The steadily increasing milk yield per cow during the last decades has been a challenge to the cow's metabolic physiology. The cycling between mobilisation in early lactation and accretion of body reserves later in lactation occurs naturally during lactation (Friggens et al. Reference Friggens, Ingvartsen and Emmans2004). Genetic selection of cows for higher milk production has resulted in greater mobilisation during early lactation, because feed intake has not increased correspondingly. Additionally, several factors may aggravate this natural cycle of body reserves by increasing the energy deficit, thereby increasing the mobilisation and lengthening the period of negative energy balance. Such factors are e.g. diseases that reduce appetite and consequently decrease energy intake, like ketosis or systemic mastitis (Bareille et al. Reference Bareille, Beaudeau, Billon, Robert and Faverdin2003), or displaced abomasum (LeBlanc et al. Reference LeBlanc, Leslie and Duffield2005). These situations have accentuated the need for daily surveillance of the individual cow in order to reduce incidence of diseases and metabolic disorders. Traditional surveillance in the herd is personal inspection, especially during the milking situation. However, daily surveillance of the herd is increasingly performed by more or less automatic in-line systems for milk analysis. Some of these in-line surveillance systems have proven ability to discover failure to thrive or mammary infections in the subclinical state, allowing for management interventions to avoid profound imbalances and disease (Nielsen et al. Reference Nielsen, Friggens, Chagunda and Ingvartsen2005a; Chagunda et al. Reference Chagunda, Friggens, Rasmussen and Larsen2006; Friggens et al. Reference Friggens, Chagunda, Bjerring, Ridder, Højsgaard and Larsen2007b).
Analyses of blood have been the preferred way to support clinical inspection of the animals. Blood variables (glucose, NEFA, BHBA etc.) reflect the rate and extent of tissue mobilisation and have been used to predict the energetic status of the animal (Bjerre-Harpøth et al. Reference Bjerre-Harpøth, Friggens, Thorup, Larsen, Damgaard, Ingvartsen and Moyes2012) or used to generate indices like physiological imbalance (Moyes et al. Reference Moyes, Larsen and Ingvartsen2013). However, blood sampling is invasive, time consuming and cannot, at least not at present, be automated. Estimation of the nutritional status of an animal via variations in milk components is attractive as milk samples can be collected automatically. Therefore, the use of biomarkers in milk to monitor the physiological and nutritional status of the animal has attracted attention. Several studies have worked with measurements directly from mid-infrared spectral data from milk (fat and protein; e.g. McParland et al. Reference McParland, Banos, Wall, Coffey, Soyeurt, Veerkamp and Berry2011). However, these macro constituents have proved not to be sufficient for reliable estimations of individual nutritional status. Friggens et al. (Reference Friggens, Ridder and Løvendahl2007a) incorporated DIM, milk yield, milk fat, protein yield and protein percentage in milk in predictions, and concluded that mean energy balance of different parities could be predicted with relative accuracy only.
This study is based on data from a production experiment (Alstrup et al. Reference Alstrup, Weisbjerg, Hymøller, Larsen, Lund and Nielsen2014), and introduces analyses of milk variables not often used as indicators in order to validate their potential to describe the nutritional status of the animal based on the quality and quantity of feed consumed. Existing parameters in commercial surveillance equipment and routine automated infrared milk analyses (lactose, fat and protein) may describe the status of the cow to a certain extent. However, it is our objective to examine whether more specific variables in milk may supplement and strengthen the prediction of the nutritional status in dairy cows.
Materials and methods
Cows, experimental design, and facilities
The experiment was carried out at the Danish Cattle Research Centre and complied with the guidelines of the Danish Ministry of Justice with respect to animal experimentation and care of animals under study.
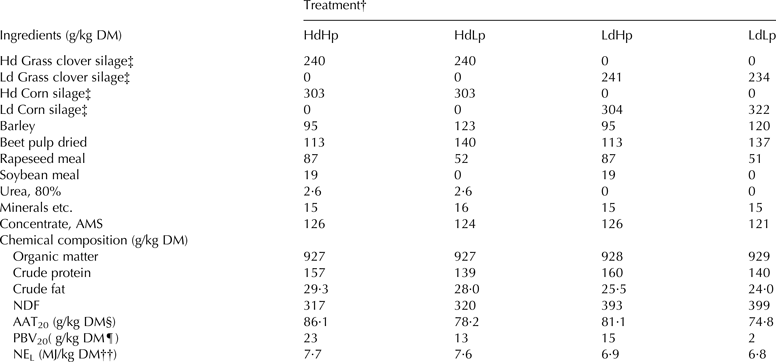
Forty-eight lactating Holstein Friesian cows were used in this study and cows were blocked according to parity (1st and older cows) and DIM, and were randomly assigned to four dietary treatments in a replicated 4 × 4 Latin square design with a 2 × 2 factorial arrangement of treatments. Each experimental period lasted 21 d. Cows were offered 3 kg concentrate daily in the automatic milking system (AMS), and a mixed ration (MR) ad libitum. The AMS concentrate offer was the same for all four treatments. The dietary treatments were MR with (1) high organic matter (OM) digestibility and high CP concentration (HdHp), (2) high OM digestibility and low CP concentration (HdLp), (3) low OM digestibility and high CP concentration (LdHp), and (4) low OM digestibility and low CP concentration (LdLp). All rations consisted of 30% corn silage, 25% grass-clover silage and 45% concentrate on DM basis (Table 1). Differences in ration digestibilities were obtained by use of silages with varying OM digestibilities (OMD): Hd grass clover silage 81·0%, Ld grass clover silage 66·2%, Hd corn silage 77·8%, and Ld corn silage 72·0% OMD. Dietary CP was increased by substituting barley and sugar beet pulp with rapeseed- and soybean meal. Rations were adjusted with urea to reach similar CP concentration (per kg DM) for the two different forage qualities.
Table 1. Ration ingredients and chemical composition of the dietary rations

† High digestibility and high protein concentration (HdHp), high digestibility and low protein concentration (HdLp), low digestibility and high protein concentration (LdHp) and low digestibility and low protein concentration (LdLp)
‡ OM digestibilities (OMD) of silages were calculated from measured in vitro digestibility according to Akerlind et al. (Reference Akerlind, Weisbjerg, Eriksson, Thøgersen, Udén, Ólafsson, Harstad, Volden and Volden2011), and were as follows: 81·0% for Hd grass clover silage, 66·2% for Ld grass clover silage, 77·8% for Hd corn silage, and 72·0% for Ld corn silage
§ AAT20 = Estimated amino acids absorbed in the small intestine at 20 kg DM intake (Volden, Reference Volden and Volden2011)
¶ PBV20 = Estimated protein balance in the rumen at 20 kg DM intake (Volden, Reference Volden and Volden2011)
†† NEL is calculated according to Weisbjerg & Hvelplund (Reference Weisbjerg and Hvelplund1993)
Cows were kept in a loose-housing system and had access to the AMS (DeLaval AB, Tumba, Sweden). The AMS was equipped with a device for automatic measurement of milk yield and milk sampling and further equipped with a device for concentrate feeding. The amount of concentrate dispensed and removed was recorded and concentrate refusals at the end of each cow visit was weighed. For automatic recording of intake of MR the Insentec RIC system (Insentec, Marknesse, The Netherlands) was used. Cows had free access to drinking water. For further details of the experimental setup see Alstrup et al. (Reference Alstrup, Weisbjerg, Hymøller, Larsen, Lund and Nielsen2014).
Experimental procedures
Feed intake
Individual intake of mixed ration and concentrate fed in the AMS was calculated on a daily basis.
Milk yield and quality
Milk yield was measured at every visit in the AMS using DeLaval Free Flow meter MM25 (DeLaval AB, Tumba, Sweden) based on optical milk flow measurement. Every week, representative milk samples were taken at each milking in 48 consecutive hours, and samples were analysed for protein, fat, and lactose content on a Milkoscan 4000 infrared analyser at Eurofins Steins (Holstebro, Denmark). Determination of SCC was performed using a standard Fossomatic cell counter (EN ISO 13366-3, Foss Electric Ltd., Hillerød, DK). The analysed milk samples and the corresponding milk yield recordings were used to calculate an average concentration of protein, fat and lactose. Lactose was measured as lactose monohydrate, and ECM (3·14 MJ/kg) was calculated with the energy factor as given by Sjaunja et al. (Reference Sjaunja, Baevre, Junkkarinen, Pedersen and Setala1991).
Milk urea was analysed using flow injection analysis (Nielsen et al. Reference Nielsen, Larsen, Bjerring and Ingvartsen2005b) using a FIAstar 5000 Analyser (Foss Tecator AB, Höganäs, Sweden). Application notes given by the manufacturer (Foss Tecator AB, Höganäs, Sweden) were followed (Nielsen et al. Reference Nielsen, Friggens, Chagunda and Ingvartsen2005a). Milk BHBA, uric acid (UA), triacylglycerol (TAG), cholesterol, and glucose and glucose-6-phosphate (Glu6P) were analysed by enzymatic-fluormetric methods (Larsen & Nielsen, Reference Larsen and Nielsen2005; Larsen & Moyes, Reference Larsen and Moyes2010; Larsen et al. Reference Larsen, Larsen and Friggens2011; Larsen, Reference Larsen2012, Reference Larsen2015; respectively).
Statistical analysis
Data on feed intake and milk yield were analysed with the MIXED procedure of the Statistical Analysis Systems (SAS® version 9.2, 2010) where model parameters included fixed effect of period (1–4), fixed effect of lactation number (l) (1–4), fixed effect of digestibility (d) (Hd, Ld), fixed effect of CP concentration (p) (Hp, Lp), interaction between digestibility and CP concentration, interaction between lactation number and digestibility, interaction between lactation number and CP concentration, and cow (1–48) was treated as a random variable. Residuals were assumed normally distributed with mean value 0 and constant variance εijkl ~ N(0,σ2). For analysis on milk yield the following parameters were excluded: interaction between lactation number and digestibility and interaction between lactation number and CP concentration; due to lack of significance (P > 0·10).
Data on milk composition, feed and energy parameters and basic milking data were mutually correlated using Pearson's method. Differences between samplings groups (day vs. night) were evaluated by two-tailed t-tests.
Random regression was used to test the effect of lactation stage (DIM) on level of cholesterol, BHBA, cholesterol/TAG and cholesterol/milk fat with the MIXED models procedure of SAS. The model included effect of DIM combined with effect of either ECM yield, BCS or BW gain/d. Intercept and DIM were set as random
Results reported in tables are, if not otherwise stated, treatment LSM and sem. P values ⩽ 0·05 were regarded as significant.
Results
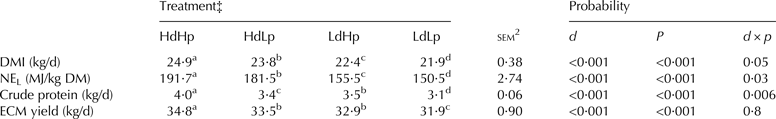
Results on feed intake and milk yield are shown in Table 2. The voluntary feed intake was stimulated by both the highly digestible OM and the high CP level (P < 0·001). Consequently, the energy and CP intake was highest in these groups (P < 0·001). The ECM yield was higher in Hd groups compared to Ld groups, and higher in Hp groups compared to Lp groups. No interaction was seen between digestibility and CP for ECM yield.
Table 2. Treatment effect on daily feed intake and energy corrected milk (ECM) yield†

d, digestibility; p, crude protein; NEL, Net energy for lactation
a-dMeans within a row with different superscripts differ between mean values (P < 0·05)
† For more detailed production data see Alstrup et al. (Reference Alstrup, Weisbjerg, Hymøller, Larsen, Lund and Nielsen2014)
‡ High digestibility and high protein concentration (HdHp), high digestibility and low protein concentration (HdLp), low digestibility and high protein concentration (LdHp) and low digestibility and low protein concentration (LdLp)
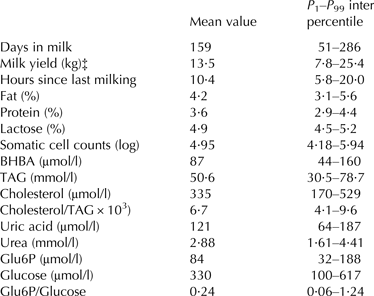
Descriptive statistics for basic milking data (DIM, milk yield, and h since last milking) and traditional milk constituents, and the variables measured in this study, are given in Table 3. All observations were done between 51 and 286 DIM; the average milk yield was 13·5 kg per milking, and on average there was 10·4 h between two milkings (range 5·8–20·0 h) indicating on average 2·3 voluntary milkings per day. Milk fat, protein and lactose concentration, and SCC revealed customary levels and narrow ranges as commonly seen in ordinary Danish herds. The ratio between the highest observation and the lowest observation was <2. The milk BHBA mean value (87 µmol/l) and range (44–160 µmol/l) indicate no serious ketosis conditions during the experimental period. Most of the additional variables in milk (Table 3) show ranges where the highest observations are 3 to 6 times higher than the smallest observations, the ratio between Glu6P and glucose had even a range near 20.
Table 3. Descriptive statistics for basic milking data and milk constituents†

TAG, triacylglycerol; Glu6P, glucose-6-phosphate
† Numbers of observations were in all instances between 817 and 842
‡ Milk yield for the actual milking
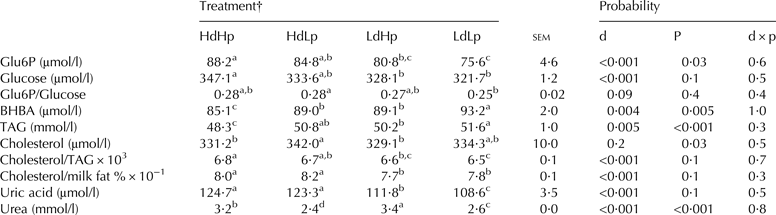
The treatment effect on concentration of the potential nutritional markers in milk is shown in Table 4. Hd causes higher levels of Glu6P, glucose, and uric acid, and additionally a higher ratio between cholesterol and TAG. On the contrary, the BHBA and the urea content are significantly lower in the Hd groups. Hp increased Glu6P, but decreased BHBA and TAG concentration, whereas urea concentration was lower in the Lp groups compared to the Hp groups. No significant interactions were seen between digestibility level and CP level.
Table 4. Treatment effect on concentration of potential nutritional markers in milk

d, digestibility; p, crude protein; Glu6P, glucose-6-phosphate; BHBA, β-hydroxybutyrate; TAG, triacylglycerol
a–dMeans within a row with different superscripts differ between mean values (P < 0·05)
†High digestibility and high protein concentration (HdHp), high digestibility and low protein concentration (HdLp), low digestibility and high protein concentration (LdHp) and low digestibility and low protein concentration (LdLp)
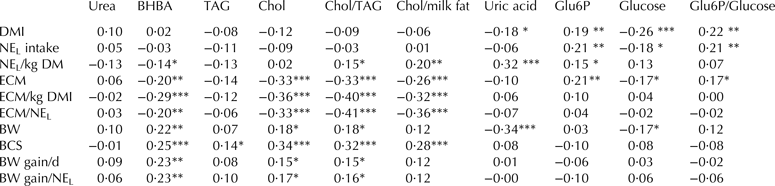
The potential indicators of energy status in milk were regressed against parameters connected to feed intake, milk output, BCS, and cows daily BW gain (Table 5). Milk urea revealed no connection to the parameters under consideration. A weak positive correlation between TAG and BCS and a stronger between cholesterol and BCS was seen. BHBA was negatively correlated to energy density in the feed, ECM, ECM/kg DMI, and ECM/NEL; while BHBA in milk was positively associated to parameters connected to BW and BCS. Milk cholesterol and cholesterol/TAG and cholesterol/milk fat was negatively correlated to ECM, ECM/kg DMI and ECM/NEL, but positively associated to BCS. Uric acid was directly correlated to the energy density of the feed (NEL/kg DM), but negatively correlated to BW of the cow. Glu6P in milk was positively correlated to NEL intake and ECM and glucose was negatively correlated to DM and energy intake and ECM; furthermore, the ratio between Glu6P and glucose correlated positively to energy intake and ECM. However, further analysis showed that stage of lactation (DIM) could explain some of the correlations between ECM yield, BW gain and BCS, and the milk measures cholesterol, BHBA, cholesterol/TAG and cholesterol/milk fat (P < 0·0001). For cholesterol, which was positively correlated to DIM, DIM explained all correlation. For BHBA, when accounted for the positive correlation to DIM, BHBA positively correlated to both BW gain (P = 0·03) and BCS (P = 0·007). For cholesterol/TAG, when accounted for the positive correlation to DIM, cholesterol/TAG tended to correlate negatively to ECM yield (P = 0·08) and positively correlated to BCS (P = 0·008). For cholesterol/milk fat, when accounted for DIM, cholesterol/milk fat correlated positively to BCS (P = 0·02) (data not shown).
Table 5. Correlation between potential indicators of energy status in milk and parameters for energy balance in the dairy cow†

BHBA, β-hydroxybutyrate; TAG, triacylglycerides; Chol, cholesterol; Glu6P, glucose-6-phosphate; DMI, dry matter intake; NEL intake, net energy intake; NEL/kg DM, net energy per kg DM feed; ECM, energy corrected milk; BW, body weight; BCS, body condition score
Coefficients of correlation supplied with *, **, or *** are significantly different from random distribution at the P < 0·05, P < 0·01 and P < 0·001 level, respectively
† The indicator variables are listed horizontally and correlated against calculated parameters connected to feed energy, energy output in milk, energetic production of milk, body condition, and weight gain relative to energy uptake. Calculations are performed on (balanced) pooled milk samples, n = 192
Samples analysed for standard variables (milk protein, lactose, fat, and SCC) and the variables tested in this study (urea, uric acid, BHBA, glucose, Glu6P, cholesterol, and TAG) were analysed for correlation mutually and against basic parameters, i.e. DIM, milk yield, and h since last milking. Several milk constituents were correlated positively to DIM: fat, TAG, cholesterol, protein, and BHBA whereas Glu6P content decreased with DIM. Fat, TAG, cholesterol, lactose, and protein were inversely correlated with milk yield. Milk fat was positively correlated to protein, BHBA, cholesterol, and TAG, while glucose was inversely correlated to BHBA and Glu6P. The details are shown in Table 6.
Table 6. Correlations between indicators in the milk and basic milking data†

Coefficients of correlation supplied with *, **, or *** are significantly different from random distribution at the P < 0·05, P < 0·01 and P < 0·001 level, respectively.
† Milk samples were analysed for standard variables and urea, uric acid, β-hydroxybutyrate (BHBA), glucose, glucose-6-phosphate (Glu6P), triacylglyderides (TAG) and cholesterol (Chol) and correlated (Pearsons correlation, r) mutually and to basic milking data (milk yield for the actual milking; h since last milking (time); and days in milk (DIM), n > 820
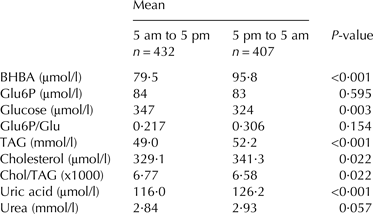
Milking in the AMS resulted in evenly distributed milkings during 24 h as indicated in Fig. 1, giving diurnal variation in the concentration of BHBA in milk. Glucose reveals a significantly higher concentration during day time (5·00 am to 5·00 pm) whereas other constituents (BHBA, TAG, cholesterol, uric acid) revealed a higher content during night time (5·00 pm to 5·00 am) (Table 7).

Fig. 1. Effect of time of milking on level of BHBA (μmol/l) in milk.
Table 7. Effect of time of milking on content of milk metabolites†

Glu6P, glucose-6-phosphate; Glu, glucose; TAG, triacylglyceride; Chol, cholesterol
† Voluntary milking was distributed even throughout 24 h (n = 840). 432 milk samples were harvested in the day time (5 am to 5 pm), while 407 samples were harvested during the evening/night (5 pm to 5 am). Differences between means of milk metabolites were tested by two-tailed t-test
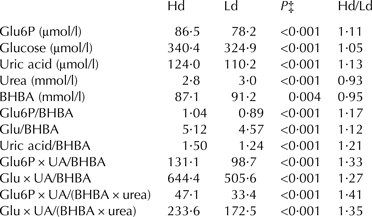
Table 8 show potential indices for discrimination between dietary treatments (status of the dairy cow). The table represents an elaboration of results shown in table 4. By combining milk indicators it is possible to obtain indices with greater potential for discrimination under practical conditions.
Table 8. Comparison between milk variables in high and low digestible rations†

Glu6P, glucose-6-phosphate; Glu, glucose; UA, uric acid
† Milk variables in a high digestible ration (Hd) were compared to the content in a low digestible ration (Ld) and ratios between the variables were elaborated. Values in the table are multiplied / divided irrespective of nature or size of the variables
‡ P values for main effect of digestibility, main model
Discussion
The higher digestibility of the feed resulted in a higher energy density per kg feed DM; this combined with an increased DMI resulted in a higher energy supply and higher milk yield, lower milk fat concentration, and higher protein and lactose concentration (Alstrup et al. Reference Alstrup, Weisbjerg, Hymøller, Larsen, Lund and Nielsen2014). This higher energy supply further affected minor components in milk, and concentrations of Glu6P, glucose, and uric acid increased, and BHBA, TAG, and urea decreased. Despite the clear effects of forage digestibility and thereby energy supply on most minor milk components when analysed as main treatment effect (Table 4), the overall correlations between energy intake and some of the same milk components in Table 5 conflicted to this in both sign and significance. Although there was a significant difference between treatment means, the BHBA level of milk samples were very moderate and below levels seen for ketosis or energy deficiency (Larsen & Nielsen, Reference Larsen and Nielsen2005), and none of the animals showed signs of imbalance. As stated in Table 3, lactation stage (DIM) in this experiment was mainly mid- and late lactation, not in the critical early lactation period where animals risk negative energy balance (Grummer, Reference Grummer1993; Drackley, Reference Drackley1999). The present treatments obviously have affected the energy status of the cows, however, they were all in positive balance. The practiced ad libitum feeding may have been contributing to prevent negative energy balances, also in the Ld group where feeding resulted in a decreased ECM yield.
As treatments affected the energy supply to the cow (Table 2) it was hypothesised that the milk metabolites could be used as indicators of the nutritional status of the cow. In this study BW gain was used as expression of the energy status. The correlation between indicators (BHBA, cholesterol, and cholesterol/TAG) and energy status measured as BW gain per d were weak. Therefore these indicators would apparently not be reliable indicators for energy status in practice. Further, separate random regression analysis indicated that lactation stage (DIM) could account for most of the correlation between the mentioned milk indicators and BW gain.
Body condition score is normally assessed by a visual scoring (Ferguson et al. Reference Ferguson, Galligan and Thomsen1994), which is both resource demanding and subjective, this fact hampers the use of BCS in management. Therefore, objective and automatic methods are desirable. The immediate statistical analyses showed a strong positive correlation between certain milk indicators (BHBA, cholesterol, cholesterol/TAG and cholesterol/milk fat) and energy status (BCS). The observed positive correlation between TAG and BCS and between cholesterol and BCS indicates that fatter cows deliver more fat milk. Similar observations were made by Roche et al. (Reference Roche, Friggens, Kay, Fisher, Stafford and Berry2009) who also found that milk fat content increased with increasing BCS at calving. However, as is the case for BW gain, the association between milk variables and BCS could mainly be explained by the correlation with DIM. These findings, however, imply that there are some possibilities in the use of milk metabolites for estimation of the energy status of the cow at a specific, comparable time point, and that eventual models should account for DIM.
As indicated in Table 3, the milk variables introduced here are found in a broader range than the traditionally measured milk variables. This circumstance potentially allows for a more sensitive description of the energy status inside the mammary cells. However, the present differences between absolute levels of the metabolites under consideration (Table 4) would hardly be distinguishable if analysed in-line by more imprecise (and robust) analytical equipment. Furthermore, bigger differences between means would be necessary if individual milk metabolites were chosen. In this situation it is tempting to strengthen the utilisation of milk metabolites as indicators under similar conditions. It is possible to use more than one metabolite as an indicator and develop an index of compounds, preferentially compounds that react oppositely to energy load. Moyes et al. (Reference Moyes, Larsen and Ingvartsen2013) tested this approach for blood variables. We tested this approach for milk metabolites. In Table 8 the ratio between glucose in milk from Hd and Ld (across CP level) is 1·05. By dividing the glucose level by the BHBA level for the different groups, the ratio became 1·10, and further, Glu × uric acid/BHBA was 1·23 and Glu × uric acid/(BHBA × urea) was 1·32. Consequently, the more (valuable) indicators incorporated into an index the greater the variation in the index values and thereby better possibility to describe differences between means.
The present study found a moderate positive correlation between free glucose and lactose which obviously reflects the conditions in the mammary cells i.e. uptake of glucose from the circulating blood system, the utilisation of glucose in lactose synthesis (various steps) and the secretion of lactose and glucose to the milk. In addition, a moderate inverse correlation between Glu6P and lactose was observed, and may relate to lactose synthesis as well as milk production since Glu6P is a precursor for lactose synthesis and also serves as an intermediate in glycolysis and the pentose phosphate cycle in the mammary cell. The fact that time since last milking was not positively correlated to free glucose and Glu6P is from an analytical and physiological point of view very important. If free glucose in milk simply was a consequence of hydrolysis of lactose or other oligosaccharides post secretion, longer deposition in the udder would be likely to enhance hydrolysis, resulting in higher concentrations of free glucose. However, that was not the case, suggesting that free glucose in milk is ascribable to conditions in the epithelial cells prior to secretion.
The present investigation may contribute to further focus on milk uric acid as an indicator of cow status. Giesecke et al. (Reference Giesecke, Ehrenreich, Stangassinger and Ahrens1994) found a significant relationship between energy intake and uric acid excretion in milk; the present study found a relationship between energy density in feed and milk urate by revealing markedly different levels between highly digestible feeds vs. low digestible feeds. The increased level of uric acid seen in cows provided with higher feed energy concentration presumably implies an increased microbial synthesis in the rumen (Timmermans et al. Reference Timmermans, Johnson, Harrison and Davidson2000). Moreover, it has been suggested that purine derivatives are metabolised to uric acid in the mammary cells also, and that the use of milk uric acid (and other purine derivatives) up till now has not been satisfactory as an indicator of duodenal flow of purine bases (González-Ronquillo et al. Reference González-Ronquillo, Balcells, Belenguer, Castrillo and Mota2004; Tas & Susenbeth, Reference Tas and Susenbeth2007). This situation could partly be due to the high diurnal variation found in the present study, which in turn may be a result of activity and eating habits. Further studies might be relevant to elucidate the situation.
Conclusion
If milk indicators of energy status and energy balance are to be useful as a management tool in practice, they need to correlate highly to the response in question, and exhibit no major systematic bias that cannot be accounted for. Apparently, DIM and also diurnal variation systematically influence several of the milk variables presented here. Therefore, to counteract this, stage of lactation and possibly time of the day should be incorporated in predictive models to be useful as management tools within the herd. Further research in this area is warranted to confirm or reject the potential of milk metabolites as predictors of practical energy status and balance expressed by ECM, BCS and weight gain per day and derived parameters.
Jens Clausen and Carsten Berthelsen are kindly acknowledged for their skillful technical assistance during the course of the work. Further we would like to thank the barn staff at DCRC for skillful assistance during the experiment. This work has been supported by the Danish National Advanced Technology Foundation, Lattec I/S, the Danish Ministry of Food, Agriculture and Fisheries, and the Danish Cattle Federation.