Long-chain fatty acids (FA) in milk are nutritionally important for the consumer providing energy and substrates for vital physiological functions. One FA that is being promoted as a potential target to modify milk fat is conjugated linoleic acid (CLA; c9 t11 18 : 2), a potential anti-carcinogen with many beneficial effects in humans (Ip et al. Reference Ip, Jiang, Thompson and Scimeca1997; McGuire & McGuire, Reference McGuire and McGuire2000; Belury, Reference Belury2002). Other FA, particularly the monounsaturated fatty acids (MUFA) and ω-3 FA, provide more opportunities to improve the FA profile of milk (German et al. Reference German, Morand, Dillard, Xu, Welch, Burns, Davis, Popay and Prosser1997). Alteration of the FA profile of milk fat is impacted by many factors such as nutrition, season, stage of lactation and hormones (Grummer, Reference Grummer1991; Palmquist et al. Reference Palmquist, Beaulieu and Barbano1993; Jenkins & McGuire, Reference Jenkins and McGuire2006). Furthermore, the dietary unsaturated FA consumed by the cow are actively biohydrogenated in the rumen by microbes, thus minimising the amount of unsaturated FA that are absorbed by the cow (Grummer, Reference Grummer1991). The stearoyl-CoA desaturase (SCD) enzyme is present in bovine tissues and is active in the mammary gland (Bionaz & Loor, Reference Bionaz and Loor2008; Jacobs et al. Reference Jacobs, van Baal, Smits, Taweel, Hendriks, van Vuuren and Dijkstra2011). Four isoforms of SCD have been identified in the mouse but only two forms have been found and characterised in humans (Ntambi & Miyazaki, Reference Ntambi and Miyazaki2004) and cattle (Lengi & Corl, Reference Lengi and Corl2007). In humans and cattle, SCD1 is the primary form in tissues other than the brain where SCD5 is more abundant (Lengi & Corl, Reference Lengi and Corl2007). Regulation of SCD1 has been well studied in rodent liver (Ntambi, Reference Ntambi1999; Ntambi & Miyazaki, Reference Ntambi and Miyazaki2004). In conjunction with NADPH, cytochrome b5 reductase and cytochrome b5 and in the presence of molecular oxygen, SCD introduces a double bond (between carbons 9 and 10) into some saturated fatty acyl-CoAs (Ntambi & Miyazaki, Reference Ntambi and Miyazaki2004). This insertion of a double bond increases the fluidity of the lipid in triacylglycerols (TAG) or phospholipids (PL), which receive the FA modified by SCD. The SCD enzyme uses myristic (14 : 0), palmitic (16 : 0) and stearic acid (18 : 0) as substrates for its actions, creating myristoleic (14 : 1), palmitoleic (16 : 1) and oleic (18 : 1) acid, respectively (Ntambi, Reference Ntambi1999). These six FA represent approximately 75% of the total FA in milk fat. Additionally, Griinari et al. (Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000) demonstrated that c9 t11 18 : 2 (CLA) could be synthesised from vaccenic acid (VA; t11 18 : 1) by the action of SCD in the mammary gland.
The activity of SCD accounted for 90% of 14 : 1, 50% of 16 : 1, 59% of 18 : 1, and 80% of c9 t11 CLA in milk from lactating cows fed typical dairy rations as determined using 13C-labelled FA in lactating cows (Mosley et al. Reference Mosley, Shafii, Moate and McGuire2006; Mosley & McGuire, Reference Mosley and McGuire2007). Further, no 13C-labelled desaturase products were detected in blood, only in milk, indicating that SCD products in milk fat may arise mainly from SCD activity in mammary tissue in the lactating cow. Jensen & Patton (Reference Jensen and Patton2000) hypothesised that fluidity of milk fat is critical for a successful lactation. Therefore, SCD is very important for milk fat synthesis in ruminants, which absorb mainly saturated FA as a result of rumen biohydrogenation. Recent data confirmed (Gervais et al. Reference Gervais, McFadden, Lengi, Corl and Chouinard2009) the original observation in which another gene (SCD5) encoding for SCD protein in the bovine was identified (Lengi & Corl, Reference Lengi and Corl2007).
Manipulation to increase the activity of SCD may lead to increased production of MUFA and reduced SFA, thereby improving the milk and tissue FA profiles. However, a clearer understanding of the expression of SCD at gene and protein levels across ruminant tissues may yield new thoughts as to the regulation of SCD activity leading to improved animal products. In the current study, the hypotheses were that a positive correlation existed between gene expression of SCD1 and occurrence of its products in various bovine tissues. Further, a positive correlation would also be present between gene expression of SCD1 and relative abundance of SCD protein across tissues. Our specific objective was to determine any association between the gene expression of SCD1 and occurrence of its products (c9 14 : 1, c9 16 : 1, c9 18 : 1, and c9 t11 CLA) in various bovine tissues.
Materials and methods
Sampling
Tissue samples including large and small intestines, intestinal adipose from the mesentery, cardiac and skeletal (sternocephalicus) muscles, mammary, lung and liver were obtained from lactating Holstein cows (n=28) at slaughter, frozen in liquid nitrogen and stored at −80 °C until analysis. Samples were not pooled at any step in processing. Cows were from a single farm and management confirmed that cows were milked within 6 h of slaughter. Lactation was confirmed by the presence of milk in the mammary gland.
Sample analyses
RNA was isolated using the RNeasy Mini Plus Kit (Qiagen, Valencia CA, USA) according to manufacturer's instructions. The quality of extracted RNA was checked on a 1% denaturing agarose gel and the concentration was determined using the absorbance at 260 nm. RNA (2 μg) was reverse transcribed to cDNA (High capacity cDNA archive kit; Applied Biosystems, Foster City CA, USA) according to manufacturer's instructions. Random hexamers were used to prime the reverse transcription.
The cDNA was used to generate clones for quantitative real time PCR analysis of the genes β-actin [BAC (NM_173979); used as a house-keeping gene] and SCD1 (AY241933). Primers (Table 1) were created using the Primer3 software (Applied Biosystems, Foster City CA, USA) and the genes were amplified using the Advantage 2 PCR Kit (Clontech, Mountain View CA, USA). The PCR reaction was purified using the QIAquick PCR Purification Kit (Qiagen) and the amplicon was ligated into a cloning vector (TOPO TA Cloning Kit Dual Promoter with Chemically Competent cells; Invitrogen, Carlsbad CA, USA). Clones containing inserts were identified by restriction enzyme analysis (EcoRI, Invitrogen) with cutting on either side of the PCR insert after isolation of plasmids (FastPlasmid Mini Prep Kit; Eppendorf, Westbury NY, USA). The insert sequence was verified by sequencing with the ABI Prism Big Dye terminator cycle sequencing ready reaction kit (Perkin Elmer, Foster City CA, USA).
Table 1. Primers used in cloning polymerase chain reaction analysis of β-actin and stearoyl-CoA desaturase 1 (SCD1) genes†

† Designed using Primer Express
For quantitative real time PCR, the quality and concentration of RNA were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland DE, USA). Total RNA was reverse transcribed into cDNA using Applied Biosystems High Capacity Reverse Transcriptase Kit (Applied Biosystems) and a PCR Sprint Thermal Cycler (Thermo Electron Corp., Milford MA, USA). The single-stranded cDNA was then used as the template for quantitative PCR to evaluate absolute expression of SCD1 and BAC. Primers for PCR amplification of SCD1 and BAC were designed by Primer Express (Version 1.5, Applied Biosystems) generating the primers detailed in Table 2. Gene expression of SCD1 and BAC was measured using the 7500 Fast Real-Time PCR System (Taqman methodology; Applied Biosystems).
Table 2. Primers and probes used in quantitative real time reverse transcribed polymerase chain reaction (rt-PCR) analysis†

† Designed using Primer Express
Extracted lipid was converted to fatty acid methyl esters and analysed by GC. Briefly, lipid was extracted from tissue (500 mg) using chloroform:methanol (2 : 1) (Clark et al. Reference Clark, Ferris, Fey, Brown, Hundrieser and Jensen1982). The extracted lipid (5 mg) was methylated using base-catalysed transesterification (Christie, Reference Christie1982) with a reaction time of 24 h. Fatty acid methyl esters were analysed by GC (Hewlett-Packard 6890 Series with auto injector) fitted with a flame ionisation detector and a 100 m×0·25 mm, with 0·2-μm film capillary column coated with CP-Sil 88 (Chrompack; Middelburg, The Netherlands). Initially, the oven temperature was 70 °C (for 3 min) and then increased to 175 °C at a rate of 3 °C per min and held for 3 min. Oven temperature was then increased to 185 °C at a rate of 1 °C per min and held for 20 min, increased to 215 °C at a rate of 3 °C per min, and then increased to 230 °C at a rate of 10 °C per min and held for 5 min. To quantify fatty acids, response correction factors were determined by the analysis of a butter oil standard with certified values (CRM 164; European Community Bureau of Reference, Brussels).
For determination of SCD1 protein content, tissue was homogenised in cold lysis buffer (50 mm–Tris, pH 7·4, 0·5% Triton X-100, 0·3 m-NaCl, 2 mm-EDTA, pH 8·0, and proteinase inhibitor), followed by centrifuging at 16 100 g at 4 °C for 15 min. Supernatants were collected for protein concentration measurements. Protein concentrations were determined using Bradford Assay (Bio-Rad, Hercules CA, USA). To ensure equal loading, samples were diluted to the same protein concentrations with Laemmli sample 78 buffer (Bio-Rad, Hercules CA, USA) and heated at 95 °C for 10 min. Proteins were separated by electrophoresis using 12% polyacrylamide gels (Cambrex Corporation, East Rutherford NJ, USA) and transferred to a PVDF membrane using a Bio-Rad trans-blot sd semi-dry transfer cell (Bio-Rad, Hercules CA, USA). Membranes were then blocked in blocking buffer (0·05 m-Tris pH 7·4, 0·2 m-NaCl, 0·1% Tween, and 5% dried non-fat milk) on a rocker for 1 h. Blots were probed with primary anti-SCD1 antibody (custom rabbit anti-bovine SCD1, Pacific Immunology, 1 : 1000) in blocking buffer at 4 °C overnight. This antibody does not detect bovine SCD5 (Corl & Lengi, unpublished results). Membranes then were washed in washing buffer (0·05 m-Tris pH 7·4, 0·2 m-NaCl, and 0·1% Tween). Following washing, membranes were incubated with horseradish peroxidase-conjugated- secondary antibody (Santa Cruz Biotechnology, Santa Cruz CA, USA) at 1 : 1000 in blocking buffer for 1 h at room temperature. Membranes were then washed again, and proteins were detected using ECL-Plus chemiluminescence substrate (Amersham Biosciences, Pittsburg PA , USA) according to manufacturer's instructions. Chemiluminescence was measured using a Chemidoc XRS digital imaging system and densitometry was quantified using Quantity One software (Bio-Rad, Hercules CA, USA).
Statistical analyses
All statistical analyses were conducted using SAS software (v. 9.2 for Windows; SAS Institute Inc., Cary NC, USA). Gene expression data were analysed by using relative measurement (ΔCt value=Cttarget gene − Cthouse-keeping gene) in the MIXED model and by using original copy number (per ng total RNA) in CORR procedure of SAS. Data for protein abundance were analysed in the MIXED model and CORR procedure (Pearson's correlation) of SAS. For descriptive purposes, means and sem were calculated for copy number of SCD1 and fatty acid data for each tissue (PROC UNIVARIATE). Fatty acid methyl ester weight percentages were used to calculate the desaturase indices ([product]/([substrate]+[product]) for all desaturase product substrate pairs (c914 : 1 and 14 : 0, c916 : 1 and 16 : 0, c918 : 1 and 18 : 0, and c9 t1118 : 2 and t1118 : 1). Results are reported as least squares means±sem. Significant differences in gene expression of SCD1 among various tissues were declared at P<0·05. Correlation analysis was used to assess any association between SCD products and mRNA expression of SCD1 within and across tissues, and significant correlations were declared at P<0·05.
Results
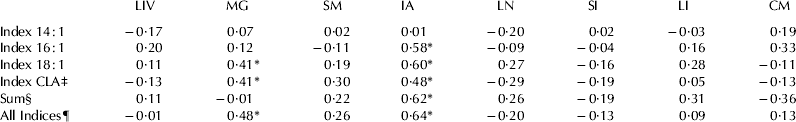
In the present study, expression of SCD1 mRNA was detected in measureable quantities in all tissues tested with greatest expression in mammary gland, intestinal adipose and cardiac muscle tissues, and lowest expression in liver when expressed in absolute quantities (Fig. 1). When SCD1 gene was expressed in relative amount (delta Ct value=Cttarget gene − Cthouse-keeping gene), its expression differed among tissue tested (Table 3) with greatest expression in skeletal muscle, mammary gland, cardiac muscle and intestinal adipose, and the lowest expression in liver. Significant variation in expression of SCD1 gene among cows was found in all tissues (data not shown). The FA profile of the tissues varied (Table 4). Interestingly, the desaturase indices for the SCD1 substrates 14 : 0, 16 : 0, 18 : 0, and t11 18 : 1 and sum of SCD1 products were not similar across all tissues (Table 5). Across all tissues tested, the desaturase indices for c9 18 : 1 (r=0·24) and sum of SCD products (r=0·20) were positively correlated (P<0·01 for both) with SCD1 gene expression (Table 6). Within each tissue, the relationship between SCD1 gene expression and the desaturase indices varied significantly (Table 6). Whereas no correlation was detected between SCD1 expression and desaturase indices in the liver, large and small intestines, lung, cardiac or skeletal muscles, positive correlations were detected between SCD1 expression and all of the desaturase indices in intestinal adipose tissue (P<0·02 for all) except for the index for c9 14 : 1. Pearson's correlation coefficient varied from r=0·48 (c9 t11 18 : 2) to r=0·64 (sum of all SCD1 indices) for adipose tissue (Table 6). In mammary tissue, c9 18 : 1, c9 t11 18 : 2 and sum of all SCD1 desaturase indices were positively correlated with SCD expression (r=0·41, 0·41, and 0·48 respectively; P⩽0·03 for all).

Fig. 1. Absolute quantification of stearoyl-CoA desaturase1 (SCD1) expression in various bovine tissues using quantitative rt-PCR analysis.
Table 3. Least Square means of ΔCt (critical threshold) values (Cttarget gene – Cthouse-keeping gene) of SCD1 gene in various bovine tissues (n=28)†

† LIV=liver; MG=mammary gland; SM=skeletal muscle (sternocephalicus); IA=intestinal adipose; LN=lung; SI=small intestine; LI=large intestine; CM=cardiac muscle
‡ Means without a common superscript differ at P⩽0·05
Table 4. Means (sd) of fatty acid composition (percent of total identified fatty acid methyl esters) of various bovine tissues (n=28)†

† LIV=liver; MG=mammary gland; SM=skeletal muscle (sternocephalicus); IA=intestinal adipose; LN=lung; SI=small intestine; LI=large intestine; CM=cardiac muscle
‡ Conjugated linoleic acid

† Indices were calculated as [(SCD product)/(SCD product)+(SCD substrate)]
‡ LIV=liver; MG=mammary gland; SM=skeletal (sternocephalicus) muscle; IA=intestinal adipose; LN=lung; SI=small intestine; LI=large intestine; CM=cardiac muscle
§ Index conjugated linoleic acid (CLA)
¶ Sum of all SCD products
Table 6. Pearson's correlation coefficients (r) for the relationship between SCD1 gene expression (copy number/ng RNA) and abundance of its products within each tissue tested†
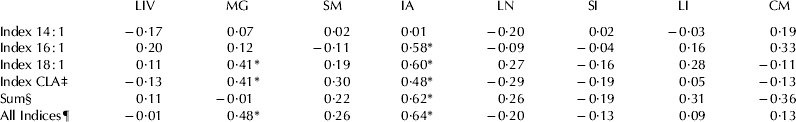
† Indices were calculated as [(SCD product)/(SCD product)+(SCD substrate)]. Asterisk indicates P-value <0·05. LIV=liver; MG=mammary gland; SM=skeletal (sternocephalicus) muscle; IA=intestinal adipose; LN=lung; SI=small intestine; LI=large intestine; CM=cardiac muscle
‡ Index conjugated linoleic acid
§ Sum of all SCD products
¶ Sum of all indices
In a qualitative approach, SCD1 protein was detected and density compared among bovine tissues tested (Fig. 2). Statistical analysis revealed that the relative abundance of SCD1 protein in the mammary gland tended to be greater than in intestinal adipose (P=0·058) but different from all other tissues tested. Intestinal adipose tissue, however, did not differ from any other tissues tested. Mammary gland, intestinal adipose, lungs, cardiac muscle, liver, large intestine, small intestine and skeletal muscle tissues contained 1358, 502, 50·3, 33·2, 24·5, 5·8, 4·6 and 0·7 arbitrary units of SCD1 protein, respectively. Protein abundance analysis (Western blotting) visually corresponded relatively well with mRNA expression data except for muscle tissues (cardiac and skeletal). Correlation procedure (PROC CORR of SAS) did not seem reliable, owing to presence of many samples with ‘no protein’ present (Spearman's coefficient of correlation r=0·26, P<0·0001). Overall, tissues with high mRNA expression of SCD1 contained greater SCD1 protein whereas detection of SCD1 protein in tissues with low SCD1 mRNA expression was very faint or absent.

Fig. 2. A representative Western blot of Stearoyl-CoA desaturase1 (SCD1) in various bovine tissues [LIV=liver; IA=intestinal adipose; LN=lung; SI=small intestine; MG=mammary gland; LI=large intestine; CM=cardiac muscle; SM=skeletal (sternocephalicus) muscle; RU=rumen]. A discussion of the band in lung is provided in the text.
Discussion
Consistent with the current findings on the tissue gene expression of SCD1, the activity of SCD1 has been detected in mammary and adipose tissue of cattle (Christie, Reference Christie and Christie1981; Vernon, Reference Vernon and Christie1981; Yang et al. Reference Yang, Larsen, Smith and Tume1999). However, rates of desaturation in liver are low (Bell, Reference Bell and Christie1981), which is in line with findings of the present study regarding gene expression (Table 3; Fig. 1) and protein abundance (Fig. 2). For instance, no desaturase activity was detected in bovine liver from Angus and Braford cattle when 18 : 0 was provided as a substrate in vitro (St. John et al. 1991). Moreover, bovine liver explants from Charolais steers did not desaturate VA to c9 t11 18 : 2 (Gruffat et al. Reference Gruffat, De La Torre, Chardigny, Durand, Loreau and Bauchart2005). Ovine intestinal mucosal preparations actively desaturate 18 : 0 (Bickerstaffe & Annison, Reference Bickerstaffe and Annison1969). Similarly, our data show that SCD1 is expressed in both small and large intestinal tissue (Fig. 1). Furthermore, Lengi & Corl (Reference Lengi and Corl2007) reported high expression of SCD1 mRNA in bovine adipose tissue while the expression was lowest in lungs and liver. These observations are in also agreement with findings of the present study (Table 3).
It is important to note that changes in SCD activity using the proxy of desaturase indices for substrate to product are closely associated with changes in SCD1 gene expression (Baumgard et al. Reference Baumgard, Matitashvili, Corl, Dwyer and Bauman2002). Thus, gene expression for SCD1 may be more important than post-transcriptional regulation and the relationships among various tissues in FA profile and SCD may be related to the importance of desaturation in each tissue. For instance, synthesising fat that contains MUFA and PUFA (lower melting point for FA with a double bond or more, as compared with saturated FA) for milk fat is a good example of how desaturation by SCD1 is in line with the necessary characteristic features of the end product. On the other hand, liver in ruminants is not a major source of circulating FA (Bell, Reference Bell and Christie1981); SCD1 gene expression and SCD1 protein abundance were lowest in liver samples in the present study, similar to that found by Lengi & Corl (Reference Lengi and Corl2007).
Gene expression of SCD1 may be affected by various factors such as species, dietary ingredients, or specific tissue. For example, when different types of unprotected oils were fed to lactating cows, mammary gland SCD1 gene expression was reduced in response to dietary soybean oil as compared with rapeseed or linseed oils, which was also associated with a reduction in C16 and C18 desaturation indices (Jacobs et al. Reference Jacobs, van Baal, Smits, Taweel, Hendriks, van Vuuren and Dijkstra2011), indicating a positive relationship between the gene expression and desaturation indices in bovine mammary gland. Similarly, when feeding various types of oils to lactating goats, expression of SCD mRNA in the mammary gland could be used as a good tool to assess SCD activity (Bernard et al. Reference Bernard, Rouel, Leroux, Ferlay, Faulconnier, Legrand and Chilliard2005). Importantly, both of these measurements were related to milk desaturation indices (Bernard et al. Reference Bernard, Rouel, Leroux, Ferlay, Faulconnier, Legrand and Chilliard2005). Our findings showed a positive correlation between the proxy measures of desaturation in the mammary gland (18 : 1 and CLA) and SCD1 gene expression as well. In contrast, Bionaz & Loor (Reference Bionaz and Loor2008) reported no significant correlation between mammary SCD1 gene expression and desaturase indices measured. The authors noted that the gene expression appeared opposite to overall desaturase index and concluded that proxy measures of desaturation are not reliably indicative of SCD1 gene expression or activity (Bionaz & Loor, Reference Bionaz and Loor2008). Archibeque et al. (Reference Archibeque, Lunt, Gilbert, Tume and Smith2005) reported a similar poor association of SCD gene expression and desaturase index in various adipose depots of beef steers fed corn, flaxseed or sorghum-based finishing diets. Another aspect that needs further investigation is the differences observed (Fig. 1 and Table 3) when two methods of quantifying gene expression of SCD1 were employed and how that may relate to physiological importance and function of each tissue. The present study is limited in that aspect as only one ‘house-keeping’ gene was assessed in all tissues tested.
The present study showed that overall, a positive relationship was detected between expression and relative protein abundance in various bovine tissues. The SCD1 antibody used for this project was a polyclonal antibody based on a 16 amino acid peptide. While non-specific binding to a protein other SCD1 cannot be ruled out, the band present in lung (Fig. 2) is probably not SCD5. The peptide used to generate the SCD1 antibody is sufficiently different from the sequence of SCD5 to prevent cross-reactivity. Additionally, if the band was SCD5, it would be closer to the SCD1 band and lower on the gel, not higher. Finally, a previous examination of SCD5 expression in the report by Lengi & Corl (Reference Lengi and Corl2007) indicated that expression in the lung was low.
Conclusion
The present study illustrated that gene expression of SCD1 and its corresponding protein were found in a number of bovine tissues. Absolute gene expression of SCD1 was greatest in the mammary gland, cardiac muscle, intestinal adipose and skeletal muscle. When gene expression of SCD1 was assessed in a relative term, outcomes were, to a great extent, similar with greatest relative expression of SCD1 in skeletal muscle, mammary gland, cardiac muscle and intestinal adipose, and the lowest expression in liver. Gene expression of SCD1 was positively related to protein abundance of SCD in most tissues. The relationship between SCD1 gene expression and SCD fatty acid indices were positive for intestinal adipose and mammary gland, but not significant for all other tissues. A clearer understanding of gene expression and activities of SCD, as a key regulatory enzyme that controls FA profile of bovine milk, may help to manipulate FA composition toward more beneficial products and improved public health.
Funding for this project was provided by the Idaho Agricultural Experimental Station and by National Research Initiative Competitive Grant no. 2006-35206-16819 from the USDA National Institute of Food and Agriculture. The authors thank S. Zaman, D. Pfeifer, W. Price, and J. E. Williams (University of Idaho, Moscow ID) for technical assistance.