33 to 1·66) slowed acidification, while concentrations over this value completely inhibited acid development.

Fig. 2. Effect of salt content on cheese pH in rind (○), outer core (□) and inner core (▵) after brining.
Changes in total solids (TS) of the different cheese portions (rind, outer and inner core) are given in Table 3. There were no significant differences (ANOVA, P < 0·05) in TS between inner and outer cheese core during the first 30 d ripening. Only TS content in rind at 30 d was significantly higher. F/TS remained almost constant throughout the ripening (50·6%).
Table 3. Total solid content (%) ±sd of the different cheese portions during the first 30 d of ripening

a, bDifferent letter in the same row indicates significant differences among ripening times (Tukey Test, P < 0·05)
A, BDifferent letter in the same column significant differences among cheese portions (Tukey Test, P < 0·05)
Effect of salt concentration on microbial composition of cheese rind
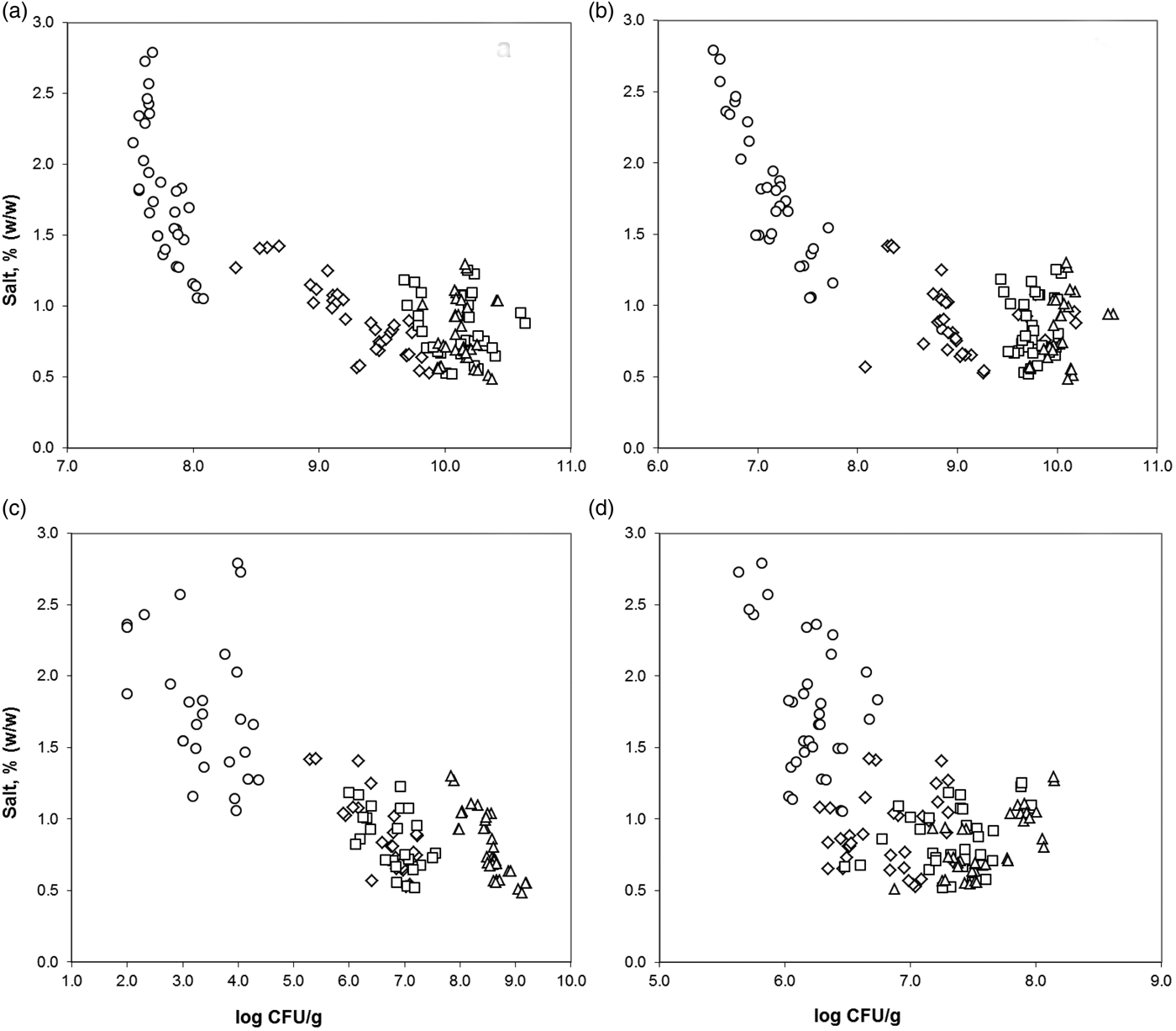
To investigate whether the brine salting influences rind microbiota, several microbial groups (mesophiles, halotolerants, pseudomonas and yeast–moulds) were determined in cheese rind. Brine salting effect over each microbial group was analysed independently by one-way ANCOVA without interaction for the factor Ripening Time, using Salt Concentration as covariate on the plate counts. Significant variables were selected by the forward selection procedure. There was no significant influence of Brining Time, Brine Concentration and Brine Temperature. ANCOVA models explained 95% of the variability in test scores for mesophiles and pseudomonas, 97% for halotolerants and 74% for yeast–moulds (adjusted R 2 from 0·740 to 0·968). Figure 3 illustrates the effect of salt concentration on each microbial population (log CFU/g) throughout the sampling period. Microorganism population significantly increased throughout the first 30 d of cheese ripening (P < 0·001) for all microbial groups except for mesophiles, which attained its maximum at 15 d. In addition, NaCl concentration significantly influenced mesophiles, pseudomonads and halotolerants population (P < 0·001). Microbial counts correlated inversely with salt concentration. Conversely, yeast–moulds growth (Fig. 3d) was not affected by NaCl at the concentrations achieved in cheese rind in the present study, which were comprised between 0·48 and 2·79% (w/w).

Fig. 3. Effect of salt content on mesophiles (a), halotorerants (b), pseudomonads (c) mould and yeast (d) counts in cheese rind during maturation. Ripening times are: 0 d (○), 7 d (◊), 15 d (□) and 30 d (▵).
Discussion
Salt concentration in cheese is affected by numerous factors. Their influence on salt absorption and its distribution in cheese differ markedly with variety. As established previously, the studied factors were able to explain most of the variability found in salt content scores (92%), so there were probably no important unconsidered variables.
The mathematical model proposed in this work suggests that the quantity of salt in cheese increased proportionally with brining time. Nevertheless, in other cheese varieties, like Ragusano, Feta or Mozzarella, salt uptake increases at an ever-diminishing rate, as the NaCl concentration gradient between the cheese and brine decreases over time (Melilli et al. Reference Melilli, Barbano, Licitra, Tumino, Farina and Carpino2003; McMahon et al. Reference McMahon, Motawee and McManus2009; Luo et al. Reference Luo, Pan, Guo and Ren2013). Probably brining time used for Torta del Casar cheese is not long enough to show a significant gradient reduction. Moreover, our results showed that higher brine concentration gave higher salt absorption even at 24°Be (~25·5% w/w). However, it is reported that concentrations above 25% (w/w) may result in lower salt uptake in some cheese types as a result of the surface layer dehydration (e.g. Melilli et al. Reference Melilli, Barbano, Licitra, Tumino, Farina and Carpino2003). Such shrinkage of the cheese rind could take place only in cheeses which are brined for longer time.
Salt equilibrium among portions (βR, βO and βI = 0) was achieved within the first 15 d, although it was reached faster when cheese was brined at 10 °C. According to Floury et al. (Reference Floury, Jeanson, Aly and Lortal2010), salt equilibration times for soft cheeses range from about 1 to 2 weeks. Similar values have been reported for other soft cheeses like Camembert and Limburger (Guinee, Reference Guinee2004; Guinee & Fox, Reference Guinee, Fox, Fox, McSweeney, Cogan and Guinee2004). From the equilibrium point onwards, NaCl concentration in cheese rind was slightly lower than in cheese core, showing at 30 d a reverse concentration gradient throughout the cheese. The lower values for the salt content could be due to surface drying (Guinee & Fox, Reference Guinee and Fox1983), which increases the percentage of salt in moisture (% S/M) in the outside layers, and thus salt migration towards the more humid portions (Santapaola et al. Reference Santapaola, Andrés, Maldonado and Fernández2012). This is in agreement with differences in moisture levels shown in Table 3, especially at 30 d.
NaCl is one of the major factors controlling and regulating cheese microflora. As a consequence, the acidifying activity in cheese and its pH are also affected. S/M values >5% abruptly decrease the acid development (Guinee & Fox, Reference Guinee, Fox, Fox, McSweeney, Cogan and Guinee2004; McMahon et al. Reference McMahon, Motawee and McManus2009). In this work, NaCl levels ≥1·5% (2·9% S/M) strongly inhibited acidification during the first ripening hours (Fig. 2). Similar results were obtained by Schroeder et al. (Reference Schroeder, Bodyfelt, Wyatt and McDaniel1988) who reported that cheese containing more than 0·85% NaCl (~2·5% S/M) supported significantly lower lactic acid bacteria. Differences observed by different authors may lie in the salt tolerance of starter cultures, which varies according to the species, strain and cheese pH.
To achieve a spreadable texture in Torta del Casar, slow rate of acidification is needed, taking days instead of hours to achieve its lowest pH. Hardening of texture appears when cheese acidification is too fast and intense. In other soft cheese varieties like Crescenza and Cremoso, the creamy texture is largely influenced by pH (Hynes et al. Reference Hynes, Delacroix-Buchet, Meinardi and Zalazar1999). Although NaCl diffuses rapidly, the initial salt gradient seems to influence the cheese pH during the first ripening days, as lower pH values were obtained from samples with lower salt content (Fig. 1b, c). Differences observed in this work between inner and outer core pH during the first month of ripening could be caused by this fact. In brine salted cheese, acidification and growth of starters are usually restrained by lactose depletion, high acidity or low temperature before an inhibitory concentration of salt is attained in the cheese centre (Sheehan, Reference Sheehan and McSweeney2007).
The above results showed that the microbial composition of the cheese rind changed during the first 30 d of maturation. The continuous growth of halotolerant, pseudomonads, yeasts and moulds throughout cheese ripening, while mesophiles attained its maximum at 15 d, suggests a competitive advantage of these microbial groups over others. Low maturation temperatures could favour pseudomonads, whereas rind dehydration, and consequently available water (a w) reduction may benefit halotolerant, yeast and moulds (van den Berg, Reference van den Berg and MacCarthy1986).
The surface microbiota plays a critical role in the development of cheese rind, as well as in its alteration. Pseudomonas spp. has been implicated in pigment-related spoilage of cheeses (Cantoni et al. Reference Cantoni, Stella, Cozzi, Iacumin and Comi2003; Martin et al. Reference Martin, Murphy, Ralyea, Wiedmann and Boor2011). Furthermore, tyrosinase production by microorganisms like pseudomonads triggers the development of pink pigments in cheese (O'Connell & Fox, Reference O'Connell and Fox2001). Salt, as food preservative, may help to control such microorganisms and to reach the correct balance within the smear microbiota. However, the amount of salt in PDO cheeses is limited, and what is more, consumer acceptance of lower sodium cheese is an upward trend. Brining treatments applied in the present work were selected so that the concentration of NaCl at retail level did not exceed 2%. Under this condition, mould and yeasts were not affected. Conversely, mesophiles, pseudomonads and halotolerants counts were significantly influenced by salt concentration, mostly during the first days of ripening. Salt diffusion into cheese lowered NaCl levels in rind, eventually reaching values below the inhibitory concentration for these microorganisms. Only pseudomonads remained influenced after 30 d. The difference in pseudomonads population between the most and least salty rind (1·28 and 0·56%) was over 1.2 log units (Fig. 3c), as the result of the low salt tolerance of this microbial group in cheese. This conclusion is supported by the work of Quigley et al. (Reference Quigley, O'Sullivan, Beresford, Ross, Fitzgerald and Cotter2012), in which Pseudomonas was not detected in cheeses with a high salt content. ·
As a major food spoilage microorganism, pseudomonads control in cheese rind by an adequate brining could be of great importance. The parameters studied in this work (brine temperature, brine concentration and brining time) had no influence. For this purpose, only final salt concentration had a significant effect. Given that the most salty cheeses obtained the best punctuation in the sensory evaluation (data not shown), salting by immersion 3 h at 10 °C in 24°B brine is recommended.
This work has been funded by the Autonomous Community of Extremadura and European Regional Development Fund (PDT09B001 grant).