β-Lactoglobulin (β-Lg), which accounts for about 50% of the total whey proteins in bovine milk, is relatively unstable to heat. At temperatures above ∼70 °C, it undergoes denaturation and aggregation which affect the heat stability and functional properties of products containing whey proteins. However, in industrial practice, thermal processing is a necessary step that is commonly applied to improve the shelf life of food products and ensure their microbial safety. Therefore, many researchers have focused on how to protect β-Lg during thermal processing to prevent its denaturation and aggregation with itself and other milk proteins, chiefly κ-casein (Hoffmann & van Mil, Reference Hoffmann and van Mil1999; Sakai et al. Reference Sakai, Sakurai, Sakai, Hoshino and Goto2000; Burrington, Reference Burrington2012).
At room temperature and at neutral or near neutral pH, β-Lg exists as a non-covalent dimer in its native form (Hoffmann & van Mil, Reference Hoffmann and van Mil1999; Surroca et al. Reference Surroca, Haverkamp and Heck2002; Creamer et al. Reference Creamer, Bienvenue, Nilsson, Paulsson, van Wanroij, Lowe, Anema, Boland and Jimenez-Flores2004). However, during thermal processing the native dimer dissociates into monomers which subsequently aggregate into oligomers and polymers. Aggregation involves a free sulphydryl (−SH) group which is normally buried within the folded globular structure of β-Lg but becomes exposed on the outer surface of the molecule when the structure is unfolded during heating. Once exposed, this −SH group becomes accessible and available for new intermolecular interactions, particularly sulphydryl−disulphide interactions that cause protein aggregation via new disulphide linkages (Surroca et al. Reference Surroca, Haverkamp and Heck2002). Previous research has shown that aggregation can be prevented by −SH-specific reagents such as dithio(bis)-p-nitrobenzoate (DTNB) and N-ethylmaleimide (NEM) which trap the free reactive −SH group (Iametti & Bonomi, Reference Iametti and Bonomi1996; Hoffmann & van Mil, Reference Hoffmann and van Mil1997, Reference Hoffmann and van Mil1999; Sakai et al. Reference Sakai, Sakurai, Sakai, Hoshino and Goto2000).
Another reagent that potentially may reduce the formation of protein aggregates is α-lipoic acid (LA, 6,8-dithiooctanoic acid, also known as thioctic acid). LA occurs naturally in milk and is associated with the fat globule membrane (Bingham et al. Reference Bingham, Huber and Aurand1967). Both, oxidised and its reduced form, dihydrolipoic acid (DHLA, 6,8-dimercaptooctanoic acid), are biological antioxidants (Ghibu et al. Reference Ghibu, Richard, Vergely, Zeller, Cottin and Rochette2009). LA has been studied extensively for its antioxidant properties and its potential use as a therapeutic agent in several diseases (Fuchs et al. Reference Fuchs, Packer and Zimmer1997; Moini et al. Reference Moini, Packer and Saris2002; Ghibu et al. Reference Ghibu, Richard, Vergely, Zeller, Cottin and Rochette2009). It is now widely available as a nutritional supplement. An investigation of the effect of LA on human fibroblast interferon showed that it had a marked ability to stabilise interferon at concentrations as low as 0·1 mm (Cartwright et al. Reference Cartwright, Senussi and Grady1977). The postulated protection mechanism was blocking the formation of disulphide bonds, resulting in the stabilisation of inactive interferon conformations.
In the work described in this paper, LA and DHLA were investigated for their stabilising effect on β-Lg during heat treatment. Knowledge of this effect may facilitate their use as novel functional ingredients for improving the heat stability of β-Lg. Furthermore, the effectiveness of DHLA in protecting β-Lg during heat treatment was compared with that of the more common –SH blocking reagents, N-ethylmaleimide (NEM) and dithio(bis)-p-nitrobenzoate (DTNB).
Materials and methods
Materials
β-Lg AB, DL-α-lipoic acid (LA), reduced DL-α-lipoic acid (DHLA), DTNB and NEM were obtained from Sigma (NSW, Australia). The electrophoresis chemicals were obtained from Bio-Rad (NSW, Australia). All other chemicals used were of analytical grade. Milli-Q water was used for all solutions (Millipore Pty Ltd. NSW, Australia).
Preparation of β-Lg solutions, with and without LA or DHLA
A β-Lg solution, with a concentration close to that in normal milk, was prepared by adding β-Lg AB to Milli-Q water to a concentration of 5 mg/ml and vortexed gently at ∼200 rpm at approximately 25 °C until fully dissolved.
LA or DHLA solutions were prepared in 50 mm phosphate buffer at pH 6·8 and added to the β-Lg solution to achieve molar ratios of LA or DHLA to β-Lg monomer of 0·5 : 1, 1 : 1, 1·5 : 1, 2 : 1 and 10 : 1. The calculations were based on the monomer of β-Lg having a molecular weight of 18·4 kDa (Wada et al. Reference Wada, Fujita and Kitabatake2006). The optimum ratio of LA or DHLA to β-Lg monomer in reducing aggregate formation of β-Lg was subsequently used in this study. An aliquot of 50 mm phosphate buffer, pH 6·8, of the same volume as that of the solutions of LA and DHLA used, was added to the β-Lg solution that did not contain LA/DHLA, to act as a control. For comparison with DTNB and NEM, DTNB and NEM solutions were prepared in a similar manner to the lipoic acid solutions. DTNB/NEM solutions were subsequently added to the β-Lg solutions at the optimum molar ratio of LA or DHLA to β-Lg monomer for reducing formation of protein aggregates.
Heat treatments
The samples (β-Lg+LA, β-Lg+DHLA, β-Lg+DTNB, β-Lg+NEM and β-Lg only) in closed 1·5 ml tubes covered with aluminium foil were heated in a thermostatically controlled waterbath (Ratek Instruments Pty. Ltd. Victoria, Australia) at different temperatures for different times. In a preliminary study, the optimum ratio of LA/DHLA to β-Lg for preventing aggregation of heated β-Lg, samples heated at 70 or 85 °C for 10 or 30 min was determined. Thereafter, samples were heated at 70, 75, 80 or 85 °C for 10, 20 or 30 min. For the comparison study of DHLA with DTNB and NEM, samples were heated at 85 °C for 10 or 20 min. After heating, samples were immediately cooled in ice water for 1 h to stop any further denaturation/aggregation of the protein. Treated samples were left to warm to room temperature (∼22 °C) prior to chromatographic and electrophoretic analyses. The control samples were a mixture of the reagent plus β-Lg but without heat treatment.
Size exclusion-HPLC (SE-HPLC)
Samples were injected into a BioSep SEC- S3000 (300×7·8 mm i.d., Phenomenex Australia Pty Ltd, NSW, Australia) column attached to a stainless steel guard column (Analytical 75×7·8 mm, Phenomenex Australia Pty Ltd, NSW, Australia) and Security Guard Cartridge (4×3·0 mm, Phenomenex Australia Pty Ltd, NSW, Australia) and run isocratically with a mobile phase consisting of 50 mm phosphate buffer, pH 6·8, containing 0·05% of sodium azide. The filtered buffer was delivered at a flow rate of 0·8 ml/min with a total run time of 20 min. The UV absorption of the eluate was measured at 280 nm using a UV–VIS detector (SPD-10 AV Shimadzu UV–VIS Detector, Kyoto, Japan).
A calibration curve for molecular weight (Mw) measurement was prepared using a method based on Irvine (Reference Irvine1997, Reference Irvine2003). A plot of peak area against concentration of the β-Lg standard (ranging from 0·5 to 5 mg/ml) was constructed and used as a standard curve to estimate the concentration of unknown β-Lg species. The concentrations and molecular weights of native β-Lg and its heat-induced aggregates were calculated from the two standard curves based on the retention times and peak areas from SE-HPLC analysis of the protein standards.
Polyacrylamide gel electrophoresis (PAGE)
Native-PAGE was carried out without the addition of a reducing agent (β-mercaptoethanol), dissociating agent (sodium dodecyl sulphate, SDS) or use of heat, according to the method of Ornstein (Reference Ornstein1964) and Davis (Reference Davis1964) but with slight modifications in the staining method. Non-reducing (NR) SDS–PAGE and reducing (R) SDS–PAGE analyses were based on the methods of Laemmli (Reference Laemmli1970) but with slight modifications as described by Oldfield et al. (Reference Oldfield, Singh, Taylor and Pearce2000). NR-SDS–PAGE was run without the addition of β-mercaptoethanol and heated at 45 °C for 5 min, whereas, R-SDS–PAGE was run with the addition of β-mercaptoethanol and heated at 95 °C for 5 min. All gels were run on a Mini-Protean III dual cell system (Bio-Rad Laboratories, Hercules, CA, USA). Mini-Protean TGX gels (12% acrylamide, 10 and 15 wells) obtained from Bio-Rad (CA, USA) were used in the analyses. The pH of the electrode buffer and sample buffer were adjusted to pH 8·3 and 6·8, respectively. The corresponding sub-samples, prepared by diluting with the appropriate sample buffer, were loaded accurately (5 μl/well) into the sample loading well using a micro pipette. Electrophoresis was carried out at 200 V. The bands were stained with 0·1% (w/v) Coomassie Brilliant Blue G250 in 34% methanol for 24 h and destained in 10% glacial acetic acid. The destaining solution was changed every 4 h to obtain a light background on the gel. Destained gels were scanned on a computing densitometer (GS-8000 Calibrated Densitometer, Bio-Rad Laboratories, NSW, Australia) and the integrated intensities of the β-Lg bands were calculated by the software program, ImageQuant (Molecular Dynamics).
Statistical analysis
Data collected from triplicate experiments were used to calculate the means and standard errors in the concentrations of proteins obtained from SE-HPLC and PAGE using Minitab 16 software (2010 Minitab Inc.). ANOVA was performed to determine the significance of differences between the samples at P=0·05.
Results and discussion
Preliminary study of effects of LA and DHLA
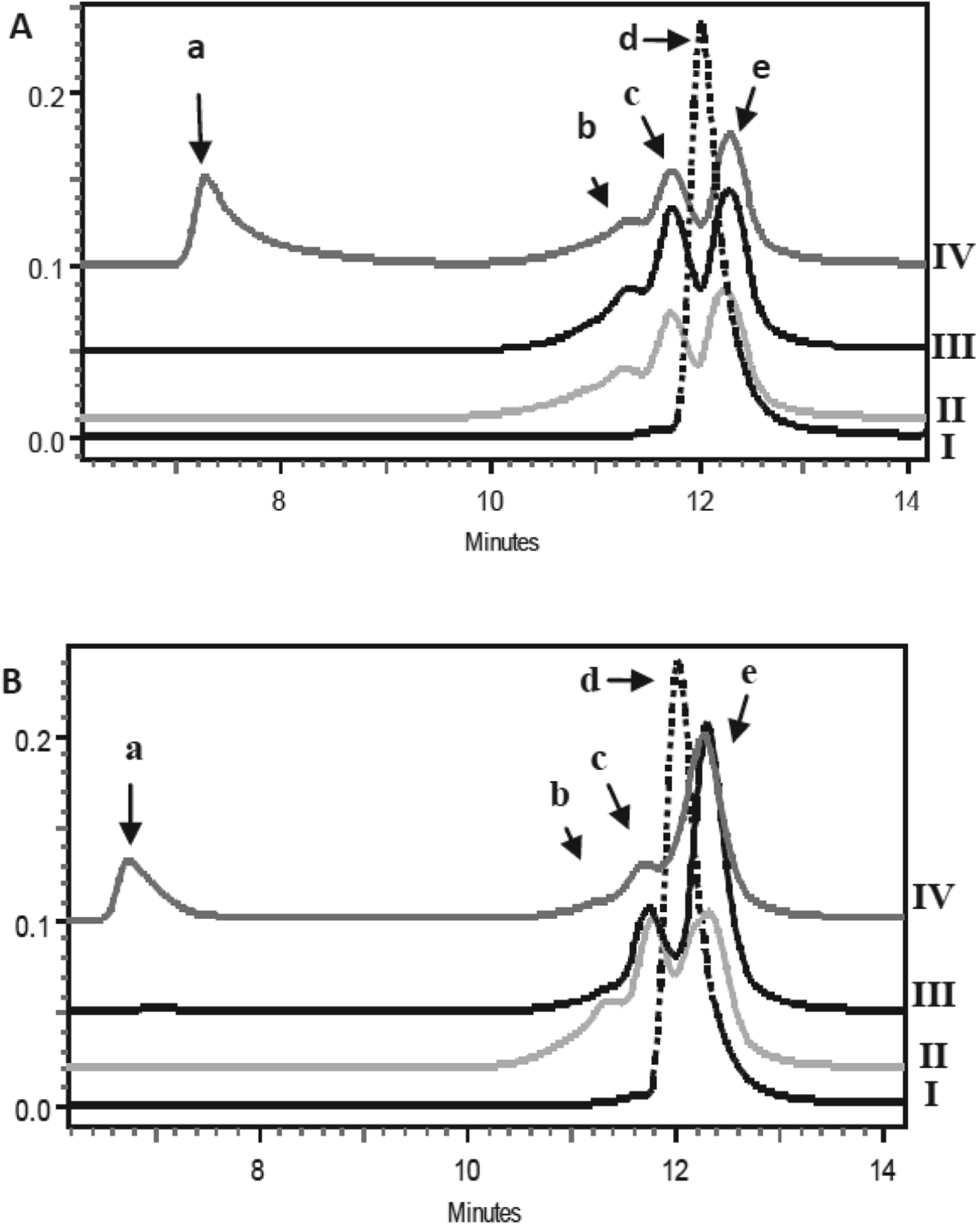
Figure 1 shows typical SE-HPLC chromatograms of the effects of LA and DHLA when heated with β-Lg at 85 °C at molar ratios of LA/DHLA to monomeric β-Lg of 1 : 1 and 10 : 1, together with unheated and heated β-Lg controls. In the unheated control, β-Lg showed a single peak which corresponds to the native dimer (Fig. 1, peak d) with a molecular weight (Mw) of ∼35 kDa. While heated β-Lg showed no dimer peak but two major peaks corresponding to β-Lg monomer (Mw ∼24 kDa, Fig. 1, peak e) and β-Lg trimers (Mw=49 kDa, Fig. 1, peak c). In addition, a minor peak attributable to a tetramer (86 kDa, Fig. 1, peak b) was present. Samples heated with LA showed very similar chromatograms (Fig. 1a), except that for the sample with the higher ratio (10 : 1) of LA to β-Lg, a peak corresponding to a much higher-molecular-weight aggregate appeared (peak a). Similar results were obtained when the samples were heated at 70 °C (results not shown). Therefore LA was not able to reduce the formation of aggregates at the LA to β-Lg ratios and temperatures studied. Based on these results, the use of LA was not further investigated.

Fig. 1. SE-HPLC chromatograms of β-Lg heated without and with LA (A) and DHLA (B) at 85 °C for 30 min. Chromatogram I, unheated β-Lg; II, β-Lg heated alone; III and IV, heated with β-Lg at molar ratios of LA/DHLA:β-Lg monomer of 1 : 1 (III) and 10 : 1 (IV). Peak a, refers to high-molecular-weight aggregates of β-Lg; peaks b, c, d and e refer to tetramers, trimers and dimers and monomers, respectively.
The SE-HPLC chromatograms of heated DHLA/β-Lg samples (Fig. 1b) were different from those with LA and showed considerably more monomer and less trimers and tetramers. These results suggest that DHLA, with two vicinal sulphydryl groups, is more reactive towards the free –SH group of the unfolded β-Lg than is LA which has a disulphide bond in a dithiolane ring structure. DHLA is able to partially trap the β-Lg monomer and reduce its interaction with other β-Lg and formation of aggregates.
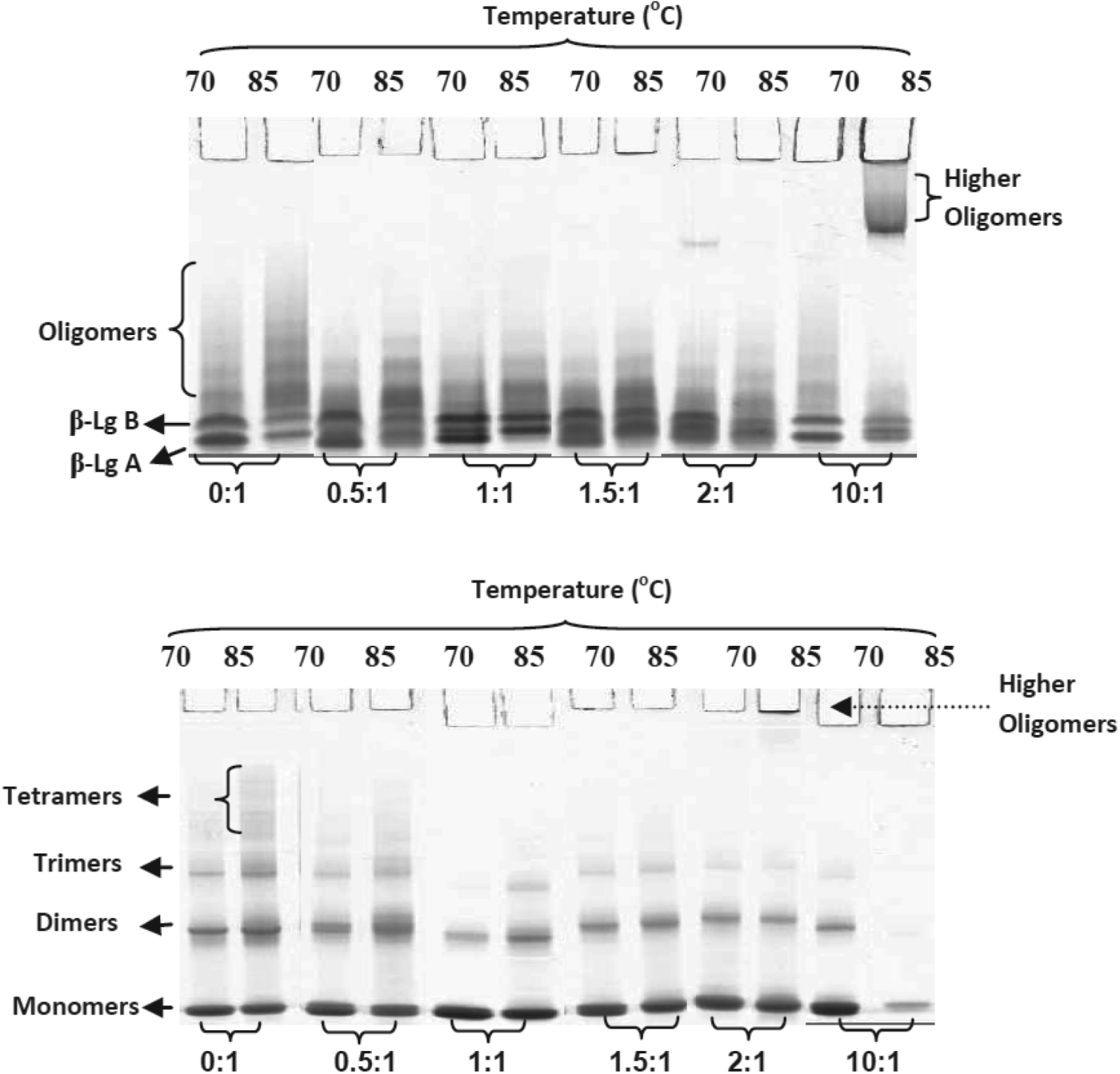
Native-PAGE and NR-SDS–PAGE gels of heated β-Lg solutions containing DHLA with DHLA:β-Lg ratios of 0 : 1 to 10 : 1 are shown in Fig. 2. In agreement with the SE-HPLC results, the samples with DHLA contained more monomeric β-Lg and less oligomeric β-Lg than the heated samples without DHLA, except for the samples with DHLA:β-Lg monomer ratios of 2 : 1 and 10 : 1. The formation of trimers and tetramers was reduced in the presence of DHLA up to a DHLA:β-Lg monomer molar ratio of 1·5 : 1 (Fig. 3). A molar ratio of 1 : 1 gave the least aggregates following heating at 70 or 85 °C for 10 or 30 min; this ratio was used in all further investigations of the potential protective effect of DHLA on heated β-Lg.

Fig. 2. Native-PAGE (top gel) and NR-SDS–PAGE (lower gel) results of β-Lg heated at 70 or 85 °C for 30 min with DHLA in ratios of DHLA:β-Lg monomer of 0 : 1 to 10 : 1.

Fig. 3. Comparison of relative concentrations of aggregates (trimers + tetramers) from Native-PAGE (■ ◆) and NR-SDS–PAGE (□◊) of DHLA/β-Lg mixtures heated at 70 and 85 °C for 30 min. The measurements were based on the relative intensities of the bands over the total intensity of each lane.
The effect of DHLA on aggregation of β-Lg: Size exclusion-HPLC
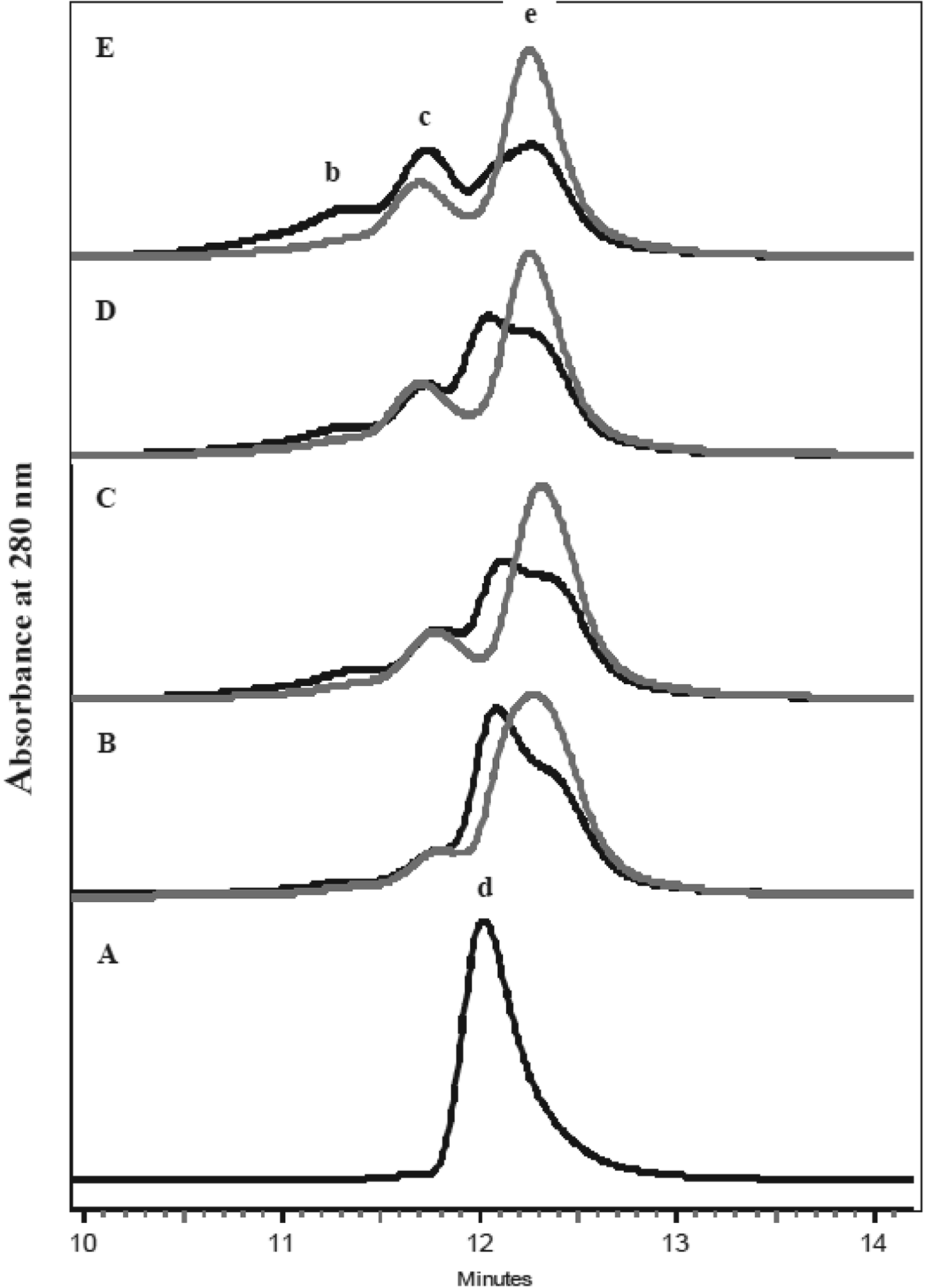
Typical SE-HPLC chromatograms in Fig. 4 show the effect of temperature on β-Lg at a DHLA:β-Lg ratio of 1 : 1, together with an unheated control. The concentrations of β-Lg monomers, dimers, trimers and tetramers were determined from the chromatogram using the standard curve constructed as described above (data not shown). The concentrations of monomers were significantly (P<0·05) higher in the presence of DHLA, with the highest concentration resulting from heating at 70 °C, while the concentration of trimers and tetramers was reduced; trimers and tetramers increased with temperature of heating (Fig. 4). In the presence of DHLA, dimers were dissociated into monomers (Fig. 4b–e), presumably with covalent binding of DHLA to the monomeric β-Lg to form a ‘modified monomer’.

Fig. 4. SE-HPLC profiles β-Lg heated without and with DHLA at various temperatures for 30 min. A=unheated β-Lg, B=70 °C, C=75 °C, D=80 °C, and E=85 °C. Black lines=without DHLA; grey lines=with DHLA. b=tetramers, c=trimers, d=dimers and e=monomers.
The effect of DHLA on aggregation of β-Lg: Electrophoresis
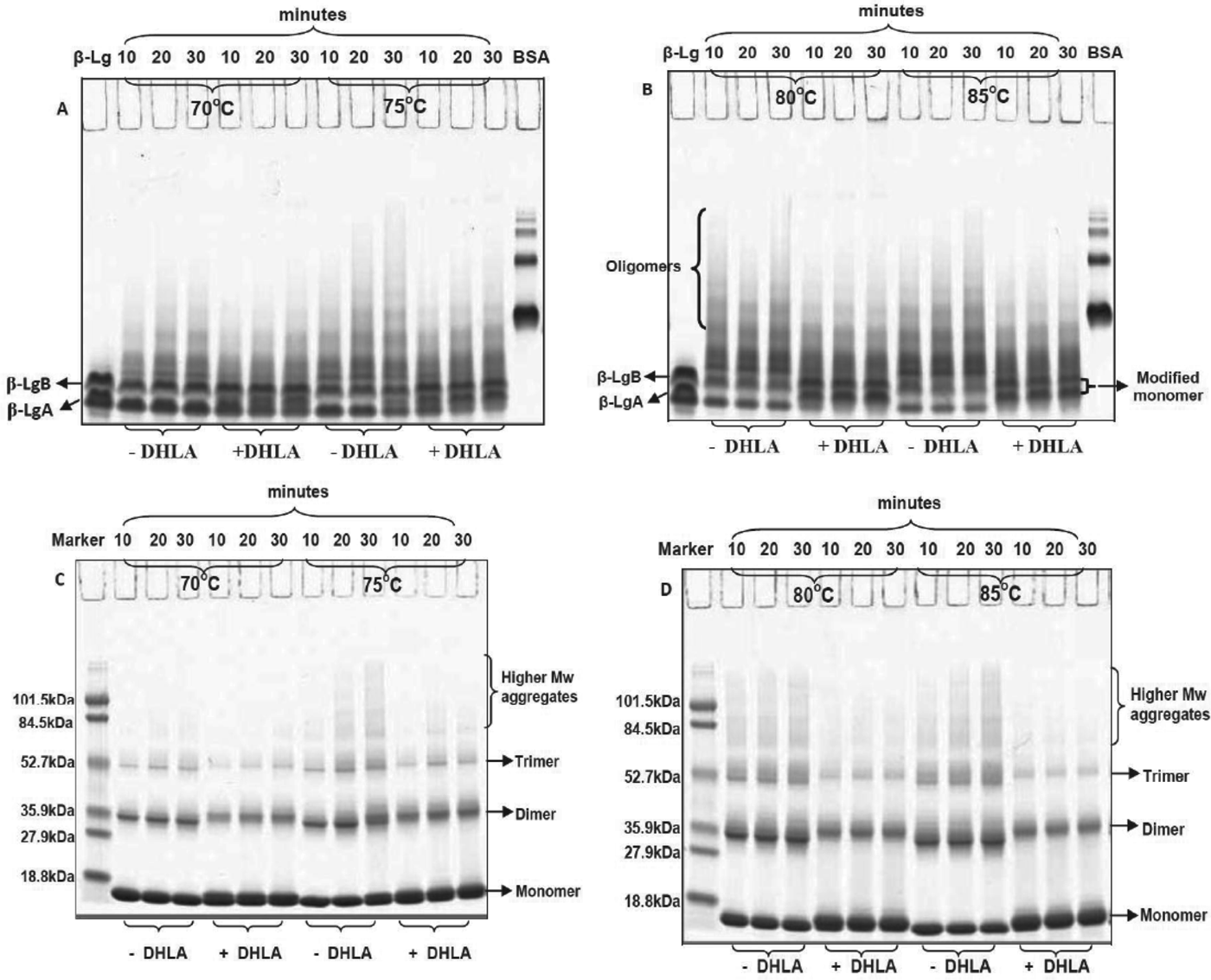
The unheated β-Lg appeared as two bands in native PAGE containing native β-Lg A and B (Fig. 5a, b, lane 1), which correspond to the molecular weight of a β-Lg monomer (∼18 kDa). The appearance of monomer rather than dimer bands in native PAGE is due to the alkaline pH (pH=8·3) of the electrophoresis buffer which results in dissociation of the native dimers into monomers (Hambling et al. Reference Hambling, McAlpine, Sawyer and Fox1992). At that pH, β-Lg A carries a larger net negative charge than β-Lg B, hence, β-Lg A migrates faster than β-Lg B (Manderson et al. Reference Manderson, Hardman and Creamer1998). On the other hand, in NR-SDS–PAGE, unheated β-Lg was resolved as a single monomer band because SDS dissociates the non-covalently linked dimers into monomers with similar mobility. These monomers are referred to as SDS-monomers (Fig. 5c, d).

Fig. 5. Native-PAGE (A, B) and NR-SDS–PAGE (C, D) gels of β-Lg samples heated at 70 or 75 °C (A, C) and 80 or 85 °C (B, D) for various times.
On native PAGE, bands for the monomers appeared at different positions for heated β-Lg samples with and without DHLA. The differences were most marked for the 80 and 85 °C samples (Fig. 5b) where the two monomer bands appear at positions of lower mobility than those of the native monomers. These bands appear to be of monomers derivatised by DHLA at the free −SH group (i.e., ‘modified monomers’). In some samples, e.g., 70 °C for 30 min and 75 °C for 10 min, a transition between the two patterns is evident indicating a mixture of native and modified monomers. These modified monomers are assumed to be disulphide-linked, an assumption supported by the fact that the monomer bands in the corresponding R-SDS–PAGE gels of all samples had the same mobility (results not shown).
The Native-PAGE gels of samples with and without DHLA showed diffuse bands corresponding to aggregates of β-Lg. These bands represent aggregates formed through both covalent and non-covalent linkages. However, the bands in the NR-SDS–PAGE gels (Fig. 5c, d) were much simplified and represent only aggregates covalently-linked via disulphide bonds.
The appearance of bands corresponding to dimers in the NR-SDS–PAGE gels (Fig. 5c, d) of heated DHLA/β-Lg is interesting given that a peak corresponding to a dimer was not observed in SE-HPLC chromatograms (Fig. 4). There are two possible explanations for this. The first is that peaks of dimers may have been hidden by the monomer and trimer peaks and the second is that some dimers may have interacted hydrophobically with monomers to form trimers and tetramers, thus, dimers were not seen in the SE-HPLC but were seen in NR-SDS–PAGE when the hydrophobic interaction was broken. Such a phenomenon has been previously reported (Manderson et al. Reference Manderson, Hardman and Creamer1998). The fact that dimer bands were seen in NR-SDS PAGE but not in R-SDS–PAGE (gel not shown) indicates that it was a disulphide-bonded dimer. The lower electrophoretic mobility of the major dimer band in the heated DHLA/β-Lg samples compared to β-Lg heated alone (Fig. 5c, d) suggests it was a non-native disulphide-bonded dimer, formed through sulphydryl–disulphide interchange reaction of a native monomer with a modified monomer containing DHLA. The resulting dimer would be expected to have lower mobility on NR-SDS–PAGE since the corresponding modified monomer has lower mobility than the native monomer.
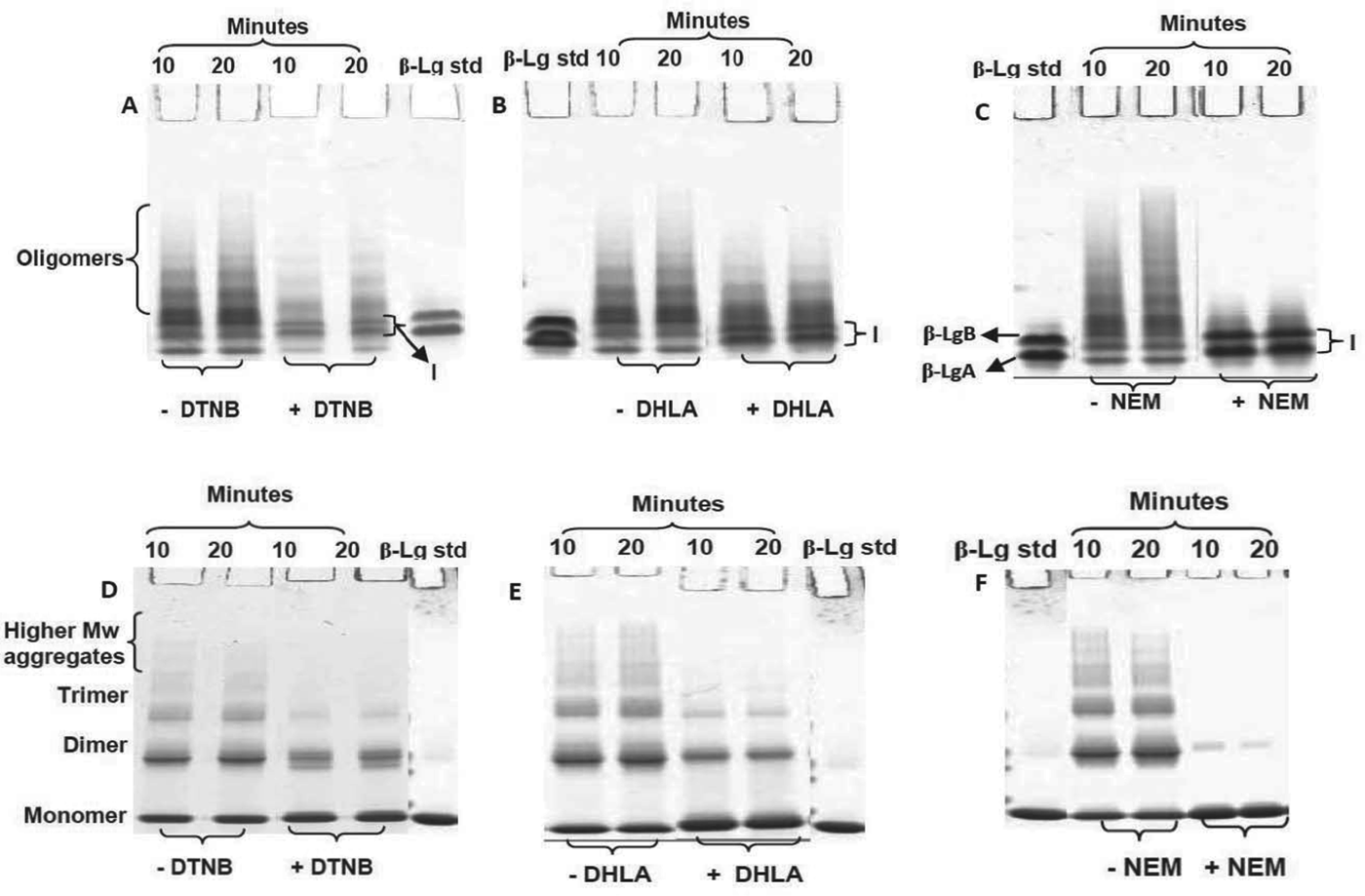
The –SH blocking activity of DHLA compared with DTNB and NEM
PAGE analysis of heated β-Lg with DHLA, DTNB or NEM in a molar ratio of 1 : 1 showed the ability of all reagents to enhance the formation of monomeric β-Lg and reduce the formation of aggregates (Fig. 6a–f). At the highest temperature used in this study, 85 °C, the abilities of these reagents to reduce the formation of higher-molecular-weight aggregates were in the order NEM>DHLA>DTNB. The presence of prominent bands for modified monomers in the Native PAGE (Fig. 6a, c, region I) and NR-SDS–PAGE gels (Fig. 6d, f, shown as thick monomer bands) suggest that DHLA reduces aggregate formation during heating of β-Lg via a similar mechanism to that of DTNB and NEM.

Fig. 6. Native-PAGE (A–C) and NR-SDS–PAGE (D–F) gels of β-Lg without and with DTNB, NEM and DHLA heated at 85 °C for 10 or 20 min. Bands marked I are of modified monomers.
In conclusion, this study found that the reduced form of lipoic acid, DHLA, but not the oxidised form, LA, was able to reduce the formation of β-Lg aggregates during heating, largely through reaction with the exposed sulphydryl group of heated β-Lg and formation of covalently-linked modified monomers. Some β-Lg aggregates still formed in the presence of DHLA and, from Native-PAGE, NR-SDS–PAGE and R-SDS–PAGE, these were shown to be formed through both covalent (disulphide) and hydrophobic linkages. A comparison study with the commonly used –SH blocking reagents, DTNB and NEM, showed that they behave in a similar manner to DHLA in limiting the formation of aggregates by trapping the intermediate reactive monomer of β-Lg and forming modified monomers. The results indicate that addition of DHLA may be useful in reducing aggregation of β-Lg during commercial heat processing; however, its efficacy in a more complex milk system requires further investigation.
The authors gratefully acknowledge Dr Lesleigh Force for her assistance with the HPLC analyses and Dairy Innovation Australia Limited for financially supporting this research.