The mammary gland (MG), the most unique feature of mammals and milk, has a vital role in growth of the offspring at their early stage of life. Production of lactose in the MG is unique to mammals and serves as the main source of carbohydrate in the nutrition of the young. Lactose is synthesised in the Golgi apparatus of mammary epithelial cells (MEC) by enzymatic condensation of glucose with UDP-galactose, which is derived from glucose. Glucose (Glu) also serves as the main source for production of ATP and NADPH, which are essential for sustaining MG metabolism and synthetic capacity (Mepham, Reference Mepham1987). Energy demands of mammalian females can be five to seven folds higher than in non-lactating dams in mice and modern cows (Hammond & Diamond, Reference Hammond and Diamond1994; Kadzere et al. Reference Kadzere, Murphy, Silanikove and Maltz2002).
Milk secretion is regulated by milk-borne negative feedback regulation system that responds to abiotic influences, such as milking frequency and heat stress (Silanikove et al. Reference Silanikove, Merin and Leitner2006, Reference Silanikove, Shapiro, Silanikove, Merin and Leitner2009) and biotic effects, such as mammary gland infection (Leitner et al. Reference Leitner, Merin and Silanikove2011a; Silanikove et al. Reference Silanikove, Rauch-Cohen, Shapiro, Arieli and Leitner2012). Casein derived peptides from plasmin activity activate the negative feed-back control on milk secretion and alter milk coagulation properties, which is mainly reflected in reduction of lactose concentration in the milk (Silanikove et al. Reference Silanikove, Merin and Leitner2006; Leitner et al. Reference Leitner, Merin and Silanikove2011a).
Earlier studies have shown that the concentration of metabolites produced by MEC in milk of humans (Arthur et al. Reference Arthur, Kent and Hartmann1987; Neville et al. Reference Neville, Hay and Fennessy1990) and ruminants (Faulkner et al. Reference Faulkner, Chaiyabutr, Peaker, Carrick and Kuhn1981; Faulkner & Peaker, Reference Faulkner and Peaker1982; Peaker & Faulkner, Reference Peaker and Faulkner1983) are closely associated with milk secretion. These earlier studies have highlighted the potential of Glu and citrate concentrations in milk for predicting changes in milk yield. More recently, it has been shown that conversion of mammary gland metabolism to glycolysis at the expense of mitochondrial metabolism is the main metabolic mechanism that translates the milk-borne negative feedback signals into reduced MEC activity and milk secretion (Silanikove et al. Reference Silanikove, Rauch-Cohen, Shapiro, Blum, Arieli and Leitner2011, Reference Silanikove, Rauch-Cohen, Shapiro, Arieli and Leitner2012, Reference Silanikove, Merin, Shapiro and Leitner2013). Thus, these studies have highlighted the potential role of metabolites produced during glycolysis, such as lactate and malate to predict changes in MEC activity and milk quality.
Subclinical mastitis (SCM) is the prevailing form of mastitis among cows, sheep and goats worldwide. The effect of coagulase-negative staphylococci (CNS) on milk yield (MY) at the whole cow level is relatively minor, most likely due to compensation effect by the uninfected glands. However, the effect of SCM by CNS on milk quality for cheese production is significant and is reflected at the whole cow level (Leitner et al. Reference Leitner, Krifucks, Merin, Lavi and Silanikove2006; Pyorala & Taponen, Reference Pyorala and Taponen2009). SCM associated with infection of Escherichia coli and Streptococcus spp. (Strep.) were found to have more influence than CNS infections in terms of their effects on MY and milk clotting parameters in goats, sheep and cows (Leitner et al. Reference Leitner, Krifucks, Merin, Lavi and Silanikove2006; Merin et al. Reference Merin, Fleminger, Komanovsky, Silanikove, Bernstein and Leitner2008).
Recently, it was reported that milk of cows which were previously clinically infected by Esch. coli and currently are free of bacteria was high in somatic cell count (SCC) and that the cow's MY was lower than could be expected, compared with previous MY data (Blum et al. 2014).
An ability to separate on-line milk of dairy cows based on its quality for cheese production was recently demonstrated (Leitner et al. Reference Leitner, Lavi, Merin, Lemberskiy-Kuzin and Katz2011b, Reference Leitner, Merin, Lemberskiy-Kuzin, Bezman and Katz2012, Reference Leitner, Merin, Jacoby, Bezman, Lemberskiy-Kuzin and Katz2013), emphasising the applicative benefit from in-advance information on the factors that rule milk quality; each increase of knowledge in that line may contribute to additional improvement in separating milk according to its quality.
The aims of the present study were to test the hypotheses that changes in milk secretion and clotting parameters of SCM infected cows are reflected by significant parallel changes in the concentration of lactose, citrate, lactate, malate, Glu and Glu-6-p in the milk of CNS-infected cows as well as in PIEc cows.
Materials and methods
Animals
Milk from four groups of high producing Israeli Holstein dairy cows (∼11 000 l milk during 305 d) free of infection (10) or infected in a single gland (quarter), with Streptococcus dysgalactiae (7); CNS, mainly Staphylococcus chromogenes (11), or ∼30 d post-clinical infection with Esch. coli (13) were selected for the study. The latter cows were free of bacterial infection at time of sampling and were defined as post-infected Esch. coli cows (PIEc). The cows were milked thrice daily (05:00, 13:00 and 20:00) in a double-sided herringbone milking parlor equipped with AfiFarm Herd Management Software using on-line Afilab milk analyser (Afimilk, Afikim, Israel), including milk conductivity (arbitrary units).
Analyses were carried at the cow level (including all four glands) or at the gland level.
A cow's udder infection was verified by three consecutive weekly bacteriological testing (Oliver et al. Reference Oliver, Gonzalez, Hogan, Jayarao and Owens2004; Leitner et al. Reference Leitner, Merin, Jacoby, Bezman, Lemberskiy-Kuzin and Katz2013). Milk samples were analysed for gross composition using the Milkoscan FT6000 (Foss Electric) and SCC by Fossomatic 360 (Foss Electric, Hillerød, Denmark) at the Israel Cattle Breeders’ Association Laboratory (Caesarea, Israel). Curd firmness (CF, Volts) and rennet clotting time (RCT, sec) were determined using the Optigraph (Ysebaert, Frepillon, France). Samples of 10 ml milk were placed in the wells and equilibrated at 30 °C before adding 0·5 ml Fromase 15 TL coagulating enzyme (Gist-Brocades nv, Delft, The Netherlands) diluted 1 : 100, aimed at achieving clotting within ∼1000 s.
The concentrations of citrate, lactate, malate, Glu and Glu-6-p were determined in whole milk samples by classical enzymatic reactions that use dehydrogenases (NAD+-depended oxidoreductases) that are coupled to conversion of NAD+ into NADH+H+. The last stage in these determinations was linked to formation of fluorochromophore (resorufin) coupled to conversion of NADH to NAD+ by diaphorase as follows:
The concentration of lactate was carried out according to Shapiro & Silanikove (Reference Shapiro and Silanikove2010). The concentration of citrate and malate was carried out according to Shapiro & Silanikove (Reference Shapiro and Silanikove2011). The concentration of Glu was carried out according to the reactions conditions described by Hicks & Carey (Reference Hicks and Carey1968) and that of Glu-6-p according to Zhu et al. (Reference Zhu, Romero and Petty2009).
Statistical analyses
All statistical analyses were carried out with JMP software (SAS, 2002).
I. Udder level analysis. The experimental unit was a cow. The analysed parameters were the 5 measurements determined for each cow: fat, protein, lactation number, SCC (×103) and log SCC. The effect of the bacterial status on the analysed parameters was determined by a one-way ANOVA in a random design. The statistical model was:
where: μ=Mean of all data, αi=The difference between the bacterial status i from the trial mean, e ij=Residual variance between measurements (Random Error). Multiple comparisons between bacterial statuses were done by Tukey HSD T-test. $$Y_{ij} = {\rm \mu} + {\rm \alpha} _i + e_{ij}$$
$$Y_{ij} = {\rm \mu} + {\rm \alpha} _i + e_{ij}$$II. Gland level analysis. The experimental unit was a gland. The analysed parameters were all the measurements determined for each gland: SCC, log SCC, Fat, Protein, Lactose, RCT, CF, lactic acid (LA), malic acid (MA), LA+MA, citric acid (CA), CA/LA+MA, Glucose, Glu-6-p, G-6-p/G and Urea. The effect of the bacterial status on the analysed parameters was determined by a one-way ANOVA in a random design with above described Model with the addition of the quarter level effect. Multiple comparisons between bacterial statuses were done by Tukey HSD T-test.
Linear correlation analyses were determined between the following parameters: CA, CA/LA+MA, glucose, lactose, SCC, Glu-6-p and Glu-6-p/g ratio and CF.
Results
Cow level
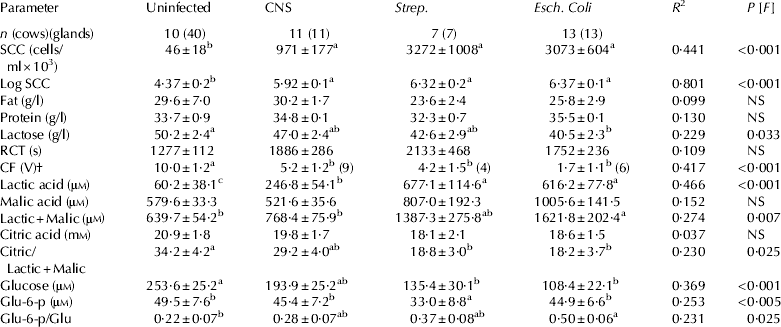
No significant differences were found on the effect of lactation number and days in milk among the 4 groups. In comparison with uninfected cows, PIEc cows had the lowest MY (80% of control), whereas in cows infected with CNS and Strep. the reduction in MY was moderate (∼95% of the control). Conductivity was inversely related to MY and the highest level was recorded in PIEc cows (P<0·05), whereas the lowest levels were recorded in the uninfected cows. However, it did not significantly differ from values measured in cows infected with CNS and Strep. The SCC significantly increased in all infected cows, which was moderate in the CNS infected cows (682×103 cell/ml) and exceeded well above a million in Strep. infections and ∼2 million in PIEc cows (Table 1).
Table 1. Effect of subclinical mastitis by coagulase negative staphylococci (CNS) and Streptococci (Strep.) and previous clinical infection with Escherichia coli (Esch. coli) on milk yield, milk conductivity and somatic cell count (SCC) on the whole cow level (mean±SE)

a,b,cValues marked by different superscript letters are statistically significant. The significance of the main (treatment; P [F]) effect is indicated on the right column
Quarter level
The increases in SCC were ∼20 fold higher in milk of glands infected with CNS and ∼60 times higher in the milk of glands infected with Strep. and PIEc cows. Fat and protein concentrations in milk from glands infected with Strep. were the lowest, but no significant differences were found between groups. Lactose concentration was consistently lower in infected glands in comparison with uninfected ones, ranging from 6·4% (CNS), 15·0% (Strep.) and 19·3% (PIEc cows)(Table 2). Bacterial infection was associated with non-coagulating milk samples that ranged from 0 of 10 in uninfected glands, 2 of 11 in cows infected with CNS, 3 of 7 in cows infected with Strep. and 7 of 13 in PIEc cows. Rennet clotting time and CF were determined only in milk that coagulated. Rennet clotting time consistently increased (but not significant) and CF consistently and significantly decreased in the infected glands in comparison with the uninfected ones, though the effect was much more prominent in the case of infection with Strep. and in PIEc than in CNS (Table 2).
Table 2. Effect of subclinical mastitis by coagulase negative staphylococci (CNS) and Streptococci (Strep.) and previous clinical infection with Escherichia coli (Esch. coli) on somatic cell count (SCC), gross milk composition (fat, protein and lactose), rennet clotting time (RCT), curd firmness (CF) and the concentrations of lactate, malate and citrate on a gland level (mean±SE)

a,b,cValues marked by different superscript letters are statistically significant. The significance of the main (infection; P [F]) effect is indicated on the right column
† In parentheses is given the number of glands that their milk coagulated
Bacterial infection was consistently associated with increased concentration of LA in milk of infected glands. The effect of CNS was significantly higher in comparison with the control, but it was more prominent and significant in Strep. and in PIEc infected cows. The concentration of MA increased in the case of Strep. and PIEc infected cows but not in CNS. In case of Strep. and PIEc infections, CA decreased ∼10%, but the effect was not significant. As a result, bacterial infection was consistently associated with decreased ratio of CA/LA+MA and though the effect of CNS on this ratio was significant, it was much more prominent in the case of Strep. and PIEc infected cows (Table 2). Bacterial infection was consistently associated with a significant decrease in the concentration of Glu in milk of infected glands compared with uninfected ones. Though the effect of CNS on Glu concentration was significant, it was much more prominent in the case of Strep. and PIEc infected cows. The concentrations of Glu-6-p in milk were always lower than Glu concentrations, but no significant differences in its absolute levels were found between the groups. The ratio between Glu-6-p and Glu ranged from 0·22 in uninfected glands and 0·28 (CNS), 0·237 (Strep.) to 0·50 in PIEc cows, the response in the PIEc cows being significantly different in comparison with the controls (Table 2).
Discussion
Earlier evidences showed similarity and parallel changes between concentrations of some of MEC metabolites in milk and in the MEC cytoplasm. The concentration of Glu in milk decreased under starvation and increased under hormonal treatment with growth hormone and IGF-1 and was closely related to milk yield (Faulkner et al. Reference Faulkner, Chaiyabutr, Peaker, Carrick and Kuhn1981; Faulkner & Peaker, Reference Faulkner and Peaker1982; Peaker & Faulkner, Reference Peaker and Faulkner1983; Arthur et al. Reference Arthur, Kent and Hartmann1987; Mepham, Reference Mepham1987; Neville et al. Reference Neville, Hay and Fennessy1990). The data reported herein suggest that lactose and minor milk constituents may provide a simple method for following mammary gland metabolism in vivo as well as for providing additional data on secretory mechanisms and milk clotting parameters. Recent reports showed that lactose concentration is a superior indicator than SCC in reflecting reduction in MY and milk clotting parameters under SCM and that when lactose concentration in milk drops to the critical level of 4% or lower, the milk does not coagulate at all (Leitner et al. Reference Leitner, Merin and Silanikove2011a). Consistently, in the PIEc cows where lactose levels dropped to ∼4%, relatively large proportion (∼50%) of milk samples did not coagulate, whereas the curd of those that did coagulated was weak.
The close correlation between lactose concentration and milk clotting parameters appears puzzling at first view, since no apparent direct chemical relation between lactose concentration and casein coagulation exist. However, this phenomenon can be explained by the findings that casein-derived peptides that serve as negative-feed-back control of milk secretion by limiting lactose secretion are also involved in impeding casein coagulation (Leitner et al. Reference Leitner, Merin and Silanikove2011a).
The increase in LA and MA concentrations and the inverse decrease in CA concentration in the infected glands and in the PIEc cows in comparison with the uninfected glands were mirrored by reduction in a milk-reflected mitochondrial/cytosol metabolic index (MRM/CI, i.e., the CA/LA+MA ratio in milk). The reduction in MRM/CI, which resemble the behaviour of cancer cells (the Warburg-like effect), suggests that the metabolic shift observed in SCM and PIEc cows is similar to that induced following lipopolysaccharide challenge (Silanikove et al. Reference Silanikove, Rauch-Cohen, Shapiro, Blum, Arieli and Leitner2011) and drying-off cows (Silanikove et al. Reference Silanikove, Merin, Shapiro and Leitner2013) and explains the simultaneous reduction in lactose secretion and milk yield.
A central metabolite in MEC is Glu and up to 85% of whole-body Glu turnover is used by MEC for milk synthesis in high producing dairy cows (Cherepanov et al. Reference Cherepanov, Danfær and Cant2000), which is in accordance with the assumption that Glu concentration in milk reflects its concentration in MEC cytoplasm (Faulkner et al. Reference Faulkner, Chaiyabutr, Peaker, Carrick and Kuhn1981; Faulkner & Peaker, Reference Faulkner and Peaker1982; Peaker & Faulkner, Reference Peaker and Faulkner1983). The present results show significant linear correlations among Glu, MRM/CI, lactose and CF (Table 3). The decrease in the MRM/CI by up to 45% in PIEc cows reflects quite a significant drop in ATP yield and overall metabolism by MEC, which eventually should be reflected by reduced uptake of Glu from the blood (Cherepanov et al. Reference Cherepanov, Danfær and Cant2000).
Table 3. Linear correlations among glucose (Glu), lactose, glucose-6-phospate (Glu-6-p), citrate/lactate+malate (CA/LA+MA), Glu-6-p/Glu, log SCC and CF. All the data set on the gland level was applied
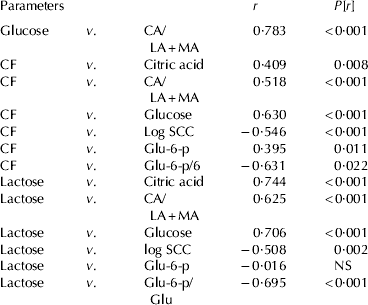
The increased Glu-6-p to Glu ratio in milk indicates that in SCM and PIEc cows larger proportion of Glu was committed for glycolysis, and thus with diminished availability of ATP and NADPH for synthesis of lactose and fat. The reduction of Glu-6-p concentration in milk was particularly evident in cows infected with Strep., which is consistent with the more severe reduction in milk fat and protein concentrations in those glands. Our results suggest that Glu-6-p/Glu ratio may serve as a milk-derived glycolytic index. Negative linear relations among that index and Glu, lactose and CF are consistent with this hypothesis (Table 3).
In general, the present results are consistent with some recent publications showing that mammary gland inflammation induced significant rise in Glu concentration in blood plasma and is reflected by maintaining relatively high production rate of Glu (Vernay et al. Reference Vernay, Wellnitz, Kreipe, van Dorland and Bruckmaier2012). Thus, a rise in plasma concentration of Glu, despite the quite significant reduction in extraction of Glu by the mammary gland may be regarded as a protective sparing mechanism (Silanikove et al. Reference Silanikove, Rauch-Cohen, Shapiro, Blum, Arieli and Leitner2011; Vernay et al. Reference Vernay, Wellnitz, Kreipe, van Dorland and Bruckmaier2012). Usually SCM is a chronic situation that reflects balance between the inability of the host to eradicate the invading pathogen and its ability to localise the infection to the mammary gland where the pathogens do not impose a life threatening situation. Supporting chronic up-regulation of the glandular immune system, as reflected here by the marked increase in SCC in milk of the SCM-infected glands and PIEc cows require metabolic resources. Thus, by maintaining high Glu production rate and reduction in extraction of Glu from blood plasma by MEC, the host can divert the spared Glu to support the increased demand of the immune system for energy.
The significant increase in milk conductivity in the PIEc cows, which principally reflects influx of electrolytes into the gland lumen, suggest that it might be related to disruption of tight junctions due to necrotic damage to the alveolar epithelial cells that might occur during the acute phase of the infection. It is well known that such damage is typical to acute infection by Esch. coli (Zhao & Lacasse, Reference Zhao and Lacasse2008). As milk is iso-osmotic to blood, large increase in electrolytes concentration should be accompanied by parallel reduction in lactose concentration (Holt, Reference Holt1993), as indeed found in the PIEc cows. In the present report, it is confirmed that previous infection with Esch. coli, which developed into clinical mastitis (i.e., PIEc cows), has sizeable long-lasting negative effects on milk composition, SCC and milk clotting parameters (Blum et al. Reference Blum, Heller and Leitner2013). The biological and economical importance of this finding relates to the fact that infection with Esch. coli is one of the most prevailing causes of clinical mastitis throughout the world. Thus, more research is needed in order to understand the physiological basis of this long-lasting response and to define its extent: months, whole lactation or for the rest of the cow's production life.
Conclusions
Milk-related respiratory index (the ratio between the concentrations of citrate/lactate+malate in milk) and the concentrations of Glu and Glu-6-p and the ratio between Glu-6-p and Glu (milk-derived glycolitic index) in milk are effective tools in predicting glandular changes in milk coagulation parameters under SCM caused by CNS, Sterp. and post-clinical consequences of clinical infection with Esch. coli and to delineate between the different effects induced by those microbes. The changes in concentration of MEC metabolites in milk reflect reduction in Glu availability for lactose synthesis and shift to glycolytic metabolism at the expense of mitochondrial metabolism. The parallel reduction in milk coagulation properties is important in preventing uncontrolled inflammation in the mammary gland lumen under milk stasis.