Cadmium is a toxic heavy metal with no reported biological functions. Its toxicity results from the coordination with biochemical ligands. As cadmium is considered a soft Lewis acid, it interacts easily with oxidisable soft ligands (i.e.: thiol groups) (Crichton, Reference Crichton2007). Complexation of cadmium with vital enzymes replaces physiological ions like zinc, calcium or nickel and inhibits the activity of enzymes that require active-site thiol groups (Carballo et al. Reference Carballo, Castiñeiras, Domínguez-Martín, García-Santos, Niclós-Gutiérrez, Sigel, Sigel and Sigel2012).
The main long-term effects of low-level cadmium exposure in humans are generally chronic obstructive pulmonary disease, emphysema and chronic renal tubular disease. Skeletal effects (i.e.: softening of bones resulting from loss of minerals) and anaemia (resulting from cadmium interference with iron absorption) are also possible. Cadmium is transported by erythrocytes and albumin, and mainly stored in liver and kidneys, bound to metallothionein (50–75% of the body burden) (ATSDR, 2008).
Cadmium can occur as residue in food because of its environmental presence, as a result of human activities, including inadequate disposal electronic waste, mining or farming, and industrial production. Foodstuffs are the main source of cadmium exposure for the non-smoking general population (Satarug et al. Reference Satarug, Garrett, Sens and Sens2010). According to the European Food Safety Association (EFSA), the tolerated weekly intake (TWI) for cadmium is 2.5 μg per kilogram of body weight. The TWI was defined as the estimated amount of cadmium that can be ingested weekly over a lifetime without appreciable health risk (WHO 1989). The World Health Organization (WHO) established a guideline value for cadmium of 3 μg/l based on an allocation of 10% of the TWI to drinking-water (WHO 2006). The current average dietary exposure to cadmium for adults in Europe is close to the TWI, but in highly contaminated areas, it may be higher (EFSA 2012).
For this reason, all processes addressed to remove it are helpful. The capacity of different genera of lactic acid bacteria to adsorb heavy metals on their cell wall surface (biosorption) or accumulate them inside the cells (bioaccumulation) has gained interest in the last years and supports their use for several biotechnology processes (Halttunen et al. Reference Halttunen, Salminen and Tahvonen2007; Mrvcic et al. Reference Mrvcic, Stanzer, Solic and Stehlik-Tomas2012). In this regard, the GRAS (Generally Recognised as Safe) status of lactic acid bacteria and probiotics strengthens their relevance in cadmium removal, as they could be included in food matrices. This supports the application of probiotics in novel detoxification therapies (Urban & Kuthan, Reference Urban and Kuthan2004; Zhai et al. Reference Zhai, Wang, Zhao, Liu, Tian, Zhang and Chen2013). In this context, Battikh et al. (Reference Battikh, Safa, Niccola and Aagha2011) showed that a complex composed of Lactobacillus acidophilus and Lb. bulgaricus decreased the negative effects of cadmium on iron distribution in liver, kidney, leg and breast meat of chicken. It has also been published that the ability of some strains of Streptococccus thermophilus and Lb. bulgaricus to bind lead and cadmium has a beneficial effect against toxic heavy metals in rats (Salim et al. Reference Salim, Badawy and Kassem2011). Furthermore, Bhakta et al. (Reference Bhakta, Ohnishi, Munekage, Iwasaki and Wei2012) reported that the cadmium sorption by probiotic Lb. reuteri, may decrease the negative effects related to cadmium bioaccumulation in fish. Moreover, Carnobacterium piscicola and Leuconostoc mesenteroides subsp. Lysis are other species of lactic acid bacteria that can tolerate high concentrations of cadmium (Rial et al. Reference Rial, Vázquez and Murado2011). Monachese (Reference Monachese2012) reported a detailed analysis on the sequestration capacity of lead, cadmium and arsenic by different species of lactobacilli present in the gut microbiota (i.e.: Lb. johnsonii, Lb. casei, Lb. plantarum and Lb. rhamnosus).
Lb. kefir has well demonstrated probiotic properties (Golowczyc et al. Reference Golowczyc, Mobili, Garrote, Abraham and De Antoni2007; Zheng et al. Reference Zheng, Lu, Wang, Yang, Pan and Huang2013) and is able to bind different heavy metals, including cadmium (biosorption) (Gerbino et al. Reference Gerbino, Mobili, Tymczyszyn, Fausto and Gómez-Zavaglia2011, Reference Gerbino, Mobili, Tymczyszyn, Frausto Reyes, Araujo-Andrade and Gómez-Zavaglia2012). In this work, we aim to get a deeper insight into the cadmium sequestering capacity of Lb. kefir. Strains of this species were incubated in presence of different concentrations of cadmium and the effect of this metal cation on growth (lag time and EC50) and morphology has been investigated. Finally, the capacity of Lb. kefir to protect HepG2 cells from cadmium toxicity was evaluated to support novel dietary strategies in detoxification of this harmful metal.
Materials and methods
Bacterial strains and growth conditions
Two strains of Lb. kefir, CIDCA 8348 isolated from kefir grains (Garrote et al. Reference Garrote, Abraham and De Antoni2001), and JCM 5818, obtained from the Japanese Collection of Microorganisms (Reiken, Japan), stored at −80 °C, were used in this work. Frozen strains were activated under aerobic conditions in a 1/2 dilution of MRS broth (de Man et al. Reference De Man, Rogosa and Sharpe1960) (Difco, Detroit, MI, USA) at 30 °C.
Cadmium tolerance growth kinetics
A 2% w/v inoculum of Lb. kefir CIDCA 8348 or JCM 5818 (5×108 CFU/ml) was added to a 1/2 dilution of MRS broth placed in 96-well plates (Greiner Bio-one, Germany) and supplemented with concentrations of Cd(NO3)2 ranging from 0 to 1 mM (Baker, Phillipsburg, New Jersey, USA). Strains were incubated 76 h at 30 °C. The optical density (OD) at 600 nm was determined every 4–10 h on a Synergy HT microplate reader (Bio-Tek Instruments, Winoski, Vermont, USA).
The increase of the lag time was determined as the difference between the lag time of Lb. kefir strains grown in presence of cadmium, and the lag time of strains grown in absence of cadmium (controls).
The total biomass obtained in each condition was referred to that of the controls in the stationary phase. The following equation was used:
where ODCd2+ is the OD of microorganisms grown 55 h in presence of cadmium and ODctrl is the OD of the controls in the stationary phase.
The effective concentration 50 (EC50), defined as the cadmium concentration that reduces 50% the biomass of a Lb. kefir culture in the stationary phase (maximum biomass obtained), was determined using GraphPad Prism 5 program (GraphPad Software Inc., San Diego, CA, 2007) as follows:
where EC50 is the effective concentration 50, %OD is the value obtained from Eq. (1), %ODmax is the maximum value of %OD at 600 nm, X is the cadmium concentration and S is the slope of the plot.
Bioaccumulation of cadmium
A 2% w/v inoculum of Lb. kefir strains was inoculated in 10 ml of 1/2 diluted MRS broth in presence of the EC50 for cadmium and incubated 48 h at 30 °C. Then, cells were washed twice in Milli Q water (Milli-Q plus; Millipore Cop., USA), harvested by centrifugation and pellets were resuspended in 1 ml cadmium-free ultrapure water. After centrifugation, cell pellets were collected and digested in concentrated HNO3-H2O2 (García et al. Reference García, Senior, El Zauahre, Morán, Acosta and Femández2006). The cadmium concentrations were determined in an atomic absorption spectrophotometer (SHIMADZU 6800). The values obtained were referred to the cadmium concentration added to the culture medium (100%). Results were expressed as percentage of bioaccumulation.
Microscopy
For optical microscopic observations, microorganisms grown in presence of cadmium at the EC50 were fixed onto a microscope slide at 37 °C for 20 min and stained with crystal violet dye to enhance contrast in the microscopic image.
For transmission electron microscopy (TEM) observations, microorganisms grown in presence of cadmium at the EC50 were prepared according to Gerbino et al. (Reference Gerbino, Mobili, Tymczyszyn, Fausto and Gómez-Zavaglia2011). Briefly, microorganisms were fixed in 20 g glutaraldehyde/l for 15 h at room temperature. Glutaraldehyde solution was prepared in phosphate-buffered saline (PBS) whose composition was: 0·144 g KH2PO4/l, 9 g NaCl/l, 0·795 g Na2HPO4/l, pH 7·2). Fixed cells were collected by centrifugation and washed three times with PBS. All samples were post-fixed with 10 g osmium tetroxide/l prepared in PBS, and dehydrated for 2 h. Samples were included in Epon 812 and sliced with a Sorvall MT 2B ultramicrotome. A JEM 1200 EX II transmission electron microscope (JEOL Ltd., Tokio, Japan) was operated at 85 kV and photographed with a camera Erlangshen ES1000W, Model 785 (Gatan Inc., Pleasanton, California, USA) from Central Service Electronic Microscopy (Faculty of Veterinary Sciences, National University of La Plata). Microorganisms grown in 1/2 MRS broth without the addition of cadmium were used as controls.
Eukaryotic cell cultures
HepG2 cells (human hepatoma cell line) were grown in Dulbecco's Modified Eagle's Medium (DMEM; Gibco BRL Life Technologies, Rockville, MD, USA) supplemented with 10% v/v inactivated (30 min/60 °C) foetal bovine serum (FBS, Gibco BRL Life Technologies, Rockville, MD, USA), 2 g NaHCO3/l, 10 mg streptomycin/l and 10 IU penicillin G/ml and incubated at 37 °C in a 5% v/v CO2 atmosphere. The culture medium was changed every 2 d to attain a concentration of 2×105 cells/ml. Cells were allowed to attach to the wells of polyestyrene plates for 24 h before treatment with Cd(NO3)2, according to Fotakis & Timbrell (Reference Fotakis and Timbrell2006).
One millilitre of 48 h cultures of Lb. kefir CIDCA 8348 or JCM 5818 grown in 1/2 MRS (containing 5×108 CFU/ml) was harvested and washed twice with ultra pure water (Milli-Q plus; Millipore Cop., USA). Bacterial pellets were resuspended in DMEM (pH=6) containing Cd(NO3)2 at different concentrations of cadmium (0–0·03 mM) and incubated 1 h at 30 °C. Five hundred microlitres of the suspensions were added to HepG2 cell monolayers previously washed with PBS. Polyestyrene plates were incubated 20 h at 37 °C in a 5% v/v CO2 atmosphere. Solutions of Cd(NO3)2 ranging 0 to 0·03 mM were used as controls. A solution of 0·06 mM KNO3 was included to test the toxic effect of nitrates, and bacteria resuspended in DMEM that were not treated with Cd(NO3)2 were used as negative controls.
Mitochondrial dehydrogenase activity
The mitochondrial dehydrogenase activity of viable cells can be evaluated from the conversion of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to an insoluble purple formazan. The cell membranes are impermeable to the formazan product and therefore it accumulates in healthy cells. The MTT assay was performed as described by Mosmann (Reference Mosmann1983). Briefly, cells were incubated in serum free DMEM containing 0·8 mg MTT/ml for 4 h and washed with PBS. Dimethyl sulphoxide was added to remove formazan from cells and absorbance was recorded at 560 nm in a Synergy HT microplate reader (Bio-Tek Instruments, Winoski, Vermont, USA). Results were expressed as percentage of the viability of non-cadmium treated cells (100% viability).
Statistical analysis
All the assays were conducted in triplicate, and average values were used for data analysis. The statistical data analysis was carried out using the statistical program GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, 2007).
Results
The growth kinetics of Lb. kefir CIDCA 8348 and JCM 5818 in presence of different concentrations of Cd(NO3)2 are shown in Fig. 1. Both Lb. kefir CIDCA 8348 (Fig. 1a) and JCM 5818 (Fig. 1b) were able to grow in presence of cadmium concentrations ranging 0 to 1 mM although with different yields in biomass and/or rates of growth. An increase of the lag time and a decrease of the maximum biomass and rate of growth (slope of the logarithmic phase) were observed as soon as the concentration of cadmium in the culture medium increased.
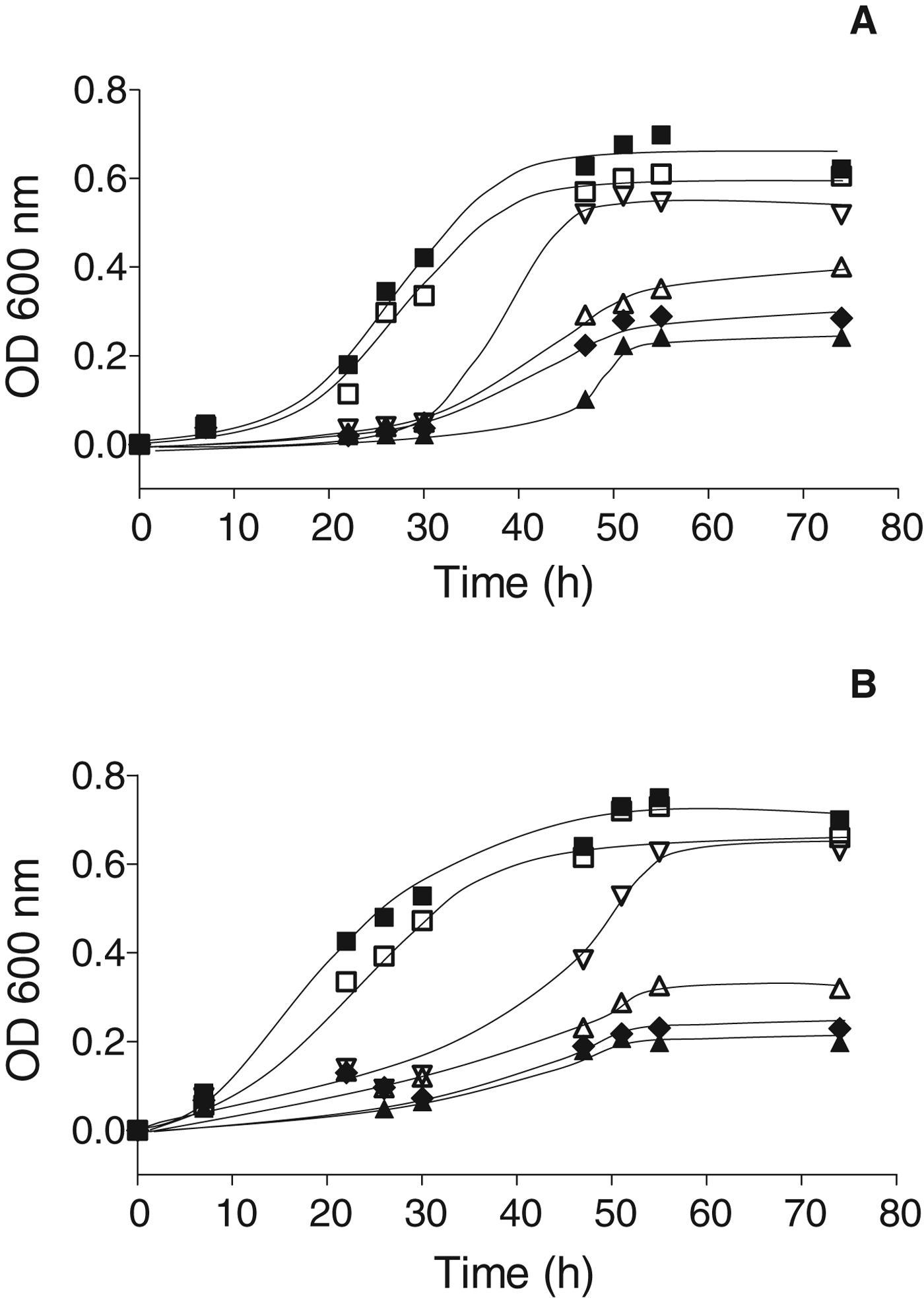
Fig. 1. Growth kinetics of Lb. kefir: CIDCA 8348 (a) and JCM 5818 (b) grown in presence of cadmium concentrations ranging 0–1 mM. ■ (0 mM); □ (0·0020 mM); ▽ (0·0078 mM); △ (0·0156 mM); ◆ (0·250 mM); ▲ (1 mM).
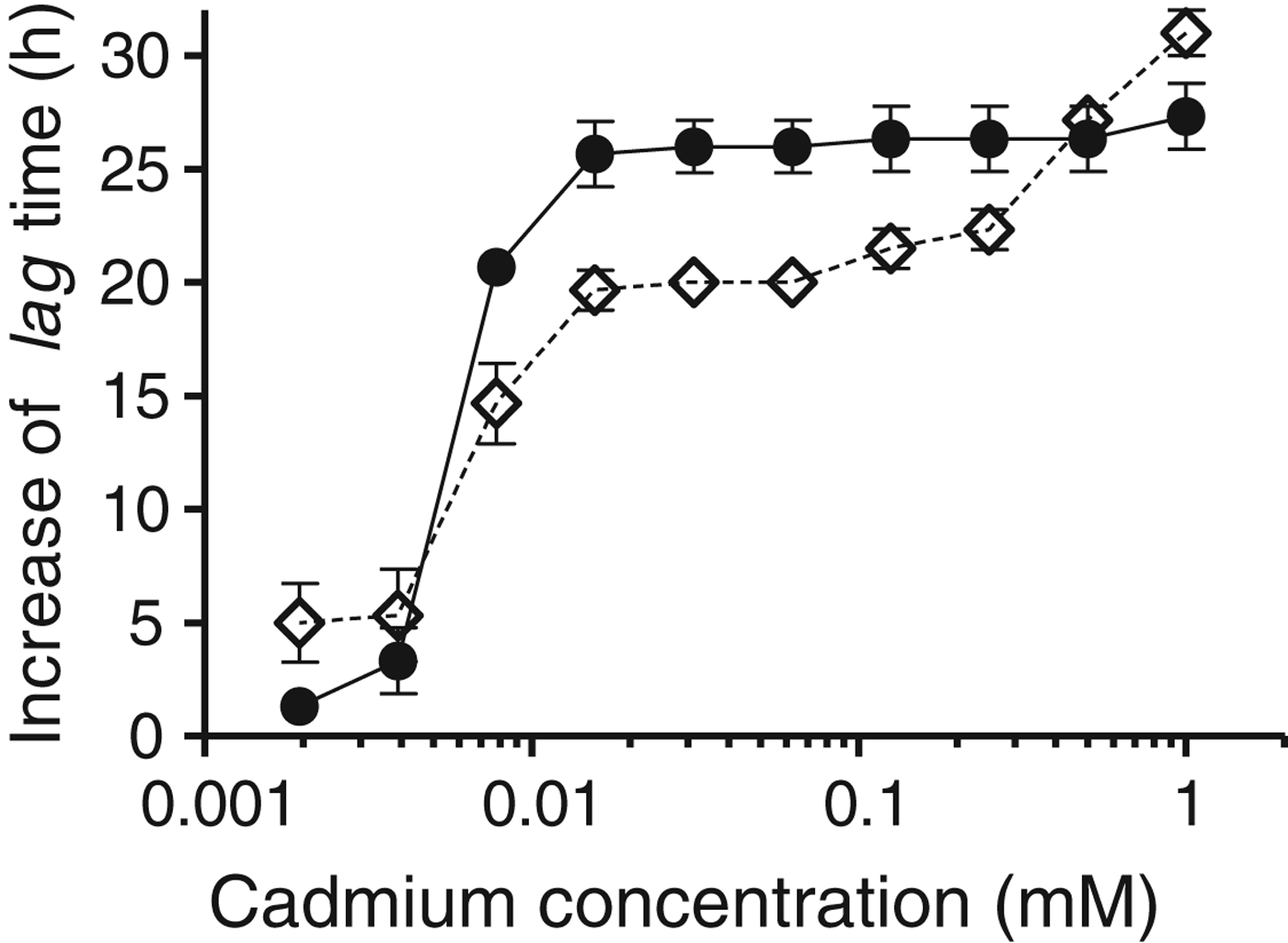
When plotting the increase of the lag time against the cadmium concentration in the culture medium, an abrupt increase of the lag time was observed for both strains at 0·0078 mM (Fig. 2). Below this value, the increase of the lag times for Lb. kefir JCM 5818 is lower than that of Lb. kefir CIDCA 8348, between 0·0078 and 0·25 mM, the behaviour is the opposite, and above 0·25 mM, strain Lb. kefir CIDCA 8348 again had a lower increase of the lag time.

Fig. 2. Semilog plot showing the increase of the lag time vs the concentration of cadmium in the growth medium. The increase of the lag time was determined as the difference between the lag times of Lb. kefir incubated with a given concentration of cadmium and the lag time of strains incubated without cadmium (control). Lb. kefir CIDCA 8348 (◊); Lb. kefir JCM 5818 (●). Error bars indicate sd.
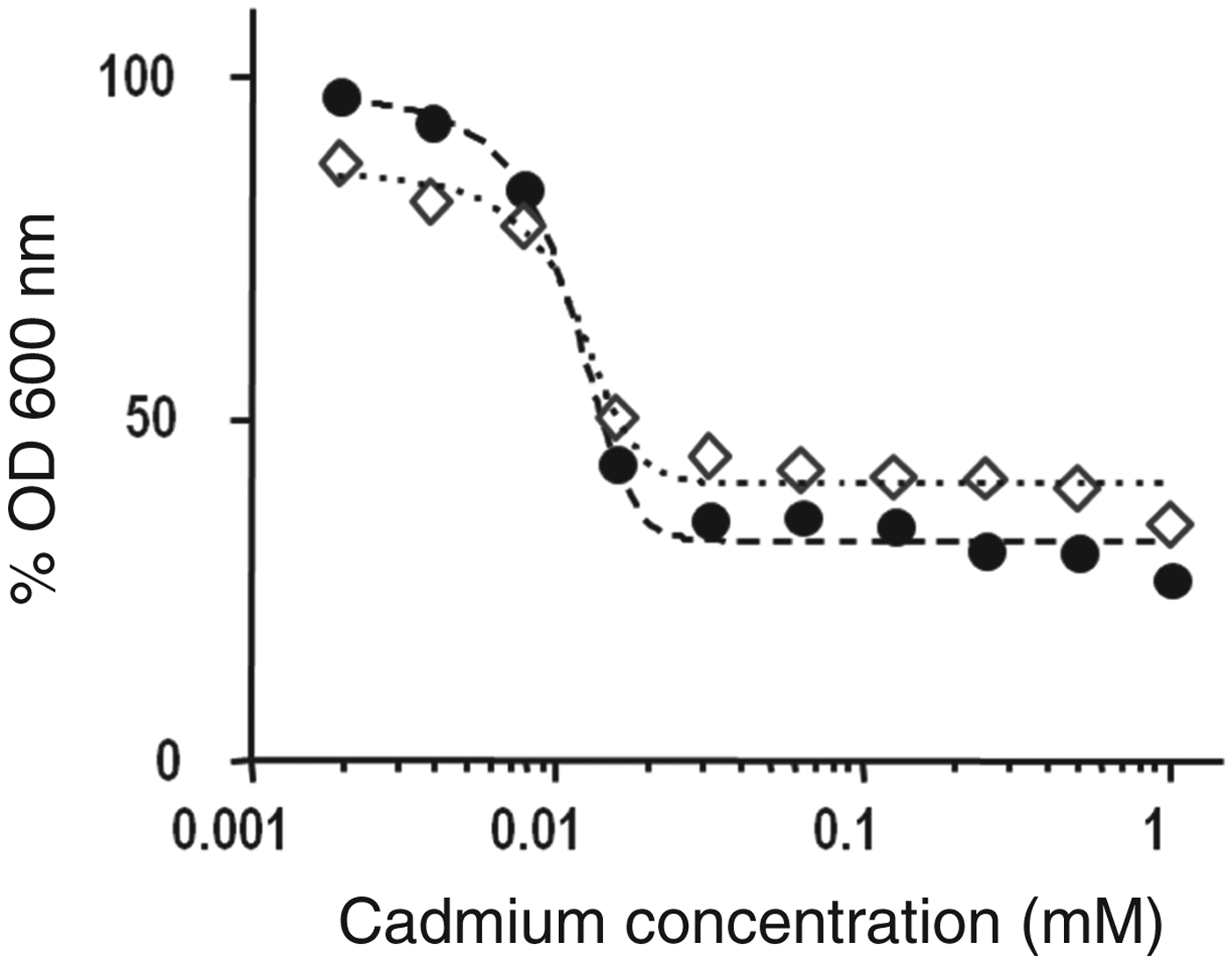
The toxicity of cadmium cations was analysed in terms of the effective concentration 50 (EC50). In the context of this work, this parameter indicates the concentration of cadmium that inhibits 50% of the bacterial growth. A dose-response graph was constructed by plotting the %OD obtained after 55 h incubation (stationary phase, taken from Fig. 1a, b) v. the cadmium concentration in the culture media (Fig. 3). Using Eq. (2), the calculated EC50 was 0·0012 and 0·0011 mM for Lb. kefir CIDCA 8348 and JCM 5818, respectively (Table 1). These concentrations were then used for bioaccumulation assays and for microscopic preparations. Table 1 also depicts the percentage of cadmium internalised by both strains, Lb. kefir JCM 5818 being the strain that incorporated it more efficiently.

Fig. 3. Dose-response plot showing the percentage of total biomass (%OD) obtained for each condition of growth after 55 h incubation vs concentration of cadmium in the growth medium. %OD is referred to OD of strains grown in the absence of cadmium (ODmax). Lb. kefir CIDCA 8348 (◊) and JCM 5818 (●).
Table 1. EC50 and percentage of cadmium bioaccumulated by Lb. kefir CIDCA 8348 and JCM 5818

Figure 4 shows the morphology of Lb. kefir CIDCA 8348 and JCM 5818 grown in presence or absence of cadmium cations. Lb. kefir CIDCA 8348 is an auto-aggregative strain (Fig. 4a) and although the cadmium treatment did not affect aggregation, rods became shorter and thicker (Fig. 4b). The non-aggregative strain Lb. kefir JCM 5818 (Fig. 4c) occurred as shorter rods after growing in presence of cadmium (Fig. 4d).

Fig. 4. Microscopic observation (1000×) of Lb. kefir CIDCA 8348 and JCM 5818 grown in absence (a and c) and presence (b and d) of the EC50. Arrows indicate the regions where thickening of lactobacilli is more evident.
Electron microscopic pictures indicate that morphological features like shape and size were affected by the cadmium treatment (Fig. 5). Indeed, a slight shortening and rounding of bacterial cells was observed in both strains (Fig. 5b, d). Moreover, dark precipitates spread in the cytoplasm of both strains were noticed (Fig. 5b, d).

Fig. 5. Transmission electron microscopy of Lb. kefir CIDCA 8348 and JCM 5818 grown in absence (a and c) and presence (b and d) of the EC50. Arrows indicate precipitation of cadmium in the cytoplasm.
The ability of Lb. kefir to protect eukaryotic cells cultures from cadmium toxicity was analysed using HepG2 cells lines. Concentrations of cadmium higher than 3×10−3 mM strongly decreased the viability of HepG2 cells (Fig. 6). When the eukaryotic cells were exposed to cadmium pre-incubated with Lb. kefir strains, the toxicity of cadmium was considerably lower. Lb. kefir JCM 5818 was more efficient at protecting HepG2 cells as cadmium concentrations lower than 3×10−3 mM did not affect the viability of eukaryotic cells.
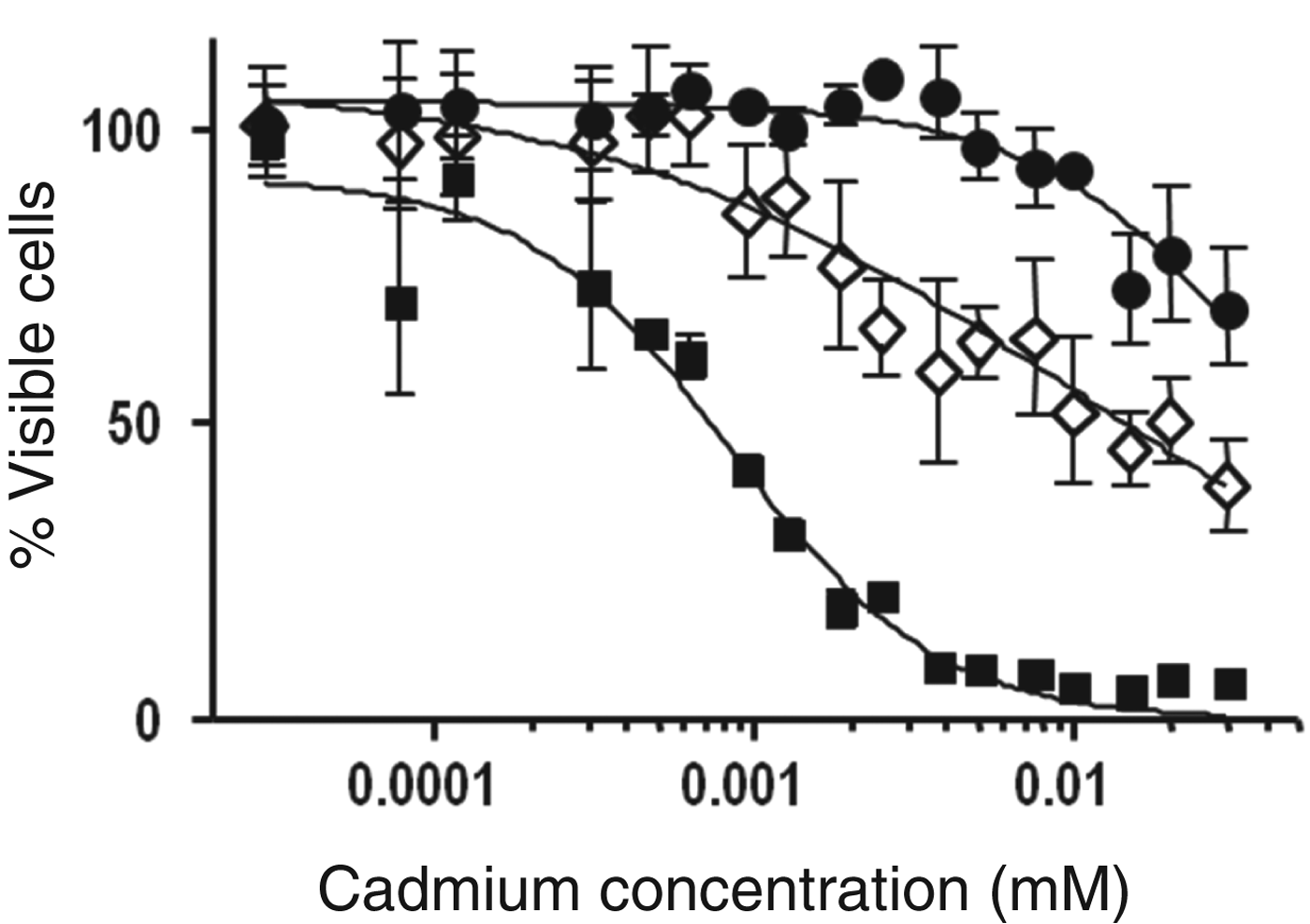
Fig. 6. Cytotoxicity of cadmium for HepG2 cells as measured by MTT assay. Percentage of viable cells vs cadmium concentration. HepG2 cells treated with suspensions of Lb. kefir CIDCA 8348 (◊) or JCM 5818 (●) previously incubated with different concentrations of cadmium. HepG2 cells directly treated with different concentrations of cadmium (■). Error bars indicate sd.
Discussion
The resistance of microorganisms to heavy metal cations is very important in different scientific domains, namely environmental and food sciences. Different mechanisms of detoxification have been described to explain the capacity of certain microorganisms to adsorb, tolerate and/or bioaccumulate toxic metals and prevent cellular damage (Cervantes et al. Reference Cervantes, Espino-Saldaña, Acevedo-Aguilar, León-Rodríguez, Rivera-Cano, Avila-Rodríguez, Wróbel-Kaczmarczyk, Wróbel-Zasada, Gutiérrez-Corona, Rodríguez-Zavala and Moreno-Sánchez2006; Halttunen et al. Reference Halttunen, Salminen and Tahvonen2007; Mrvcic et al. Reference Mrvcic, Stanzer, Solic and Stehlik-Tomas2012). Detoxification mechanisms are particularly relevant in lactobacilli, as they may act as potential adjuncts for reducing the toxicity of cadmium in humans and animals.
The results obtained in this work showed for the first time that Lb. kefir strains can grow and tolerate cadmium concentrations up to 1 mM. Even when the addition of cadmium to the culture medium increased the lag time, only at concentrations above EC50, a decrease of the total biomass was observed (Figs. 1–3). The greater lag time indicates an increase in the time required by microorganisms for the adaptation to a harsh environment containing cadmium cations. Deleterious effects of cadmium include damage of DNA, inhibition of protein synthesis and competition for enzymatic reactive sites (Hynninen, Reference Hynninen2010).
The microscopic observations reported in this work are compatible with intracellular precipitation of cadmium (Fig. 5). Different mechanisms have been proposed to explain the microbial tolerance to heavy metals. Most of them lead to reduced metal mobility, for example production of metal-binding peptides and proteins, organic or inorganic precipitation, intracellular metal sequestration, low bacterial transformation, and metal sorption by cell wall and other superficial structures (Adarsh et al. Reference Adarsh, Mishra, Chowdhury, Sudarshan, Thakur and Chaudhuri2007; Panwichian et al. Reference Panwichian, Kantachote, Wittayaweerasak and Mallavarapu2011). Formation of intracellular particles of cadmium was also reported for different lactobacilli and is a widespread phenomenon that could play a role in metal resistance because it decreases the harmful effect of cadmium. The formation of cadmium complexes with sulphur rich proteins or segregation into vesicles have been proposed as mechanisms (Prasad & Jha, Reference Prasad and Jha2010; Monachese, Reference Monachese2012). The morphological changes (Fig. 4) and cytoplasmic dark precipitates (Fig. 5) observed in both strains of Lb. kefir can be explained on this basis. Taking into account the probiotic properties of Lb. kefir, the high bioaccumulation capacity of both strains (Table 1) encourages their food use, thereby decreasing the concentration of free cadmium cations and preventing their toxicity in humans and animals.
HepG2 cells are generally used to investigate cadmium toxicity because, due to the high hepatotoxicity of cadmium, they provide a good approximation for subsequent studies in vivo (Fotakis & Timbrell, Reference Fotakis and Timbrell2006). In our previous work, we reported that both Lb. kefir strains are able to detoxify water of cadmium (Gerbino et al. Reference Gerbino, Mobili, Tymczyszyn, Frausto Reyes, Araujo-Andrade and Gómez-Zavaglia2012). In this work, we showed that a prior interaction of cadmium with Lb. kefir decreases the toxicity of cadmium in HepG2 cells (Fig. 6). The higher efficiency of strain JCM 5818 is consistent with its major capacity to bioaccumulate cadmium cations (being a non-aggregative strain, a larger surface to interact with cadmium is available) (Table 1) and its higher binding capacity measured in mg cadmium per g dry biomass (q max=61·6 mg/g) (Gerbino et al. Reference Gerbino, Mobili, Tymczyszyn, Frausto Reyes, Araujo-Andrade and Gómez-Zavaglia2012). This way, a direct interaction of cadmium with the host microbiota containing different species of lactobacilli may result in a decrease of cadmium availability by immobilisation and/or uptake of the metal. Zhai et al. (Reference Zhai, Wang, Zhao, Liu, Tian, Zhang and Chen2013) reported that a strain of Lb. plantarum decreases the intestinal absorption of cadmium in mice and consequently, increases its faecal excretion in the first 24 h after acute oral exposure. The removal of cadmium by adsorbing it at the bacterial surface is one of the mechanisms proposed to explain this effect. In fact, the binding capacity is related to a protective effect against acute cadmium toxicity (Zhai et al. Reference Zhai, Wang, Zhao, Liu, Tian, Zhang and Chen2013). Both Lb. kefir strains used in this work are able to bind cadmium fast (after 1 h incubation) and efficiently (Gerbino et al. Reference Gerbino, Mobili, Tymczyszyn, Frausto Reyes, Araujo-Andrade and Gómez-Zavaglia2012). This, together with the capacity of lactobacilli to stimulate intestinal motility (Del Piano et al. Reference Del Piano, Carmagnola, Anderloni, Andorno, Ballarè, Balzarini, Montino, Orsello, Pagliarulo, Sartori, Tari, Sforza and Capurso2010) supports the use of these strains to increase the faecal excretion of cadmium after acute exposure. The viability of eukaryotic cells exposed to Lb. kefir strains incubated with cadmium was not affected, indicating that adsorbed cadmium was not released in these experimental conditions.
Finally, it is noteworthy that the EC50 determined in this study (∼3·55 mg/l) (Table 1) far exceeded the tolerated weekly intake of cadmium for food (EFSA, 2012) and water addressed for human consumption (3 μg/l) (WHO, 2006). The high tolerance and binding capacity of Lb. kefir strains to cadmium (even in concentrations that are incompatible with human life) is an important added value when thinking about health-related applications.
As consumption of harmful metals is not likely to decrease in the near future, the regular administration of formulations containing these strains appears to be an adequate and easy to implement strategy to prevent the harmful effects of cadmium in populations exposed to this toxic metal.
This work was supported by the Argentinean Agency for the Promotion of Science and Technology (Projects PICT/2008/145 and PICT/2011/0226), the Argentinean National Research Council (CONICET) (Project PIP 114-201101-00024). AGZ and EET are members of the research career CONICET (Argentina). EG and PC are postdoctoral and doctoral fellows from CONICET, respectively.