Lutein, one species of the carotenoid, is abundant in fruits and vegetables, such as bananas, potatoes and chrysanthemum (Mangels et al. Reference Mangels, Holden, Beecher, Forman and Lanza1993). A great number of biological functions have been claimed for lutein, including preventing oxidant-induced lipid peroxidation (Wang et al. Reference Wang, Jiao, Li, Liu, Shi and Ma2013), enhancing immunity (Moraes et al. Reference Moraes, Ribeiro, Santin and Klasing2016) and protecting the eye against light-induced oxidative damage (Bernstein et al. Reference Bernstein, Khachik, Carvalho, Muir, Zhao and Katz2001). Lutein has been widely used as a feed additive in livestock production. In chicken, lutein has been fed as an additive to improve laying performance and deepen the yolk color (Levy, Reference Levy2001). In dairy cows, our research demonstrated that dietary lutein could enhance milk production and improve health status (Xu et al. Reference Xu, Wang, Yang, Wang, Duan, Wang, Liu and Lao2014). However, the effects of dietary lutein on the mammary gland metabolism in dairy cows remain unknown.
Proteomics approach is an effective tool to profile various tissue proteins. In this study, we utilized a 2-DE/MS proteomics approach to reveal the impacts of lutein on protein profiles of the mammary gland in dairy cows and then try to understand the possible mechanisms of dietary lutein in regulating lactation of dairy cows.
Materials & methods
Experimental animals and protein sample preparation
Husbandry details of healthy lactating Holstein cows have been described previously (Xu et al. Reference Xu, Wang, Yang, Wang, Duan, Wang, Liu and Lao2014). Six dairy cows were chosen from groups given 0 or 200 g lutein preparation/d per head, according to our previous results that milk yield of dairy cows with 200 g lutein addition was the highest (Xu et al. Reference Xu, Wang, Yang, Wang, Duan, Wang, Liu and Lao2014). AbsoLUTEIN, was provided by Kemin Industries (Zhuhai, China). A portion of mammary gland was collected after the cows were killed, washed with ice-cold PBS and then snap-frozen in liquid nitrogen. The mammary gland samples were prepared as described before (Wu et al. Reference Wu, Zhou, Zhang, Zheng, Jiang, Shi, Wei and Wang2009). Protein concentrations were measured with Bradford protein assay kit (Sangon Biotech) and bovine serum albumin as standard according to the manufacturer's protocol. The supernatant was stored at −80 °C for later process.
Two-dimensional gel electrophoresis and image analysis
Two-dimensional gel electrophoresis was operated as formerly described (Wu et al. Reference Wu, Zhou, Zhang, Zheng, Jiang, Shi, Wei and Wang2009). Mammary gland protein samples (100 µg) were mixed with rehydration buffer containing 7 M urea, 2 M thiourea, 2% (w/v) CHAPS, 50 mM DTT, 0·2% pharmalyte 3/10, and 0·005% bromophenol blue and then loaded to 24 cm, pH 3–10 nonlinear immobilized pH gradient strips in the PROTEAN IEF cell apparatus (Bio-Rad). After rehydration at 30 V for 12 h, the isoelectric focusing program was carried out with the following parameters: 500 v 1 h; 1000 v, 1 h; 8000 v, 8 h; 500 v, 4 h. After equilibration, the strips were placed on 12·5% SDS-polyacrylamide gel for electrophoresis. The gels were stained with silver stained method and were visualized using scanner (UMax Powerlook 2110 XL, GE Amersham). Three cows were performed for biological replications and three gels were run in each group for technical replication. Spot detection, spot matching and quantitative intensity were assessed automatically with Imagemaster 2D software supplied by the manufacture. The quantity of each spot was normalized by total protein quantity and data were analyzed by Student's t-test. Differentially protein spots (P < 0·05) with a greater than 2·0-fold change were subjected to identification by MS.
In-gel digestion and protein identification
The steps were performed as previously described (Wu et al. Reference Wu, Zhou, Zhang, Zheng, Jiang, Shi, Wei and Wang2009). The interesting protein spots were excised and washed, then dried in a SpeedVac (Eppendorf, Hamburg, Germany) under vacuum. The gel was digested by trypsin (Promega, Madison, America) and the digestion was terminated by 1% v/v trifluoroacetic acid. The protein spots were lyophilized. Subsequently, the peptide mixtures were mixed with matrix solution CHCA (Sigma) 60% CAN and 0·1% TFA. The samples were analyzed using a 4800 Plus MALDI TOF/TOF™ analyzer. Parent mass peaks with 800–4000 Da of mass range and 50 of minimum S/N were selected for tandem TOF/TOF analysis. A protein match with a score greater than 70 were considered statistically significant and confidence interval ⩾95% were accepted.
Bioinformatic analysis
The pathway enrichment analysis was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway maps.
Statistical analysis
All experiments were performed with three replicates. Data were statistically analyzed by the SAS software (SAS Institute, Car, NC, USA) using ANOVA with Turkey's multiple range tests. P < 0·05 was considered as a significant difference.
Results and discussion
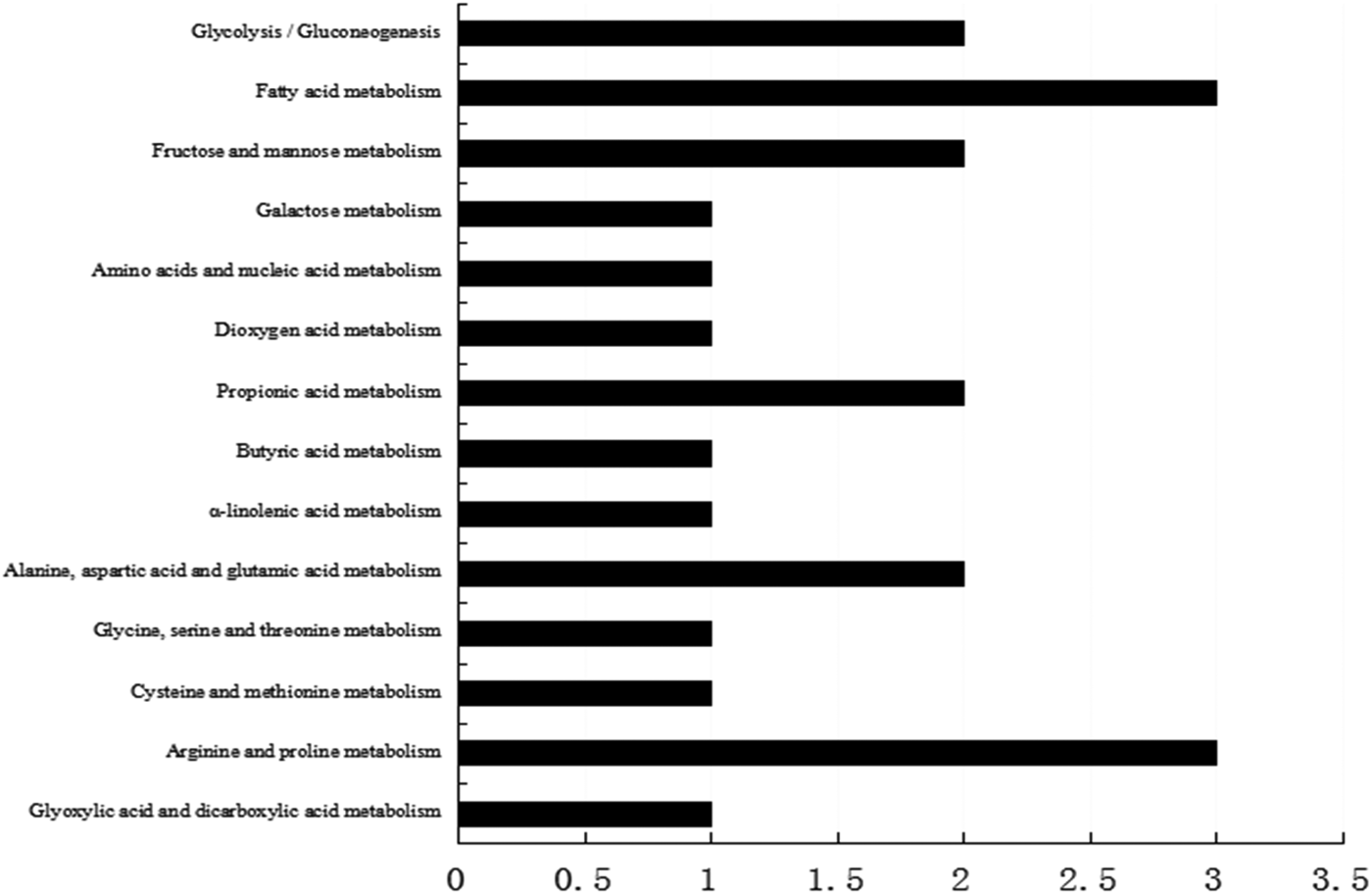
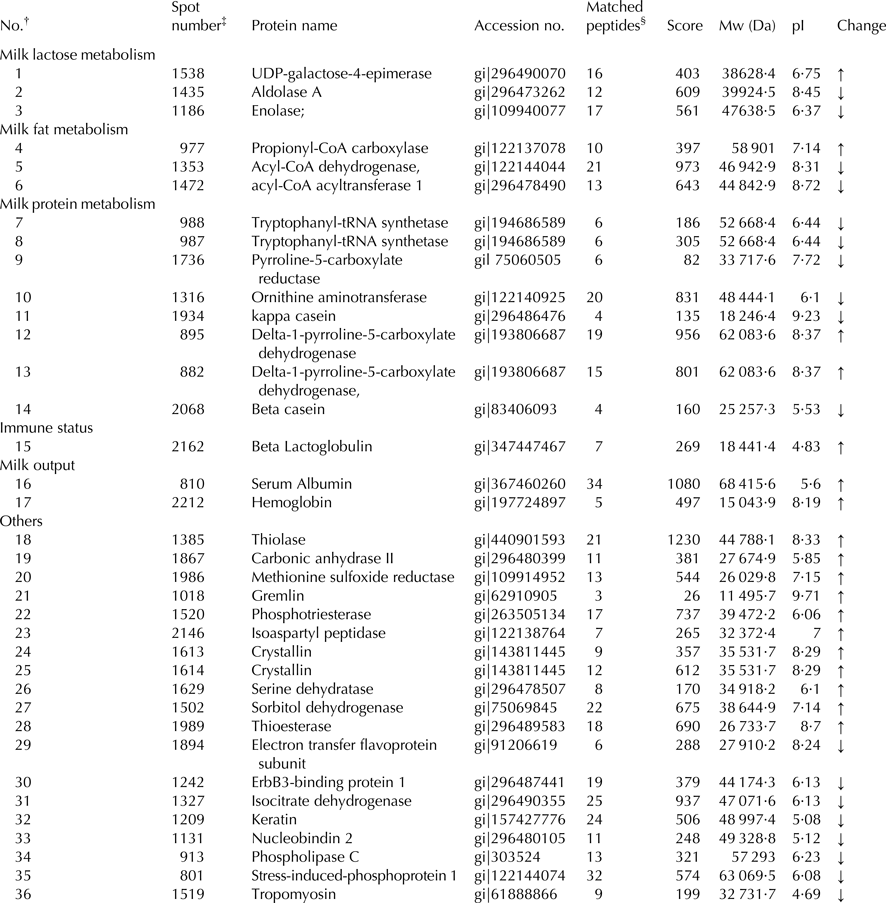
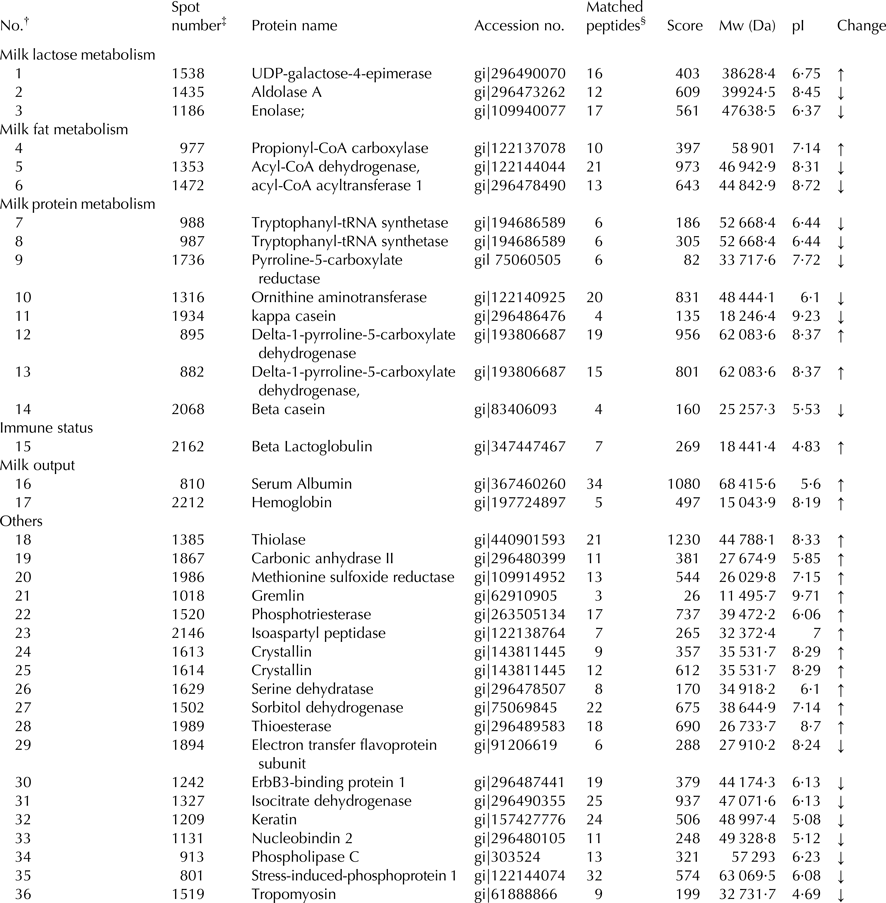
Our previous results found that dietary lutein increased milk yield, lactose content, and fat content of milk and enhanced the antioxidant capacity of dairy cows (Xu et al. Reference Xu, Wang, Yang, Wang, Duan, Wang, Liu and Lao2014). We hypothesized that lutein may increase milk production and improve the health status through regulating mammary gland metabolism. To screen proteins related to the effect of lutein on mammary gland metabolism, we revealed that 33 proteins were changed in lutein group (Table 1). Among these proteins, 15 proteins were upregulated and 18 proteins were downregulated (Table 1). The upregulated proteins include UDP-galactose-4-epimerase (GALE), propionyl-CoA carboxylase (PCC), beta lactoglobulin (β-LG), serum albumin, hemoglobin and delta-pyrroline-5-carboxylate dehydrogenase (P5CDH). The downregulated proteins include aldolase A (ALDOA), enolase (ENO1), acyl-coenzyme dehydrogenase (ACADM) and acyl-coenzyme acyltransferase 1 (ACAA1). The altered proteins were related to glucose metabolism, fatty acid metabolism and immune function of dairy cows (Fig. 1).
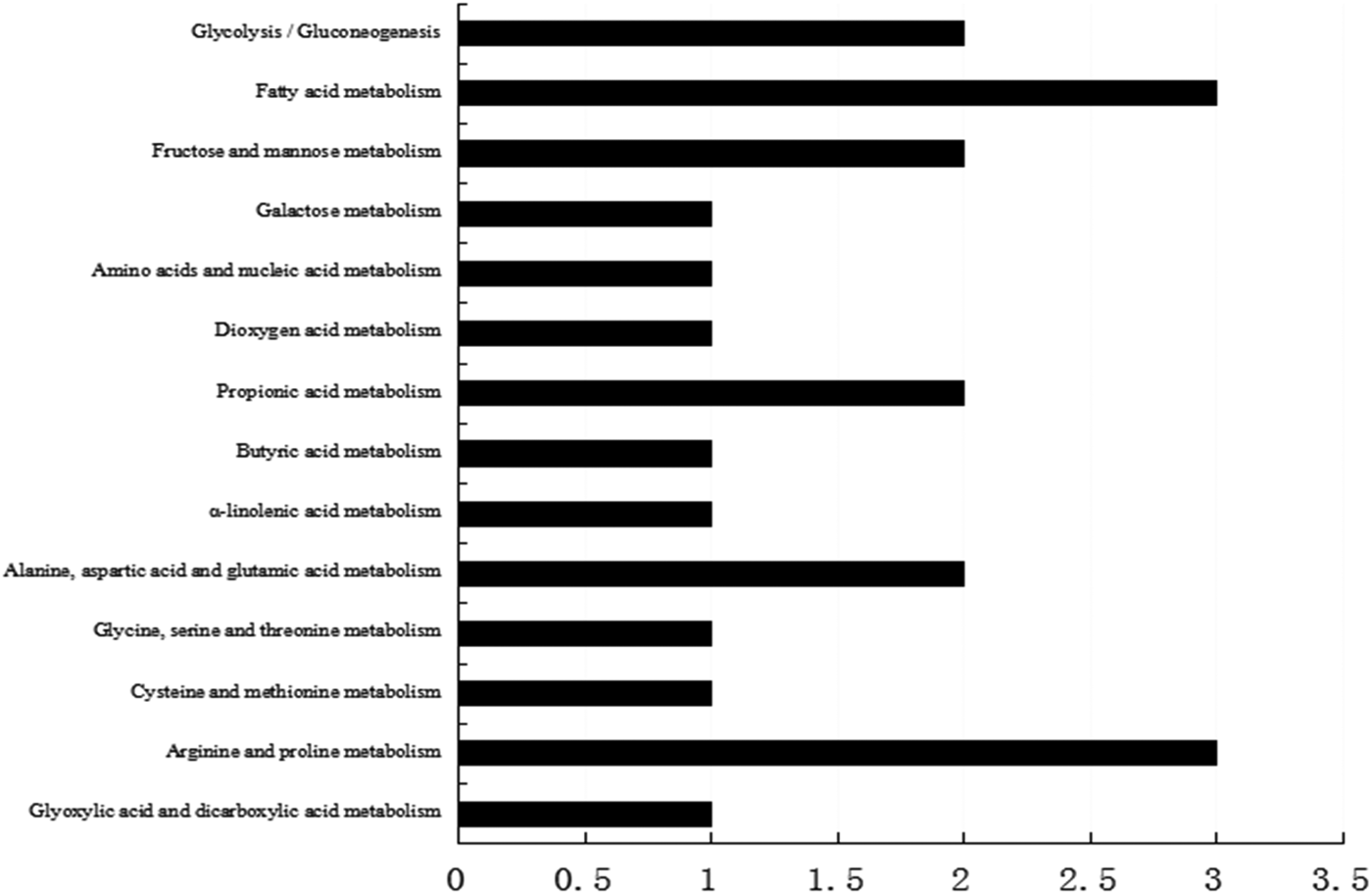
Fig. 1. KEGG pathway analysis of differentially expressed proteins.
Table 1. Identification of differentially expressed mammary gland proteins
† Represents the total proteins identified.
‡ The number corresponds to the 2-DE gel in Fig. 1.
§ The number of peptide identified.
↑ Represents proteins were upregulated; ↓ represents proteins were downregulated.
Bovine serum albumin is the most abundant protein in the bovine blood plasma, and maintains the stability of osmotic pressure and pH of blood (Carter & Ho, Reference Carter and Ho1994). Moreover, it can transport many compounds in blood including fatty acids, metal, amino acids, steroids and drugs (Carter & Ho, Reference Carter and Ho1994). Bovine hemoglobin is the iron-containing oxygen-transport protein in the red blood cells (RBC) and it makes up about 35% of RBC content (Weed et al. Reference Weed, Reed and Berg1963). It plays a major role in transporting oxygen to tissue and provides energy in cell metabolism (Weed et al. Reference Weed, Reed and Berg1963). Our previous study found that milk yield of dairy cows was increased in lutein group, which may due to the increased expression of serum albumin and hemoglobin, indicating that lutein could increase blood flow rate to increase milk output.
ENO1 and ALDOA are two key enzymes involved in glycolytic pathway (Zommara et al. Reference Zommara, Toubo, Sakono and Imaizumi1998) ENO1 contributes to catalyzing the formation of phosphoenolpyruvate from 2-phosphoglycerate (Zommara et al. Reference Zommara, Toubo, Sakono and Imaizumi1998). ALDOA converts fructose-1, 6-biophosphate to dihydroxyacetone phosphate and glyceraldehyde-3-phosphate (Zommara et al. Reference Zommara, Toubo, Sakono and Imaizumi1998). In the present study, the downregulation of ENO1 and ALDOA is likely to inhibit the glycolysis process resulting in more glucose in blood that is the major precursors for milk lactose synthesis. Besides, the increased GALE in lutein group could catalyze the interconversion of UDP-galactose and UDP-glucose in the process of lactose synthesis (Chen et al. Reference Chen, Kowal, Hamad, Fan and Wang1999). Therefore, we speculate that lutein in diet may generate more glucose and then transfer it from blood to mammary gland, leading to the increase of milk lactose synthesis in dairy cow.
Milk fat content and composition, important indicators measuring the quality of milk, are significantly influenced by diet (Bauman & Griinari, Reference Bauman and Griinari2003). Triglyceride is the dominating component of milk fat and is synthesized from free fatty acid in mammary gland. In our proteomic analysis, proteins associated with fatty acid metabolism including PCC were upregulated, whereas ACAA1 and ACADM were downregulated. PCC is a multimeric protein localized to the mitochondrial matrix which can function in the catabolism of isoleucine, threonine, methionine, valine, and odd-chain fatty acids (Browner et al. Reference Browner, Taroni, Sztul and Rosenberg1989). ACAA1, an acyltransferase located in peroxisome, could catalyze the first step of fatty acid β-oxidation. ACADM, one of four acyl-CoA dehydrogenases, catalyzes the initial dehydrogenation step in the mitochondrial β-oxidation of fatty acids. The downregulated proteins involved in fatty acid oxidation may result in more fatty acid by decreasing fatty acid catabolism after feeding lutein to dairy cows. Therefore, the increased milk fat in lutin group (Xu et al. Reference Xu, Wang, Yang, Wang, Duan, Wang, Liu and Lao2014) may be due to the adequate substrate for milk fat synthesis.
Our previous data have revealed that lutein diet can increase the milk quality. The proteomic results have shown that β-LG in the lutein group was increased. β-LG, the main component of bovine milk whey protein, accounts for 10–15% of the total milk and is the globular protein containing 162 amino acids (Liu et al. Reference Liu, Chen and Mao2007). Beyond the nutritional contribution of β-LG, it has many biological functions including preventing lipid oxidation and increasing liver glutathione in rats (Zommara et al. Reference Zommara, Toubo, Sakono and Imaizumi1998). The upregulation of β-LG indicated that lutein might increase the antioxidant capacity of milk and increase the milk quality.
Conclusion
A gel-based proteomics approach was used to monitor the changed protein profile of mammary gland in lutein-fed dairy cows. This study suggested four biological process contributing to the enhanced milk production and improved health status in lutein-diet fed dairy cows: enhanced blood flow, decreased glycolysis and increased lactose anabolism, depressed fatty acid oxidation, increased expression of antioxidant-related proteins. It provides a new clue to understanding the possible mechanisms of dietary lutein in regulating dairy cow lactation.
This study was supported by National Natural Science Foundation of China (No. 31372336), and the Zhejiang Provincial Key Science and Technology Innovation Team (No. 2011R50025).