Refrigeration has an important impact on the microbiological quality of raw milk, by decreasing the presence of mesophilic bacteria, the main cause of acidification. However, the refrigeration temperature at the milk farms often ranges between 5 and 10°C, which allow the development of psychrotrophic microorganisms (Cousin, Reference Cousin1982; Eneroth et al. Reference Eneroth, Christiansson, Brendehaug and Molin1998). Although most psychrotrophic bacteria are destroyed by the conventional thermal treatments employed in the dairy industry, such treatments have minor effects on their enzymes (Tondo et al. Reference Tondo, Lakus, Oliveira and Brandelli2004). The intracellular enzymes and those associated with the cell wall can be released to the milk when bacterial lysis takes place after the thermal treatment and, in this way, together with the extracellular enzymes, hydrolyze milk components (Chen et al. Reference Chen, Daniel and Coolbear2003). In this context, the multiplication of psychrotrophic microbiota producing thermostable enzymes is a major factor influencing the quality of dairy products produced with raw milk stored at 7°C or less for prolonged periods (Søraugh & Stepaniak, Reference Søraugh and Stepaniak1997). Milk gelation and deterioration of sensory characteristics are harmful effects caused by psychrotrophic bacteria (Shah, Reference Shah1994; Datta & Deeth, Reference Datta and Deeth2003).
In addition, the elevated growth of psychrotrophic microorganisms may generate an adequate support for adherence of bacteria resulting in biofilms (Costernon et al. Reference Costernon, Stewart and Greenberg1999; Kusumaningrum et al. Reference Kusumaningrum, Riboldi, Hazeleger and Beumer2003). Gram-negative bacteria like Pseudomonas are largely recognized by their capability to produce large amounts of exopolysaccharides, which contribute to adhesion and biofilm growth (Drenkard & Ausubel, Reference Drenkard and Ausubel2002). The cell physical interaction with a surface through extracellular materials like polysaccharides or proteins, produced by the bacterium, withstands biofilm formation (Carpentier, Reference Carpentier1997). Thus, microorganisms continue to grow, generating a highly appropriate substratum for the adherence of other bacteria, including pathogens (Costernon et al. Reference Costernon, Stewart and Greenberg1999). This explains why surfaces of equipment and tools used in food production are focuses of bacterial contamination (Holah & Thorpe, Reference Holah and Thorpe1999). Besides, organisms in biofilms are more resistant to the action of chemical and physical agents (Costernon et al. Reference Costernon, Stewart and Greenberg1999; Sinde & Carballo, Reference Sinde and Carballo2000).
Psychrotrophic gram-negative bacteria have been related to deleterious effects on milk, but research has been mostly focused on Pseudomonas spp. (Enertoh et al. 1998; Martins et al. Reference Martins, Pinto, Rocha, Araújo and Vanetti2006). There is growing evidence that other genera may have similar relevance by producing strong proteolytic activity (Nörnberg et al. Reference Nörnberg, Friedrich, Weiss, Tondo and Brandelli2010). The aim of this work was to evaluate the proteolytic activity of a Burkholderia cepacia strain isolated from refrigerated raw milk, and its capability of milk-coagulation and adhesion to stainless steel surfaces.
Materials and Methods
Microorganism
A psychrotrophic and proteolytic Bur. cepacia strain isolated from refrigerated raw milk was used (Nörnberg et al. Reference Nörnberg, Friedrich, Weiss, Tondo and Brandelli2010). The strain was maintained in BHI broth containing 20% (v/v) glycerol at −20°C. The bacterium was cultivated twice in TSB at 37°C and then plated in milk agar plates before experiments.
Bacterial growth
Bur. cepacia was cultured in sterile milk at 10°C for up to 72 h. Bacterial growth was monitored by serial dilutions to 10−8 in sterile NaCl solution, and plating in triplicate onto plate count agar (Difco, Detroit, USA). Plates were incubated for 24 h at 37°C and counts performed among 30–100 colonies. Samples were monitored for proteolytic activity as described elsewhere (Tondo et al. Reference Tondo, Lakus, Oliveira and Brandelli2004).
Production of protease
A colony from milk agar plate was inoculated in mineral medium (0·5 g NaCl/l, 0·4 g K2HPO4/l, 0·3 g K2HPO4/l), containing 10 g casein/l, pH adjusted to 7·0. The production of protease was in 250 ml flasks containing 50 ml of the medium by incubation for 72 h at 37°C in an orbital shaker. The culture was centrifuged at 10,000 g for 10 min and the supernatant was used as crude enzyme.
Proteolytic activity
Proteolytic activity was assayed using azocasein (Sigma, St. Louis, MO, USA) as substrate (Thys et al. Reference Thys, Lucas, Riffel, Heeb and Brandelli2004). The enzyme solution (100 μl) was mixed with 100 μl 0·1 m-sodium phosphate buffer pH 7. Then, 100 μl 10 mg azocasein/ml was added. The mixture was incubated for 60 min at 37°C and the reaction was stopped by adding 500 μl trichloroacetic acid (TCA) 30% (w/v). After centrifugation at 10,000 g for 5 min, 800 μl supernatant were mixed with 200 μl 1·8 m-NaOH. The absorbance at 420 nm was measured in a Shimadzu UV1240 spectrophotometer (Shimadzu, Tokyo, Japan). One enzyme unit was defined as the amount that caused an increase of 0·01 in absorbance at 420 nm in the assay conditions (Thys et al. Reference Thys, Lucas, Riffel, Heeb and Brandelli2004).
Characterization of proteolytic activity
The effects of temperature and pH on the proteolytic activity were determined. Thermal stability was evaluated by pre-incubation of the enzyme for up to 25 min at 40, 45 and 50 °C. The enzyme was also incubated at either 37, 76, 100 °C for 30 s or 142°C for 10 s, and then residual activity was measured as described above. The assay for optimum pH was developed using 0·1 mol/l of either sodium citrate buffer (pH 4 and 5), sodium phosphate buffer (pH 6, 7, and 8), or sodium carbonate buffer (pH 9 and 10).
Milk coagulation
Bur. cepacia was grown in TSB by 24 h a 37°C. The culture was centrifuged at 10,000 g for 5 min and the supernatant was filtered through a 0·22 μm membrane. Different amounts of this crude enzyme preparation (80 U/ml protease) were added to either whole or skim UHT milk. These samples were incubated at room temperature for up to 7 days. Milk coagulation was visually inspected at regular intervals of 24 h. Aliquots of thermally inactivated enzyme (100°C/10 min) served as controls.
Aliquots of the samples were adjusted to pH 4·6 with 2 m-HCl, followed by centrifugation at 11,600 g for 20 min to precipitate caseins and denatured soluble whey proteins. The concentration of amino acids in the resulting supernatants was measured by the ninhydrin method (Moore & Stein, Reference Moore and Stein1954).
Adherence to stainless steel
Stainless steel AISI 316 (Metalbras, Porto Alegre, Brazil) coupons of 2×2 cm and 0·1 cm thick were used. Before the adhesion tests, coupons were degreased with a neutral detergent (3%, v/v) for 1 h, rinsed with 70% (v/v) ethanol, and then washed with MilliQ water. After that, the coupons were dried at 60°C for 2 h and autoclaved at 121°C, for 15 min in sealed tubes (Rossoni & Gaylarde, Reference Rossoni and Gaylarde2000).
The coupons were immersed in 10 ml TSB containing 8 log CFU/ml Bur. cepacia. Three coupons of stainless steel were immersed in the cultures for 15, 30 and 60 min, without shaking, at room temperature (Kusumaningrum et al. Reference Kusumaningrum, Riboldi, Hazeleger and Beumer2003). After that, the coupons were washed with PBS (phosphate buffer saline; pH 7·2) to remove the poorly adhered cells. The stainless steel coupons were immersed in 10 ml PBS before sonication process using an ultrasound bath (Unique USC 700) with a frequency of 40 kHz. Each coupon was sonicated for 2 periods of 10 min, aiming the release of adhered cells from coupons surfaces.
Decimal dilutions of PBS containing each sonicated coupon were made, and 20 μl of each dilution were plated in TSA. The plates were incubated for 24 h at 37°C and CFU/cm2 were determined (Hood & Zottola, Reference Hood and Zottola1997). All the counts were made in triplicate and each experiment was repeated twice.
Biofilm growth assay
The capability of Bur. cepacia to form biofilms was determined using the microplate assay involving crystal violet staining (Burton et al. Reference Burton, Yakandwala, Lo Vetri and Madhyastha2007). A 20 μl inoculum of Bur. cepacia (105 CFU/mL) was added to 280 μl TSB in 96-well polystyrene microplates (Nunc, Rockville, USA). Microplates were incubated at 4, 10, or 25°C for up to 96 h. After incubation, the medium was gently removed and microplates were washed three times with distilled water. Microplates were stained for 15 min with a 4 mg/ml crystal violet solution, washed three times with distilled water, and air-dried for 60 min. The stain was then dissolved in 400 μl 95% (v/v) ethanol and the absorbance at 570 nm was measured (O'Toole & Kolter, Reference O'Toole and Kolter1998).
Results and Discussion
Proteolytic activity of Bur. cepacia
Bur. cepacia was able to grow and produce proteolytic activity during cultivation at 10°C in milk (Fig. 1). Maximum proteolytic activity was observed after 24 h, but it was also detected from 8 h. This indicates that this bacterium can secrete proteolytic enzymes during incubation at refrigeration temperatures. After 24 h growth, protein precipitation was observed indicating that coagulation occurred during growth of Bur. cepacia in milk.

Fig. 1. Time course of protease production during growth of Bur. cepacia in UHT milk. The cultures were developed in 100 ml flasks containing 20 ml sterile milk at 10°C. Bacterial counts (CFU/ml; squares) and protease activity (circles) were determined at the indicated times.
Further studies on the proteolytic system of Bur. cepacia were developed after growth on casein broth. The proteolytic activity increased for up to 72 h, when a maximum activity of 85·9 U/ml was reached. Notable activity was also detected at 24 h (48 U/ml) and 48 h (72·4 U/ml).
The optimum pH for proteolytic activity of Bur. cepacia was investigated in the range of pH 4 to 10. Maximum activity was observed between pH 6 and 7, and a reduced level of activity was observed under more acidic and alkaline pH values (Fig. 2A).

Fig. 2. Effect of pH (A) and temperature (B) on proteolytic activity of Bur. cepacia.
The effect of temperature on the activity of Bur. cepacia protease was evaluated. The maximum proteolytic activity was observed within the range 35–45°C, with accentuated decrease outside this range (Fig. 2B). The thermal stability was evaluated within 25 min at the temperatures of 40, 45, and 50°C. When the enzyme was incubated at 40°C, a high level of activity was observed within the established time maintaining at least 80% of the initial activity (Fig. 3A). By regression analysis, the residual activity correlates with time through a polynomial equation (R 2=0·994). At the temperature of 45°C, the enzyme showed a linear reduction (R 2=0·913) of its activity during the incubation time (Fig. 3A). At 50°C, there was a rapid reduction of the activity within 5 min, with about 52% of the initial activity remaining (Fig. 3A). Continued incubation at 50°C caused little additional inactivation and at least 40% residual activity was observed. A polynomial relationship was observed with a R 2=0·981. In addition, the enzyme maintained about 80% its initial activity after 30 s incubation at 76°C, and 40% its initial activity at 142°C for 10 s (Fig. 3B). These results suggest that the enzyme is relatively heat resistant and is not completely inactivated by heat treatments conventionally applied to milk, in particular to pasteurization conditions.

Fig. 3. (A) Thermal stability of Bur. cepacia protease at 40°C (circles), 45°C (squares) and 50°C (triangles). (B) The enzyme was incubated at either 37, 76, 100°C for 30 s or 142°C for 10 s and then residual activity was measured.
Milk coagulation
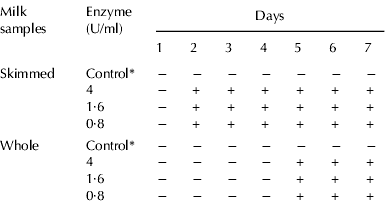
The effect of Bur. cepacia protease on milk coagulation was evaluated throughout 7 consecutive days, by visual inspection of skimmed and whole UHT milk samples artificially spoiled with the enzyme. The results are shown in Table 1. The enzyme added to the skimmed milk, independently of the amount (0·8, 1·6, or 4 U/ml), promoted milk coagulation from day 2, while the milk samples with inactivated enzyme (control group) did not coagulated. Concerning the whole milk samples, coagulation was also observed, but it started from day 5, while control samples did not coagulate until the day 7 of the evaluation. The fact that proteolysis occurs with higher velocity in skimmed milk samples than in whole UHT milk samples was also observed by Garcia-Risco et al. (Reference Garcia-Risco, Ramos and López-Fandiño1999), indicating that the rate of proteolysis is influenced by the fat level present in milk, in accordance to research by Lopez-Fandiño et al. (Reference López-Fandiño, Olano, Corzo and Ramos1993). The concentration of free amino acids increased during incubation time, whereas the values were nearly constant for control incubated samples (Fig. 4). These results indicate that the enzyme effectively hydrolyzed milk proteins and an increased amount of free amino acids and soluble peptides are present in the milk serum. This suggests that the enzyme continues to hydrolyze proteins after coagulation.

Fig. 4. Concentration of amino acids of whole (circles) and skimmed milk (squares) incubated with 4 U/ml protease (black symbols) or heat-inactivated protease (white symbols).
Table 1. Coagulation of skimmed and whole UHT milk samples by Burkholderia cepacia protease

* Control, 4 U/ml enzyme inactivated at 100°C for 10 min
Gelation is one of the main problems that affect UHT milk quality. The UHT process promotes the increase of milk viscosity, but not its coagulation, since heating time is short. During storage, milk viscosity increases until there is gel formation (gelatinization) showing that the product is no longer suitable for consumption. The mechanisms involved in the gelatinization phenomenon comprise basically alteration of milk proteins, association and dissociation of calcium ions, formation of polymers derived from Maillard reaction, formation and dissociation of κ-casein complex with whey proteins, and the participation of innate milk enzymes (plasmin) and proteases of psychrotrophic bacteria (Chen et al. Reference Chen, Daniel and Coolbear2003). The gelatinization phenomenon is induced, initially, by the action of thermal-resistant enzymes (proteases) present, naturally, in the milk or derived from bacteria, mainly those of the psychrotrophic group (Tondo et al. Reference Tondo, Lakus, Oliveira and Brandelli2004). These enzymes present the ability of degrading caseins and promote the aggregation of micelles. When the psychrotrophic bacteria counts reaches a level about 106 CFU/l, protease production by such microorganisms is able to degrade considerable quantities of casein. According to Shah (Reference Shah1994), UHT milk samples, stored at 30°C, showing protease levels higher than one ng/ml can present a bitter flavour and undergone gelatinization. Degrading action of proteases from psychrotrophic microorganisms is distinct among milk protein fractions. κ-Casein is the most susceptible to the action of these enzymes, while whey proteins are relatively protease-resistant. Koka & Weimer (Reference Koka and Weimer2000), reported that the action of these psychrotrophic bacteria, mainly the proteases from the Pseudomonas spp., is similar to that presented by chymosin, an enzyme employed in enzymatic coagulation for cheese production. Faster appearance of the phenomenon of gelatinization in UHT milk is associated to the severity of heating process and to the levels of contamination due to the presence of psychrotrophic microorganisms.
Adherence capacity and biofilm formation
The capacity of Bur. cepacia 1A4 to adhere to stainless steel was investigated. The adherence levels on the stainless steel coupons were around 107 CFU/cm2, independent of the different immersion times evaluated (P<0·05). The values observed for 15, 30, and 60 min were 2·56×107, 4·0×107 and 1·52×107 CFU/cm2, respectively.
Microbial adherence to surfaces can occur directly through contact with contaminated materials or indirectly through bacterial particles present in the atmosphere, when the process of bacterium adhesion to the substratum is initialized (Kusumaningrum et al. Reference Kusumaningrum, Riboldi, Hazeleger and Beumer2003). Some studies report that bacterial adhesion consists of cell attraction by the surface, followed by adsorption and later by adherence by the bacterium cell (Katsikogianni & Missirlis, Reference Katsikogianni and Missirlis2004). Surfaces with more free energy, such as steel and glass, are less hydrophobic. These surfaces generally allow less bacterial adherence than hydrophobic surfaces, such as teflon, nylon, and the vast variety of polymers, including polyethylene (Sinde & Carballo, Reference Sinde and Carballo2000). However, elevated counts of Bur. cepacia were found on stainless steel substratum, indicating that this strain has a high capability to adhere and potentially form biofilms.
The biofilm formation by Bur. cepacia was evaluated by crystal violet staining of extracellular polysaccharides. This strain showed an increased biofilm formation on microplates, in a temperature-dependent manner (Fig. 5A). Although low absorbance values were observed at 4°C, it suggests that this bacterium could adhere and form biofilms even at refrigeration temperatures. This strain of Bur. cepacia showed capability similar to other recognized food spoilage bacteria like Pseudomonas putida, Citrobacter freundii and Proteus vulgaris, to form biofilms at low temperatures (Michaels et al. Reference Michaels, Ayers, Celis and Gangar2003). Biofilms are formed in dairy processing lines despite cleaning-in-place procedures, representing a source of post-pasteurization contamination (Austin & Bergeron, Reference Austin and Bergeron1995). The potential for biofilm formation increases the resistance to usual sanitization and cleaning procedures, which represent a risk to food industry.

Fig. 5. Crystal violet staining of biofilms formed by Bur. cepacia. Values were determined after 24 (black bars), 48 (gray bars), 72 (dashed bars) and 96 h (white bars) incubation at the indicated temperatures.
Conclusions
Burkholderia cepacia, isolated from refrigerated raw milk, produced proteolytic activity during growth at low temperature. The enzyme was relatively heat resistant and caused coagulation of skimmed and whole milk samples within 2 and 5 days, respectively. Bur. cepacia showed a high level of adherence to stainless steel and capability to form biofilms even at refrigeration temperatures, which may represent a hazard to food industry. This is a concern for milk industry because the potential contamination of equipment and tools, since stainless steel is widely used.
This work was supported by CNPq and CAPES (Brazil).