Sodium chloride (NaCl) is one of the most important ingredients for many foods because of its low cost and several important properties (Albarracín et al. Reference Albarracín, Sánchez, Grau and Barat2011). The salt plays an important role in food safety (Kremer et al. Reference Kremer, Mojet and Shimojo2009) – as a direct consequence of reduced water activity – (Albarracín et al. Reference Albarracín, Sánchez, Grau and Barat2011), in flavour enhancing (Rulikowska et al. Reference Rulikowska, Kilcawley, Doolan, Alonso-Gomez, Nongonierma, Hannon and Wilkinson2012) – due the capacity to influence the enzymatic activity of some enzymes that are responsible for the development of different organoleptic parameters – (Albarracín et al. Reference Albarracín, Sánchez, Grau and Barat2011) and it influences the nutritional value, composition and functionality of the foods (Guo et al. Reference Guo, Hekken, Tomasula, Shieh and Tunic2011).
Epidemiological studies indicate that the dietary salt intake has positive association with blood pressure and hypertension, been directly related to the development of cardiovascular disease (Dahl, Reference Dahl1972; Weinsier, Reference Weinsier1976; Law Reference Law1997; SACN, 2003; WHO, 2003). For these reasons, there is great consumer demand for low-salt products (Katsiari et al. Reference Katsiari, Alichannidis, Voutsinas and Roussis2001; Matthews & Strong, Reference Matthews and Strong2005; Ruusunen & Puolanne, Reference Ruusunen and Puolanne2005; Guàrdia et al. Reference Guàrdia, Guerrero, Gou and Arnau2006; Albarracín et al. Reference Albarracín, Sánchez, Grau and Barat2011). According to Appel & Anderson (Reference Appel and Anderson2010), 75% of dietary salt comes from processed foods, so the aim of the food industry should be focused on the reduction of the salt content in these products.
According to Al-Otaibi & Wilbey (Reference Al-Otaibi and Wilbey2006), 0·2 g sodium/day (approximately 0·5 g NaCl) is the minimum quantity required by a healthy adult, and according to World Health Organisation (WHO), the healthy limit is 5 g sodium chloride/day (approximately 2000 mg of sodium) (WHO, 2007). Research by the Brazilian Health Ministry (2011) noted that the Brazilians consume an average of 12 g sodium chloride/day (4800 mg NaCl/day). Because of concerns about excess sodium intake, the Health Ministry and the Brazilian food industry reached an agreement to reduce the sodium content of various categories of processed foods by 2016. The UK Food Standards Agency also revised its salt reduction targets for food processors, and Food and Drug Administration (FDA) are working with the US food industry to increase salt reduction in foods (Food and Drugs Administration Press Release, 2010).
The development of low-salt products can be possible using different sodium chloride substitutes, such as other chloride salts (e.g., KCl, CaCl2 and MgCl2) (Gou et al. Reference Gou, Guerrero, Gelabert and Arnau1996; Aliño et al. Reference Aliño, Grau, Toldrá, Blesa, Pagán and Barat2010), phosphates (Ruusunen & Poulanne, Reference Ruusunen and Puolanne2005), transglutaminase (Romero de Ávila et al. Reference Romero de Ávila, Ordóñez, de la Hoza, Herrero and Cambero2010) or flavour enhancers such as monosodium glutamate (Desmond, Reference Desmond2006).
For the salt substitutes to successfully replace sodium chloride in food formulations it is necessary to carry out initial studies to obtain knowledge regarding the appropriate concentration of sodium substitutes and their salt equivalence compared with sodium chloride. According to Souza et al. (Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011) to obtain this information the most common method, widely used for sweeteners, is the estimation of the magnitude and a graphic representation of standardised results through the Law of Stevens, or the ‘Power Function’ (Stone & Oliver, Reference Stone and Oliver1969; Moskowitz, Reference Moskowitz1970).
The sodium chloride substitutes may have unpleasant taste (Cruz et al. Reference Cruz, Faria, Pollonio, Bolini, Celeghini, Granato and Shah2011), thus it is also important to know sensory characteristics of sodium chloride replacements to determine the salt substitute or blend that has a sensory profile more similar to NaCl.
Temporal dominance of sensations (TDS), proposed by Pineau et al. (Reference Pineau, Cordelle, Imbert, Rogeaux and Schlich2003), is a recent methodology that provides the sequence of sensory attributes perceived over time. Tasters assess which sensation is dominant over time until the sensation ends or another appears as dominant (Labbe et al. Reference Labbe, Schlich, Pineau, Gilbert and Martin2009; Pineau et al. Reference Pineau, Schlich, Cordelle, Mathonnière, Issanchou, Imbert, Rogeaux, Etiévant and Koster2009). According to Albert et al. (Reference Albert, Salvador, Schlich and Fiszman2012), the sensory profile obtained by this technique can be related to acceptance.
The objective of this study was to determine the equivalent amount of different sodium chloride replacements (potassium chloride, monosodium glutamate, potassium phosphate, magnesium chloride, calcium chloride, calcium lactate and potassium lactate) required to promote the same degree of ideal saltiness in butter and to study the sensory profile of sodium chloride and the substitutes using Temporal Dominance of Sensations (TDS) analysis.
In commercial products the low or reduced sodium content of the products is achieved by reduction or partial replacement of sodium chloride by substitutes, in particular, potassium chloride. This study aimed to test different sodium chloride substitutes and to evaluate the possibility of reducing total sodium chloride in butter by these substitutes. Thus, this research provides novel scientific information to the dairy industry and research fields, to expand the knowledge of the salting potency and sensory characteristics of different sodium chloride replacement, often still not used, and also to provide information of the feasibility of the total replacement of sodium chloride in butter.
Materials and methods
Materials
The following is a list of materials used in the food preparations in this study: milk cream with 38% fat, potassium chloride – 99% (Vetec®), monosodium glutamate – 99% (Aji-no-moto®), potassium phosphate – 99% (CRQ®), magnesium chloride – 99% (Vetec®), calcium chloride – 99% (Doremus®, calcium lactate – 99% (Purac®), potassium lactate – 99% (Purac®) and sodium chloride – 99% (Vetecc®).
Preparation of butter
The cream with 38% fat, which was standardised with skim milk, was pasteurised at 80 °C for 20 min. It was then cooled and subjected to physical aging at 10 °C for 24 h, followed by churning for 50 min. The butter was washed with chilled drinking water at 2 to 4 °C, and the sodium chloride or a salt substitute was added to the butter. Immediately after the addition of the salt or salt substitute, the butter was whipped. Afterward, the butter was packaged in polypropylene plastic and stored at 4 °C until the time of analysis.
Sensory analysis
Equivalent salting
To reach the equivalent saltiness for the various salt replacements relative to the salt taste of sodium chloride in butter, sensory evaluations were conducted at various stages. The procedures followed in each stage was based on the work of Souza et al. (Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011).
Selection of panellists
Were recruited 25 butter consumers who were interested in determining the equivalent salt of butter compared with sodium chloride and had available time and no restrictions as to the consumption of this product (Souza et al. Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011).
The sequential method proposed by Wald (Amerine et al. Reference Amerine, Pangborn and Roessler1965) – where a number of tests triangular are applied – was used to select panellist with a good ability to discriminate samples (Meilgaard et al. Reference Meilgaard, Civille and Carr1999).
In the triangular tests two samples of butter were used with 1% significance difference, comparing the salty taste. The samples were: butter with 1·0% sodium chloride and butter with 1·25% sodium chloride.
From the defined parameters (P=0·30, p1=0·70, α=0·10 and β=0·10) the Wald graph was constructed and judges were selected or rejected according to the number of correct tests analysed in the triangular graph (Souza et al. Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011).
With 8 triangular tests, 12 judges were selected. The selected panellists were college students aged between 18 and 27 years and included 8 females and 4 males.
Training session
The panellists selected were trained to use magnitude scales according to Souza et al. (Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011). In the training session the panellists received three samples of butter (0·5, 1 and 2% sodium chloride), and were asked to determine the potency of this samples with respect to a reference sample (butter with 1% sodium chloride). The ideal concentration of 1% sodium chloride was determined based on information regarding commercial butter and pretests.
Determination of the equivalent salt
The selected and trained panellists received a reference sample (with the optimal concentration of sodium chloride, 1%) with a potency designated by an saltiness value of 1, followed by several butter samples that were coded and presented in a balanced manner (Macfie et al. Reference Macfie, Bratchell, Greenhoff and Vallis1989). Then, the panellists were asked to estimate the intensities of the salty taste of the butter samples compared with the reference.
To determine the equivalent saltiness of the sodium chloride substitutes relative to sodium chloride, the series of concentrations presented in Table 1 was used. The central concentrations of the sodium chloride substitutes were based on pretests. To calculate the other concentrations, a multiplication factor of 1·6 was used, following Cardoso et al. (Reference Cardoso, Battochio and Cardello2004) and Marcellini et al. (Reference Marcellini, Chainho and Bolini2005).
Table 1. The concentrations of sodium chloride and each sodium chloride substitute used to determine the equivalent saltiness in butter compared with 1% sodium chloride
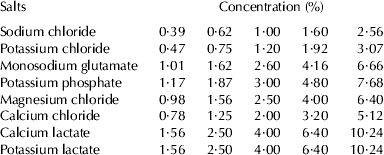
During the pretests, it was found that the calcium lactate and potassium lactate have very low salting power. The pre-tests found that the intermediate concentration (4%) was not sufficient to salt the butter, but we chose this concentration because the resulting highest concentration is exceptionally high (10·24%).
After appropriate treatment of the data, as described by Souza et al. (Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011), the ‘Power Function’ for sodium chloride and each sodium chloride substitute were obtained. The power function (‘Power Function’) has the following characteristic:
S: sensation perceived, C: concentration of the stimulus, a: antilog of the y-intercept of the linear function and n: slope of the linear function
Based on the power function of sodium chloride and each substitute and from the ideal concentration of sodium chloride in butter (1%), the equivalent concentration of each sodium chloride substitute was estimated, as described by Souza et al. (Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011).
Determination of the potencies of the sodium chloride substitutes
The potency of each sodium chloride substitute was calculated by the ratio between the ideal concentration of sodium chloride (1%) and the equivalent concentration of the sodium chloride substitute in the butter (Souza et al. Reference Souza, Pinheiro, Carneiro, Pinto, Abreu and Menezes2011).
Temporal dominance of sensations (TDS)
We recruited ten participants from the panel of the salting equivalence test to participate in the TDS analysis. Two preliminary sessions were conducted, as described by Albert et al. (Reference Albert, Salvador, Schlich and Fiszman2012). In the first session, the panellists were introduced to the notion of the temporality of sensations (TDS) and were introduced to the data acquisition program SensoMaker (Nunes & Pinheiro, Reference Nunes and Pinheiro2012). In the second session, the panellists participated in a simulation of a TDS session with several samples of butter to answer any questions of the participants and so they could get used to the computer program and methodology. This session also defined the total duration time of the experiment to be 20 s, and the attributes selected by the panel were salty, bitter, sweet, umami, sour, spicy, astringent and off-taste.
Finally, the panel evaluated three replicates of each of the four samples of butter: the standard butter sample with sodium chloride (1%) and three samples of butter with sodium chloride substitutes (potassium chloride, monosodium glutamate and potassium phosphate) at the concentration that promoted the same degree of saltiness as sodium chloride, 1%.
The participants were requested to select the dominant taste over the time. To avoid possible misunderstandings, it was clearly explained that the dominant taste is the taste that is perceived with greater clarity and intensity among others. Then, the panellists were requested to put the sample of butter (around 5 g) in the mouth and immediately start the evaluation.
The samples were served one by one, and the assessors were asked to rinse their mouth with water between each sample. The presentation was made in monadic order (Macfie et al. Reference Macfie, Bratchell, Greenhoff and Vallis1989) in disposable white plastic cups coded with three-digit numbers.
The methodology of Pineau et al. (Reference Pineau, Schlich, Cordelle, Mathonnière, Issanchou, Imbert, Rogeaux, Etiévant and Koster2009) was used in the software SensoMaker to compute the TDS curves. In brief, two lines are drawn in the TDS graphical display, the ‘chance level’ and the ‘significance level’. The ‘chance level’ is the dominance rate that an attribute can obtain by chance and the ‘significance level’ is the minimum value this proportion should equal to be considered to be significantly (Pineau et al. Reference Pineau, Schlich, Cordelle, Mathonnière, Issanchou, Imbert, Rogeaux, Etiévant and Koster2009). It is calculated using the confidence interval of a binomial proportion based on a normal approximation, according Pineau et al. Reference Pineau, Schlich, Cordelle, Mathonnière, Issanchou, Imbert, Rogeaux, Etiévant and Koster2009 (2).
Ps: lowest significant proportion value (a=0·05) at any point in time for a TDS curve; n: number of subjects *replication.
Results
Equivalent saltiness
Of all the salt substitutes tested (potassium chloride, monosodium glutamate, potassium phosphate, magnesium chloride, calcium chloride, calcium lactate and potassium lactate), calcium lactate, potassium lactate, calcium chloride and magnesium chloride were impractical to use in butter. As expected, lactates (calcium lactate and potassium lactate) did not salt the products. As the scores of all of the panellists for all of the concentration levels used for these salts were below 1, the mean salty taste was less than the standard (butter with 1% sodium chloride). Therefore, for both lactates, concentrations of up to 10·24% (an extremely high concentration) in the butter were not sufficient to salt it.
Calcium chloride and magnesium chloride were infeasible due to an extreme undesirable taste that masked the possible perception of a salty taste. In addition to all of the panellists scoring these salts below 1 (salty taste less than the standard) at all concentrations used, as the concentration of these salts increased, the panellists further decreased the scores compared with the standard. According to these panellists, this occurred because the higher concentration of these salts resulted in more intense ‘bitter’, ‘acid’ and ‘astringent’ tastes, which makes the perception of salty taste even more difficult. The panellists classified the addition of these salts, even at lower levels, as ‘unsuitable for consumption’. According to Grummer et al. (Reference Grummer, Karalus, Zhang, Vickers and Schoenfuss2012), CaCl2 and MgCl2 produced significant off-flavours (bitter, metallic, unclean and soapy) in cheese. According to Armenteros et al. (Reference Armenteros, Aristoy, Barat and Toldrá2012), divalent cations, may contribute to the sensory rejection since they are characterised by bitter tastes, producing metallic, astringent and irritating sensations.
Thus, it was possible to determine the equivalence of salt with only the substitutes potassium chloride, monosodium glutamate and potassium phosphate. A linear regression of the points obtained for sodium chloride and its substitutes was then made, and a straight-line equation was obtained for each of the salts and their combinations (Fig. 1).

Fig. 1. Linearised power function for butter salted with sodium chloride, potassium chloride, monosodium glutamate and potassium phosphate The x-axis shows the logarithm of the concentration of the sodium chloride and the substitutes (%), while the y-axis shows the logarithmic values of the estimated magnitudes, appropriately normalised.
From the equations for sodium chloride and each sodium chloride substitute (Fig. 1), a simple power function was obtained (Table 2). From the power functions obtained for sodium chloride and each substitute, the equivalent amount of saltiness required to provide the same salty taste as 1% sodium chloride in butter was calculated; the potency was also calculated (Table 3).
Table 2. Antilog of the y-intercept (a), intercept on the ordinate (n), linear coefficient of determination (R 2) and power function (Power Function) of the results to determine the equivalent saltiness of sodium chloride, potassium chloride, monosodium glutamate and potassium phosphate relative to the 1% sodium chloride in butter. S: Salt sensation perceived

Table 3. Equivalent concentrations and potencies of potassium chloride, monosodium glutamate and potassium phosphate relative to the 1% sodium chloride in butter

Temporal dominance of sensations
Figures 2–5 show the TDS profiles for the four butters evaluated in the study. Each curve represents the change in the dominance rate of an attribute over time. The TDS analyses show that in the butter with sodium chloride (Fig. 2), the salty taste was the dominant taste throughout the measured time. In the butter with potassium chloride (Fig. 3), the salty taste and bitter taste are dominant, and salty was perceived with greater dominance rate than bitter up to approximately 8 s, but after that time, the bitter taste dominated.

Fig. 2. A graphical TDS representation for the butter with sodium chloride.

Fig. 3. A graphical TDS representation for the butter with potassium chloride.
In the butter with potassium phosphate (Fig. 4), a sour taste was significantly noticed beyond the salty taste. A bitter taste was also noted above the chance level, but it did not exceed the significance level. The salty taste was perceived with high dominance rate to an approximate time of 7 s. After that time, the sour taste dominated.

Fig. 4. A graphical TDS representation for the butter with potassium phosphate.
In the butter with monosodium glutamate (Fig. 5), the dominant tastes were salty and almost insignificantly sweet and umami tastes were perceived. A similar number of participants evaluated sweet and umami as dominant at the same time period. The salty taste dominated with high dominance rate until approximately 7 s. The sweet and umami tastes had low intensities between approximately 8 and 14 s.

Fig. 5. A graphical TDS representation for the butter with monosodium glutamate.
The TDS curves also show that the butter with sodium chloride achieved a maximum dominance rate of 0·7 (the maximum of testers who selected saltiness as dominant was 70%) for saltiness, and the other sodium chloride substitutes reached a maximum saltiness dominance rate of 0·6. The duration and the maximum dominance rate of the salty taste was greater in the butter with sodium chloride than for the other substitutes.
Discussion
Equivalent saltiness
Comparing the sodium chloride substitutes (Fig. 1), it was observed that a higher concentration of monosodium glutamate and potassium phosphate was needed to reach the same salty taste sensation, and these two salt substitutes appear to have similar salting power. Sodium chloride and potassium chloride have a similar salting power, which means that a similar concentration of these salts produces the same sensation of salty taste.
The potassium chloride required a lower concentration and consequently had a high potency, but was the closest substitute to that of sodium chloride. The two other salts, monosodium glutamate and potassium phosphate, showed similar equivalent concentrations/power, but both salts were only capable of saltiness of approximately 65–70% lower than that of sodium chloride, i.e., to achieve the same level of saltiness as sodium chloride, it is necessary to use approximately three times the amount to achieve the same salty taste sensation.
The salty perception of sodium chloride is attributed to the cation (70–85%) and to the anion (30–15%) (Formaker & Hill, Reference Formaker and Hill1988; Mattes, Reference Mattes2001) and involves the passage of the ions through a narrow ionic channel. According to Mccaughy (Reference Mccaughy, Guinee and O'Kennedy2007), this passage through these channels is a specificity of the ions of sodium chloride, being difficult to find other substances with this capability, except toxic ions. Thus, the salting capacity depends on the type of cation/anion present in the substance (Ye et al. 1991, 1993). Compared with sodium chloride, other cations (potassium, magnesium and calcium) and other anions (phosphates and citrates) may have off taste and have less salty perception (Mooster, Reference Mooster, Kare, Fregly and Bernard1980).
According to Albarracín et al. (Reference Albarracín, Sánchez, Grau and Barat2011), the diffusion of larger ions through a narrow ionic channel is limited. Therefore, salts with larger anions are less effective stimuli (Delwiche et al. Reference Delwiche, Halpern and DeSimone1999), so when the cation or anion of NaCl is substituted with a higher molecular weight compound, it results in a less intense salty taste (Guàrdia et al. Reference Guàrdia, Guerrero, Gou and Arnau2006).
Temporal dominance of sensations
As already mentioned, the salty perception of NaCl is attributed either to the cation (70–85%) and the anion (30–15%) (Formaker & Hill Reference Formaker and Hill1988; Mattes Reference Mattes2001), and according to Albarracín et al. (Reference Albarracín, Sánchez, Grau and Barat2011), cations and anions have an effect on the taste properties of different types of salt. Compared with sodium chloride, other cations (potassium, magnesium and calcium) and other anions (phosphates and citrates) interfere in the taste, resulting in a perception of unpleasant taste, as sourness and residual metallic taste (Mooster, Reference Mooster, Kare, Fregly and Bernard1980). According to Horita et al. (Reference Horita, Morgano, Celeghini and Pollonio2011), potassium chloride is widely used in low-sodium products but in high concentration produces a bitter and metallic taste, resulting in sensory rejection (Seman et al. Reference Seman, Olson and Mandigo1980; Askar et al. Reference Askar, El-Samahy and Tawfik1994; Guàrdia et al. Reference Guàrdia, Guerrero, Gelabert, Gou and Arnau2008; Armenteros et al. Reference Armenteros, Aristoy, Barat and Toldrá2012).
In butter with monosodium glutamate, there were no undesirable tastes (bitter, sour or off-taste). In this salt, the predominant tastes were umami, sweet and salty. Considering the dominant profile sensations of the salts studied, glutamate appears to have the greatest potential for use as a substitute for sodium chloride. According to Brandsma (Reference Brandsma2006) the flavour enhancers – compounds that activate the receptors in the mouth and throat – may be of great interest to the food industry, because it can help in the reduction of the levels of sodium chloride. However, considering that the glutamate salty power (31·59) is well below the sodium chloride, the use of this sodium chloride substitute alone probably will not significantly reduce the level of sodium. In addition, the use of glutamate to specifically replace chloride sodium even if combination with other salt substitutes may not be relevant for consumers expecting only salty taste in butter and not sweet and umami tastes.
According to the results of TDS analysis, the total replacement of sodium chloride by potassium chloride, monosodium glutamate or by potassium phosphate in butter is not recommended, because these substitutes have undesirable tastes and/or low salty power in this product.
The sensory characteristics, functional properties and shelf life, are the characteristics most affected by reducing salt in foods (Guinee & O'Kennedy, Reference Guinee, O'Kennedy, Guinee and O'Kennedy2007). In the development of products with sodium reduction, it is important to consider: the nature and the composition of the product, the type of processing and the conditions of manufacturing (Ruusunen & Puolanne, Reference Ruusunen and Puolanne2005). Although there are many salt substitutes in the market, according to Cruz et al. (Reference Cruz, Faria, Pollonio, Bolini, Celeghini, Granato and Shah2011) only sodium chloride is really recognised as a pure salty taste.
It is important to emphasise that recent studies have been able to develop products with reduced sodium and good acceptability, but in all cases, the partial substitution of sodium chloride was used and even the replacement of sodium chloride with different blends of sodium (Katsiari et al. Reference Katsiari, Alichannidis, Voutsinas and Roussis2001; Guinee & O'Kennedy, Reference Guinee, O'Kennedy, Guinee and O'Kennedy2007; Reps et al. Reference Reps, Wisniewska and Kuzmicka2009; Horita et al. Reference Horita, Morgano, Celeghini and Pollonio2011; Ayyash et al. Reference Ayyash, Sherkat and Shah2013, Gomes et al. Reference Gomes, Cruz, Cadena, Caleghini, Faria, Bolini, Pollonio and Granato2011). In the present work, sodium chloride was completely replaced to obtain the salting power and the sensory characteristics of each particular sodium chloride substitute in butter. Thus, it would be extremely important to evaluate these salts in butter at different levels of substitution of sodium chloride and even in different combinations to mitigate/reduce unwanted tastes, especially bitter, sour and off-tastes.










