Consumers of milk and milk products are now attaching more importance than ever to health and safety. Indeed, particular attention is currently paid to the health-promoting antibacterial compounds of dairy products. The natural proteins of milk, whether antimicrobial or not, can be degraded into a variety of antibacterial peptides through enzymatic hydrolysis (Park, Reference Park and Park2009). A phosphoprotein of milk is casein whose bioactive peptides possess antimicrobial, antithrombotic, antihypertensive, opioid, and immunomodulatory properties (Silva & Malcata, Reference Silva and Malcata2005). A member of the casein group is κ-casein, which is hydrophobic, has a clearly amphipathic structure, and is devoid of the anionic phosphate cluster in its polar domain (Swaisgood, Reference Swaisgood and Fennema1996).
Much research has focused on the causes of antibacterial activity of peptides. Almost all antibacterial peptides have less than 100 amino acid residues, mainly in the range of 3 to 50 (Rutherford & Moughan, Reference Rutherford-Markwick and Moughan2005). Another characteristic of antibacterial peptides is that they are positively charged (Epand & Vogel, Reference Epand and Vogel1999). Furthermore, typically 50% or more of the residues of an antibacterial peptide are hydrophobic (Barzyk et al. Reference Barzyk, Campagna, Wieclaw, Korchowiec and Rogalska2009). Lastly, antibacterial peptide chains are usually amphipathic and can form linear, random coil molecules (Ouellette, Reference Ouellette and Schafer2006).
A wide variety of antibacterial peptides have been derived from bovine milk proteins. These proteins include lactoferrin (Bellamy et al. Reference Bellamy, Takase, Yamauchi, Wakabayashi, Kawase and Tomita1992), αS1-casein (Lahov & Regelson, Reference Lahov and Regelson1996), αS2-casein (Zucht et al. Reference Zucht, Raida, Adermann, Magert and Forssman1995; Recio & Visser, Reference Recio and Visser1999), α-lactalbumin (Pellegrini et al. Reference Pellegrini, Thomas, Bramaz, Hunziker and von Fellenberg1999), and β-lactoglobulin (Pellegrini et al. Reference Pellegrini, Dettling, Thomas and Hunziker2001). There are also reports of antibacterial peptides liberated from human or bovine κ-casein (Matin et al. Reference Matin, Monnai and Otani2000; Liepke et al. Reference Liepke, Zucht, Forsmann and Standker2001; Malkoski et al. Reference Malkoski, Dashper, O'Brien-Simpson, Talbo, Macris, Cross and Reynolds2001; López-Expósito et al. Reference López-Expósito, Minervini, Amigo and Recio2006).
Releasing antibacterial peptides from κ-casein is typically achieved using enzymes such as pepsin, trypsin, and chymosin. However, to the best of our knowledge, no study has been carried out to identify antibacterial peptides in bovine κ-casein using plasmin. The present research focused on this enzyme because it is an endogenous proteinase associated with casein in bovine milk. Plasmin is a heat-stable alkaline serine proteinase and is optimally active at a pH of about 7·5 and a temperature of 37 °C (Fox, Reference Fox1991). Plasmin has an affinity for both lysine and arginine residues, but it prefers to cleave Lys-X bonds (Bastian & Brown, Reference Bastian and Brown1996).
Another point is that much of the previous research into antibacterial effects of peptides has been limited to pathogenic bacteria (e.g., Pellegrini et al. Reference Pellegrini, Thomas, Bramaz, Hunziker and von Fellenberg1999; McCann et al. Reference McCann, Shiell, Michalski, Lee, Wan, Roginski and Coventry2006), and indeed scant attention has been paid to the effect of peptides on probiotic bacteria (Lahov et al. Reference Lahov, Edelsten, Sode-Mogensen and Sofer1971, and Lahov & Regelson, Reference Lahov and Regelson1996, to the best of our knowledge). If peptides are shown to have an inhibitory effect on probiotic bacteria, then a possible conclusion would be that they may act against intestinal microbiota and have a negative effect on human body. This led us to investigate the effect of antibacterial peptides on probiotic bacteria.
This study is aimed at exploring antibacterial effects of bovine plasmin, the plasmin digest of bovine κ-casein, and the fractions liberated from the plasmin digest of κ-casein on a number of pathogenic and probiotic bacteria. Then, the antibacterial peptides were identified and compared with nisin in terms of their antibacterial potential.
Materials and methods
Materials
Bovine κ-casein, bovine plasmin (EC Number: 3.4.21.7), and nisin were supplied by Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Sodium dihydrogen phosphate and sodium monohydrogen phosphate, used to prepare the buffer solution, were obtained from Merck (Darmstadt, Germany). The RP-HPLC solvents were Trifluoroacetic acid (TFA) and Acetonitrile (grade A), both purchased from Merck. For microbiological tests, Brain-Heart Infusion (BHI) agar, BHI broth, Man, Rogosa, and Sharpe (MRS) agar, and MRS broth were obtained from Merck. Cultures of Escherichia coli (PTCC 1399) and Staphylococcus aureus (PTCC 1431), pathogenic bacteria, came from the Iranian Research Organization for Science and Technology (IROST) in Tehran. Cultures of Lactobacillus. acidophilus (PTCC 1643) and Lb. casei (PTCC 1608), as probiotic bacteria, were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen [German Collection of Microorganisms and Cell Cultures] in Germany.
Enzymatic proteolysis
To produce the plasmin digest of κ-casein, 3 mg of bovine κ-casein was dissolved in 1 ml 10 mm phosphate buffer at pH 6·8. Bovine plasmin was added to the solution at an enzyme/substrate ratio of 1:150 (v/v). The resulting peptide was placed in a shaking incubator at 30 °C for 44 h (Dalasgaard et al. Reference Dalasgaard, Heegaard and Larsen2008).
Fractionation of the plasmin digest of κ-casein
Following Dalasgaard et al. (Reference Dalasgaard, Heegaard and Larsen2008), the plasmin digest of κ-casein was fractionated using Unicam crystal 200 series RP-HPLC (Cambridge, United Kingdom), which consisted of a G1315B diode-array absorption detector and a G1312A binary pump equipped with an automatic injector. Aliquots of the peptide were injected on a C18 reversed phase column (15×2·1 mm, 5-μm particle size). Two solvents were used: (A) 0·1% TFA in water and (B) 80% acetonitrile and 0·1% TFA. The flow rate of the liquid samples was 0·250 ml per min. The samples were eluted in the 20 °C thermostated column. We applied a linear gradient of solvent B, with the time schedule being as follows: 2 to 10 min: 40%, 15 min: 50%, and 45 to 50 min: 100%. In the end, the column was equilibrated with solvent A for 10 min. The UV detector recorded results at 241 nm. The RP-HPLC separation was repeated three times, and the peptide fractions were collected automatically.
Measurement of concentration of peptide fractions
The concentration (mg/ml) of peptide fractions at 241 nm was determined by comparing the percentage of the area under their peaks with that of the standard peptide (following Josan et al. Reference Josan, Vagner, Handl, Sankaranarayanan, Gillies and Hruby2008). Specifically, the percentage of the area under each peptide peak was multiplied by the concentration of the standard peptide divided by the percentage of the area under the peak of the standard peptide.
Determination of antibacterial activity
Plasmin, the plasmin digest of κ-casein, and peptide fractions were tested to see if they had antibacterial effects on Esch. coli, Staph. aureus, Lb. acidophilus, and Lb. casei.
For the effect of peptide fractions, first the overnight culture of each bacterium was prepared. Then, the colony forming unit (CFU) of each culture was determined, and the proportion of the CFU to the optical density was calculated. After that, each culture was diluted to a concentration of approximately 106 cells/ml. Finally, for each sterile Eppendorf vial, around 50 μl of the 0·45 mg/ml peptide fraction solution and 10 μl of the bacterial culture were added to 450 μl of the BHI broth or the MRS broth. The plasmin sample and the sample for the plasmin digest of κ-casein contained 50 μl of the respective solution.
The control sample contained 50 μl of the 20 mm phosphate buffer in place of the experimental solutions. In order to make sure the phosphate buffer does not affect bacterial growth, we prepared a blank sample in which the phosphate buffer replaced both experimental solutions and bacterial cultures.
The vials containing pathogenic bacteria (Esch. coli and Staph. aureus) were placed in a normal incubator at 37 °C for 18 h while the vials with probiotic bacteria (Lb. acidophilus and Lb. casei) were placed in a CO2 incubator under the same conditions. The optical density of each test sample was measured at 620 nm using Cecil 7400 UV-Visible Spectrophotometer (Cambridge, United Kingdom). The difference between mean values of surviving bacteria was statistically determined using Duncan's New Multiple Range Test (MRT), P value <0·05, on SPSS 19 (2010).
Determination of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) was determined only for the antibacterial peptides and the plasmin digest of κ-casein. MIC is defined as the lowest concentration of an antimicrobial that will inhibit the growth of a microorganism after overnight incubation at an absorbance of 620 nm (McCann et al. Reference McCann, Shiell, Michalski, Lee, Wan, Roginski and Coventry2006).
Different concentrations of the peptide solution (ranging from 0 to 300 ppm), and around 10 μl of the bacterial culture (approximately 106 cells/ml) were added to 450 μl of the BHI or the MRS broth.
The control sample contained different concentrations of the phosphate buffer in place of the peptide solution, and the blank sample contained the phosphate buffer instead of both peptide solutions and bacterial cultures.
The vials containing pathogenic bacteria were incubated in a normal incubator at 37 °C for 18 h, whereas the vials having probiotic bacteria were placed in a CO2 incubator under the same conditions. The optical density of all samples was measured at 620 nm using Cecil Spectrophotometer. The experiments were repeated three times for each sample.
Lastly, the results were compared with the MIC of nisin (an antibacterial peptide produced by fermentation using the bacterium Lactococcus lactis). Nisin was chosen because it is the approved bacteriocin used in food industry (Jay, Reference Jay2000). Antibacterial test of nisin was performed by adding nisin powder to 0·02 M hydrochloric acid and centrifuging it at 7000 g for 10 min at 4 °C. This solution was filtered through a 0·2 μm filter for sterilisation purposes.
Identification of antibacterial peptides
Antibacterial peptides were identified according to molecular mass. Purified antibacterial peptides were mixed with a solution containing 80% (v/v) acetonitrile and 20% (v/v) water before they were analysed for their molecular mass using an Agilent LC-MS 6410 QQQHPLC Series 1200 (Santa Clara, USA), which consisted of a model G1312 D binary pump, a model G1329A auto sampler, and a model G1315 D diode-array detector. The detector was equipped with an electrospray ionisation source (capillary voltage at 3000 V). The MS spectra of the samples were recorded in Full SCAN (from mass/charge 50 to 2000), in positive mode (ESI+), a fragmentation voltage of 120 V, and a collision voltage of zero. Nitrogen was used as the drying gas at a flow rate of 12 l/min, a nebulising gas pressure of 60 psig (413·7 kPa), and a temperature of 300 °C. The Mass Hunter software (Agilent, Santa Clara, USA) was used to calculate the mass of the antibacterial peptides.
Following Danicik et al. (Reference Danicik, Addona, Clauser, Vath and Pevzner1999), the determined molecular masses of the antibacterial peptides were compared with those of peptides generated by the theoretical enzymatic digests of the original milk proteins using the ExPASy database (http://us.expasy.org). As this database does not contain any data for plasmin, we decided to use the trypsin results instead because the two enzymes function similarly.
Results
Determination of antibacterial activity
Digestion of κ-casein by plasmin after 44 h of incubation yielded several fractions. The RP-HPLC chromatogram contained five peaks numbered from 1 to 5 (Fig. 1). The fractions associated with these peaks were named κC1, κC2, κC3, κC4, and κC5. These fractions had retention times of 12·1, 13·2, 15·5, 16·9, and 18·2 min and concentrations of 0·63, 0·86, 0·45, 0·54, and 0·33 mg in every 200 μl.
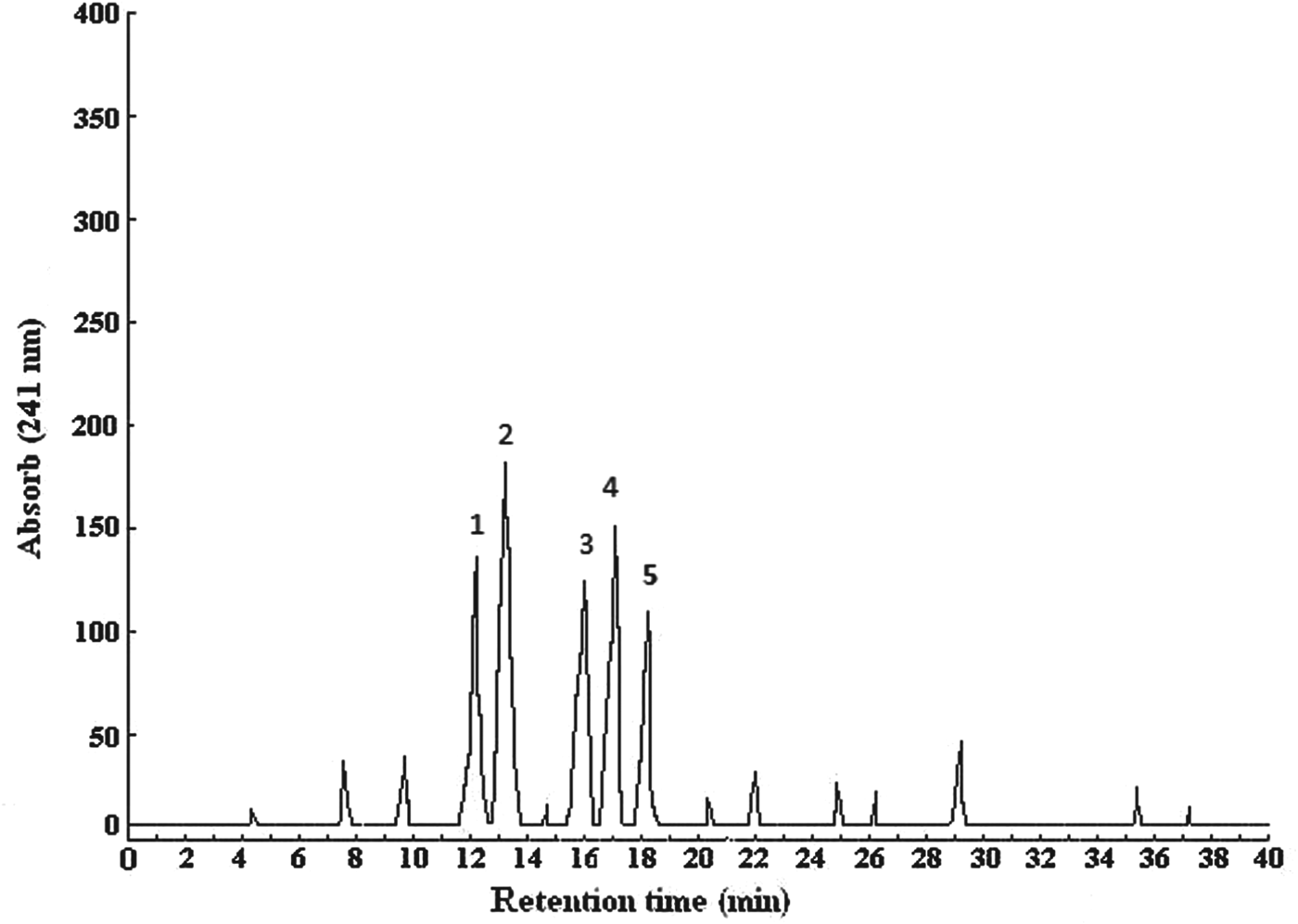
Fig. 1. Chromatograms obtained by RP-HPLC with UV detection at 241 nm of peptides after plasmin digestion, of κ-casein.
Peptide fractions, the plasmin digest of κ-casein, and plasmin were compared in terms of their effect on bacterial strains. The number of bacteria which survived exposure to the fractions is given in Table 1. The κC2 and κC5 fractions had negligible antibacterial effects, but κC1, κC3, and κC4 showed a significant antibacterial activity. These latter fractions are hereafter called antibacterial peptides. However, it should be noted that these peptides did not completely inhibit bacterial growth but only partially decreased bacterial growth (at a concentration of 0·45 mg/ml). There was a statistically significant difference in mean number between the bacteria surviving in the presence of antibacterial peptides and those present in the control samples (P<0·05). Also, significant differences (P<0·05) were observed between the four tested bacteria in terms of their responses to antibacterial peptides.
Table 1. Numbers (log10 cfu/ml) of surviving bacteria following exposure to fractionated peptides
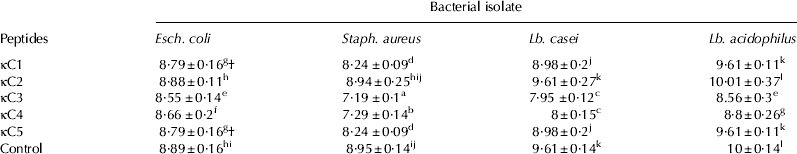
† Numbers (log10 cfu/ml) of surviving bacteria with different letters are significantly different (P<0·05)
The plasmin digest of κ-casein decreased Esch. coli growth (more successfully than the antibacterial peptides) and completely inhibited the growth of the other three bacteria. The plasmin sample revealed no antibacterial activity, an observation which was made at a maximum concentration of 200 unit per ml.
Determination of minimum inhibitory concentration
The MICs of antibacterial peptides (κC1, κC3, and κC4) and the plasmin digest of κ-casein were determined against the target bacteria, and the results were compared with the MIC of nisin (Table 2). As can be seen, the MIC of κC3 ranged from 60 to 70 μg/ml against the Gram-positive bacteria (Staph. aureus, Lb. casei and Lb. acidophilus) and was 100 μg/ml against the Gram-negative bacterium (Esch. coli), indicating that κC3 is active against all the bacteria tested. κC4 had an MIC of 70 to 80 μg/ml against the Gram-positive bacteria and an MIC of 125 μg/ml against the Gram-negative bacterium, suggesting that it is generally more destructive of the Gram-positive bacteria. The MIC of κC1 was 90 μg/ml against the Gram-positive bacteria and 200 μg/ml against the Gram-negative bacterium, revealing that this peptide was active against the Gram-positive bacteria, but not so against the Gram-negative bacterium.
Table 2. Minimum inhibitory concentration (MIC) of antibacterial compounds and nisin against selected bacteria incubated at 37 °C for 18 h (μg/ml unite)
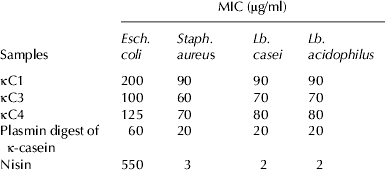
The MIC of the plasmin digest of κ-casein was 20 μg/ml against the Gram-positive bacteria and 60 μg/ml against the Gram-negative bacterium, resulting in inhibited growth.
Nisin showed a relatively low MIC (ranging from 2 to 3 μg/ml) against Gram-positive bacteria, but a very high MIC (550 μg/ml) against the Gram-negative bacterium, indicating that nisin was highly destructive of Gram-positive bacteria, but less active against the Gram-negative bacterium.
Identification of antibacterial peptides
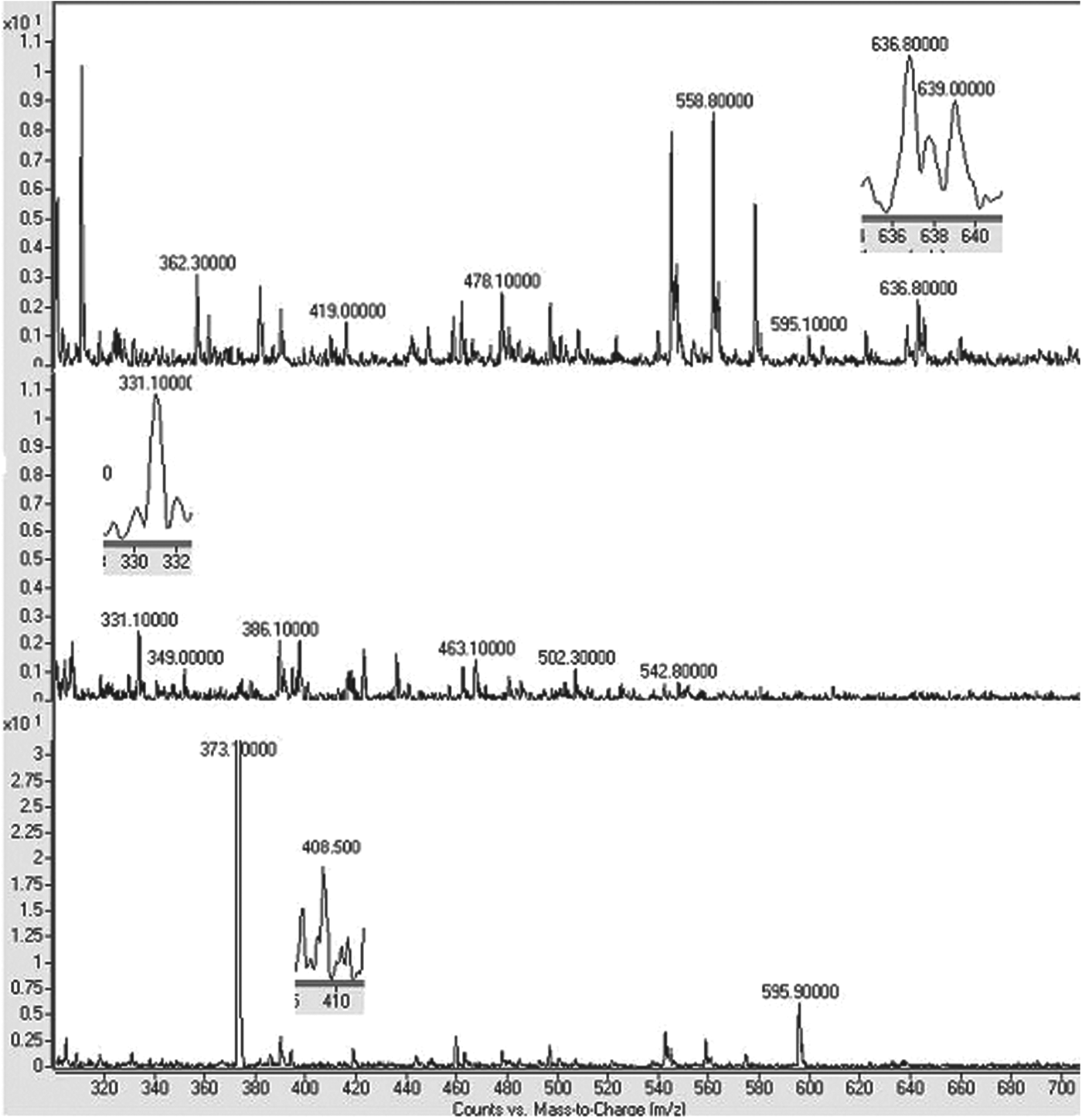
Table 3 lists the properties of the antibacterial peptides according to their molecular mass. Figure 2 shows that κC1 had a molecular mass of 639·1 Da, which is close to the theoretical peptide mass (643·2 Da). The molecular mass of this compound exactly corresponds to the f(17–21) of bovine κ-casein, with an amino acid sequence of FFSDK and a theoretical pI of 5·84. The cleavage of κ-casein at Lys21-Ile 22 was essential for the formation of neutral and hydrophilic κC1.

Fig. 2. Molecular mass diagram of κC1, κC3 and κC4 Peptides.
Table 3. Characterisation of antibacterial peptides

The κC3 peptide had a molecular mass of 331·700 Da (Fig. 2), which corresponds to the theoretical peptide mass (330·428 Da). This compound's molecular mass fully matches the f(22–24) of bovine κ-casein, with its amino acid sequence being IAK and its theoretical pI being 8·75. The formation of cationic and hydrophobic κC3 required the cleavage of κ-casein at Lys24-Tyr 25.
The molecular mass of κC4 turned out be 408·500 Da (Fig. 2), corresponding to the theoretical peptide mass (408·575 Da). The molecular mass of this compound covered the f(1–3) region of bovine κ-casein, with an MMK amino acid sequence and a 10·1 theoretical pI. In order for the cationic and hydrophobic κC4 peptide to form, it is necessary to cleave κ-casein at Lys3-Ser4.
Discussion
This research fractionated κ-casein into κC1, κC2, κC3, κC4, and κC5 through plasmin hydrolysis. This contrasts with the conclusion of Eigel (Reference Eigel1977) that κ-casein is almost non-digestible after 1 h of incubation by plasmin. Like our research, Dalasgaard et al. (Reference Dalasgaard, Heegaard and Larsen2008) were able to fractionate plasmin-hydrolysed κ-casein. They came up with four peptides (named C1, C2, C3, and C4) using a relatively long incubation period (44 h). However, they did not investigate the antibacterial properties of the obtained peptides.
Three peptides (kC1, kC3, and kC4) of the plasmin digest of bovine k-casein proved to be antibacterial. The κC3 peptide was the most active of antibacterial peptides, corresponding to the f(22–24) sequence of bovine κ-casein. κC3 has not been previously reported as an antibacterial peptide, to the best of our knowledge. However, its amino acid sequence (IAK) is part of a 7-amino acid sequence (FSDKIAK), or the residue region f(18–24), reported by López-Expósito et al. (Reference López-Expósito, Minervini, Amigo and Recio2006). The κC4 peptide is a novel antibacterial domain as its amino acid sequence (MMK) has not been previously reported. The amino acid sequence of antibacterial κC1 (FFSDK) has previously been reported by Matin et al. (Reference Matin, Monnai and Otani2000), who also found cytotoxic activity for this peptide in addition to an antibacterial effect on Staph. aureus.
Our findings showed that plasmin had an affinity for lysine residue. This is because plasmin attacks Lys-X bonds in all the antibacterial peptides. The observed affinity of plasmin for lysine residue has also been reported by Bastian & Brown (Reference Bastian and Brown1996), who found that this enzyme hydrolyses Lys-X and Arg-X bonds and preferentially attacks the former.
Another issue of interest was the relationship between peptide charge and antibacterial activity. The κC3 and κC4 peptides, with one positive charge, revealed more activity against the bacteria tested than did κC1. However, the observation that neutral κC1 did reveal some degree of antibacterial activity is inconsistent with the finding of Epand & Vogel (Reference Epand and Vogel1999) that the positive charge of a peptide is a key factor in antibacterial activity.
The current research also explored the relationship between hydrophobicity and antibacterial activity. κC3 and κC4 as hydrophobic peptides proved to be more antibacterial than κC1 with hydrophobicity of less than 50%. However, it is worthy of note that hydrophilic κC1 did exhibit some degree of antibacterial activity, an observation which disagrees with the finding of Barzyk et al. (Reference Barzyk, Campagna, Wieclaw, Korchowiec and Rogalska2009) that 50% or more of the residues of an antibacterial peptide should be hydrophobic.
We also investigated the relationship between peptide length and antibacterial activity. The antibacterial peptides identified in this work were short (between 3 and 5 amino acid residues), and they exhibited weaker antibacterial properties, at least for the bacterial strains tested, than longer peptides such as lactoferrin and αS2-casein–derived peptides reported in the previous studies (Bellamy et al. Reference Bellamy, Takase, Yamauchi, Wakabayashi, Kawase and Tomita1992; Recio & Visser, Reference Recio and Visser1999). This is consistent with the study of López-Expósito et al. (Reference López-Expósito, Minervini, Amigo and Recio2006) who reported that shorter antibacterial peptides should have weaker antibacterial activity.
Furthermore, an investigation of the amino acid sequences of the antibacterial peptides in this research showed that their C-terminal residue is a basic amino acid, lysine. This residue may be a deciding factor in antibacterial activity. This finding is in agreement with those of Matin et al. (Reference Matin, Monnai and Otani2000) and Brown & Hancock (Reference Brown and Hancock2006), who reported that cationic side chains of arginine, lysine, and histidine can mediate in the interaction between peptides and negatively-charged bacterial membranes or cell walls, including lipopolysaccharide.
For antibacterial peptides, the MIC values ranged from 60 to 90 μg/ml against the Gram-positive bacteria and from 100 to 200 μg/ml against the Gram-negative bacterium. These MIC values are consistent with a study by McCann et al. (Reference McCann, Shiell, Michalski, Lee, Wan, Roginski and Conventry2005) that reported the MIC of αS2-casein to be in the range of 21 to 168 μg/ml for f(181–207), 10·7 to 171·2 μg/ml for f(175–207), and 4·8 to 76·2 μg/ml for f(164–207) against a wide variety of Gram-positive and Gram-negative bacteria. Similarly, McCann et al. (Reference McCann, Shiell, Michalski, Lee, Wan, Roginski and Coventry2006) reported an MIC of 125 μg/ml against Gram-positive bacteria and 125–500 μg/ml against Gram-negative bacteria for bovine αS1-casein, f(99–109).
The MIC results for the plasmin digest of κ-casein showed that this compound has a higher antibacterial potential than any of the antibacterial peptides, an observation explainable by synergism between the peptides in the plasmin digest of κ-casein. This finding differs from the study of McCann et al. (Reference McCann, Shiell, Michalski, Lee, Wan, Roginski and Conventry2005) which demonstrated the MIC of the chymosin digest of sodium caseinate (CrMIX) to be very high against their target bacteria.
Gram-positive bacteria were more sensitive than the Gram-negative bacterium to the action of the κC1, κC3, and κC4 peptides and the plasmin digest of κ-casein. The higher resistance of Gram-negative bacteria might relate to the structural complexity of their cell membrane since their cell wall contains, in addition to a cytoplasmic membrane, an outer membrane consisting of lipopolysaccharide, phospholipid, lipoprotein, and protein, all supporting the bacterial cell (Hancock & Lehrer, Reference Hancock and Lehrer1998).
Nisin proved to be stronger than antibacterial peptides and the plasmin digest of κ-casein in terms of antibacterial activity against Gram-positive bacteria. Its MIC value against Staph. aureus was higher (3 μg/ml) than that found by Martinez et al. (Reference Martinez, Obeso, Rodriquez and Garcia2008) (0·75 μg/ml) and similar to the value reported by Pilar et al. (Reference Pilar, Beatriz, Lorena and Ana2010) (3–6 μg/ml). Nisin was not so effective against the Gram-negative bacterium (550 μg/ml). This finding is similar to the results reported in McCann et al. (Reference McCann, Shiell, Michalski, Lee, Wan, Roginski and Conventry2005), with the difference being that they found a lower MIC value (400 μg/ml). The inactivity of nisin against the Gram-negative bacterium can be attributed to its relatively large size, which prevents it from penetrating the outer membrane of the Gram-negative cell wall (Heike & Sahl, Reference Heike and Sahl2000).
The present research also studied the effects of antibacterial peptides and the plasmin digest of κ-casein on pathogenic and probiotic bacteria. Antibacterial peptides were found to be more effective in inhibiting the survival of Gram-positive bacteria, and most probiotic bacteria strains are Gram-positive.
The effect of antibacterial peptides on pathogenic bacteria increases the safety of raw milk and dairy products (McCann et al. Reference McCann, Shiell, Michalski, Lee, Wan, Roginski and Conventry2005; López-Expósito et al. Reference López-Expósito, Minervini, Amigo and Recio2006). However, the problem is that these antibacterial compounds are also active against probiotic bacteria. Now, given the fact that the most important qualitative parameter of probiotic microorganisms is their viability in the final product until the time of consumption (Mortazavian & Sohrabvandi, Reference Mortazavian, Sohrabvandi and Mortazavian2006), it is necessary to evaluate the effect of antibacterial peptides on the viability of probiotic microorganisms in the final product under different conditions (Lahov et al. Reference Lahov, Edelsten, Sode-Mogensen and Sofer1971; Lahov & Regelson, Reference Lahov and Regelson1996). From a medical point of view (in the human body), consumption of antibacterial peptides can control gasterointestinal infection, but it also affects the natural bacteria of the intestines. As a result, consumption of antibacterial peptides should be controlled and enriched with probiotic bacteria (Zhou et al. Reference Zhou, Pillidge, Gopal and Gill2005).
The support of the following organizations is gratefully acknowledged: the Science and Research Branch of the Islamic Azad University (SRBIAU), the Biochemistry Research Department of the University of Kharazmi in Tehran, the Microbiology Department of the Central Tehran Branch of the Islamic Azad University, and the Department of Medical Sciences of the University of Shahid Beheshti, Tehran.