Whey is a by-product of cheese production and casein manufacture in the dairy industry. The disposal of whey represents a major source of environmental pollution because of its high organic load and the large amounts produced. Furthermore, the high nutritional value of this by-product has challenged the industry to develop uses for it. Since whey is a source of lactose and other nutrients essential for microbial growth, recent research has focused on the development of technologies that employ whey as a raw material for producing foods or food additives of added value (Panesar et al. Reference Panesar, Kennedy, Gandhi and Bunko2007).
Kefir is a sour milk slightly carbonated with a low alcohol content that is obtained through the use of kefir grains. These grains are clusters of lactic- and acetic-acid bacteria along with yeasts in a structural matrix of polysaccharides and proteins. The microorganisms present are responsible for the lactic, acetic, and alcoholic fermentation of milk that yields a product with characteristic organoleptic properties (Garrote et al. Reference Garrote, Abraham, De Antoni, Mozzi, Raya and Vignolo2010).
Since certain yeasts along with the LAB from kefir are able to utilise lactose, whey has been proposed as a substrate for the production of various bioproducts in order to lead to its increased market value. The cultivation of kefir microorganisms in whey has been suggested for producing alcohol (Athanasiadis et al. Reference Athanasiadis, Boskou, Kanellaki, Kiosseoglou and Koutinas2002; Kourkoutas et al. Reference Kourkoutas, Psarianos, Koutinas, Kanellaki, Banat and Marchant2002; Koutinas et al. Reference Koutinas, Athanasiadis, Bekatorou, Psarianos, Kanellaki, Agouridis and Blekas2007), baker's yeast (Harta et al. Reference Harta, Iconomopoulou, Bekatorou, Nigam, Kontominas and Koutinas2004; Plessas et al. Reference Plessas, Pherson, Bekatorou, Nigam and Koutinas2005, Reference Plessas, Bekatorou, Kanellaki, Koutinas, Marchant and Banat2007), polysaccharides (Rimada & Abraham Reference Rimada and Abraham2001, Reference Rimada and Abraham2003, Reference Rimada and Abraham2006), cell proteins (Paraskevopoulou et al. Reference Paraskevopoulou, Athanasiadis, Kanellaki, Bekatorou, Blekas and Kiosseoglou2003; Koutinas et al. Reference Koutinas, Athanasiadis, Bekatorou, Iconomopoulou and Blekas2005), fermented beverages (Athanasiadis et al. Reference Athanasiadis, Paraskevopoulou, Blekas and Kiosseoglou2004; Assadi et al. Reference Assadi, Pourahmad and Moazami2008; Magalhães et al. Reference Magalhães, Pereira, Nicolau, Dragone, Domingues, Teixeira, de Almeida Silva and Schwan2010, Reference Magalhães, Dragone, Gilberto, de Melo Pereira, Oliveira, Domingues, Teixeira, Almeida Silva and Schwan2011a, Reference Magalhães, Dias, de Melo Pereira, Oliveira, Domingues, Teixeira, de Almeida e Silva and Schwanb), starters for cheese production (Kourkoutas et al. Reference Kourkoutas, Kandylis, Panas, Dooley, Nigam and Koutinas2006; Dimitrellou et al. Reference Dimitrellou, Kourkoutas, Banat, Marchant and Koutinas2007, Reference Dimitrellou, Kourkoutas, Koutinas and Kanellaki2009; Katechaki et al. Reference Katechaki, Panas, Rapti, Kandilogiannakis and Koutinas2008, Reference Katechaki, Panas, Kourkoutas, Koliopoulos and Koutinas2009; Koutinas et al. Reference Koutinas, Papapostolou, Dimitrellou, Kopsahelis, Katechaki, Bekatorou and Bosnea2009, Reference Koutinas, Bekatorou, Katechaki, Dimitrellou, Kopsahelis, Papapostolou, Panas, Sideris, Kallis, Bosnea, Koliopoulos, Sotiropoulos, Panteli, Kourkoutas, Kanellaki and Soupioni2010), protein hydrolysates (Ferreira et al. Reference Ferreira, Pinho, Monteiro, Faria, Cruz, Perreira, Roque and Tavares2010), and antimicrobial compounds (Londero et al. Reference Londero, Quinta, Abraham, Sereno, De Antoni and Garrote2011).
Recent studies have contributed to an understanding of the chemical changes that occur during the fermentation of whey by kefir cultures such as proteolysis, lactose consumption, and organic-acid and volatile-compound production (Athanasiadis et al. Reference Athanasiadis, Paraskevopoulou, Blekas and Kiosseoglou2004; Ferreira et al. Reference Ferreira, Pinho, Monteiro, Faria, Cruz, Perreira, Roque and Tavares2010; Golfinopoulos et al. Reference Golfinopoulos, Kopsahelis, Tsaousi, Koutinas and Soupioni2011; Magalhães et al. Reference Magalhães, Dragone, Gilberto, de Melo Pereira, Oliveira, Domingues, Teixeira, Almeida Silva and Schwan2011a, Reference Magalhães, Dias, de Melo Pereira, Oliveira, Domingues, Teixeira, de Almeida e Silva and Schwanb). Whey fermentation by kefir cultures at higher temperatures increases the consumption rate of lactose, the main constituent of whey that contributes to its high organic-pollutant load (Golfinopoulos et al. Reference Golfinopoulos, Kopsahelis, Tsaousi, Koutinas and Soupioni2011). Rimada & Abraham (Reference Rimada and Abraham2001) reported that kefiran, a polysaccharide with immunomodulatory properties, is released into whey in larger amounts at higher incubation temperatures during whey fermentation with kefir grains. Little attention, however, has been placed on the microbiologic composition of kefir grains grown in whey or fermented whey, or on how variations in incubation temperature affect that property. In addition to the chemical changes effected by kefir microflora during whey fermentation, certain LAB and yeasts isolated from kefir have been reported to promote human health (Golowczyc et al. Reference Golowczyc, Mobili, Garrote, Abraham and De Antoni2007, Reference Golowczyc, Gugliada, Hollmann, Delfederico, Garrote, Abraham, Semorile and De Antoni2008; Romanin et al. Reference Romanin, Serradell, González, Lausada, Garrote and Rumbo2010).
Recently, Magalhães et al. (Reference Magalhães, Pereira, Nicolau, Dragone, Domingues, Teixeira, de Almeida Silva and Schwan2010) found that no differences occur in the dominant microbiota of kefir grains and their corresponding fermented beverage when milk is replaced with either whole- or deproteinized-cheese whey as a fermentation substrate. Nevertheless, a critical point to analyse is if the microflora is maintained in kefir grains and the fermentation products after successive subcultures in whey since the constancy of that microbial and chemical composition is of extreme relevance to the implementation of any of the presently considered industrial applications of kefir grains to the fermentation of whey.
The aims of this study were thus to evaluate the microbiological and chemical composition and fermentation capacity of kefir grains after successive subcultures in whey at different incubation temperatures and to analyse the microbiological profile of the fermented products.
Materials and Methods
Kefir grains
Traditional kefir grains CIDCA AGK10, belonging to the collection of the Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA), La Plata, Argentina, were maintained by subculture in milk at 20°C.
Milk and whey fermentation
Commercial nonfat milk (Sancor S.A, Santa Fe, Argentina) and sweet-whey powder (Lactogal S.A., Portugal) reconstituted in water at 100 g/l were used. The composition of reconstituted whey was: 91·3 g humidity and 0·8 g ash per 100 ml and 1 g protein, <0·3 g lipids, and 6·5 g lactose per 100 g, pH 6·3. Kefir grains were added to either milk or whey at a concentration of 100 g/l. The fermentation was conducted for 24 h at 20°C in milk and at 20, 30, or 37°C in whey. The kefir grains were then separated from the fermentation products by filtration through a sieve.
Increment in kefir-grain biomass
After fermentation, the kefir grains were separated by filtration, washed with sterile water, and dried to constant weight between tissue papers. They were then weighed on a Precisa 205A analytical scale (precision ±0·1 mg).
Kefir-grain chemical composition
The water content of the grains was determined after drying them to constant weight at 100°C. The total sugar content was assayed by the anthrone method with glucose as a standard (Southgate, Reference Southgate and Southgate1976). For protein-content determination, 0·5 g kefir grains were heated for 45 min in a boiling-water bath in phosphate buffer (0·1 m KH2PO4, 0·1 m NaOH, pH 7·0±0·2) containing 3 m urea. The protein content was then measured by the Pierce's BCA Protein Assay Reagent Kit (Rockford, Illinois, USA) according to the manufacturer's instructions.
Acidification kinetics
At regular time intervals during a fermentation, samples were taken and the pH measured with a pH meter model pH211 equipped with an HI 1131B microelectrode (Hanna Instrument, USA).
Enumeration of viable microorganisms
Kefir grains were disrupted in a mortar and resuspended in an aqueous tryptone solution (1 g/l). The concentrations of viable microorganisms in the kefir grains and in the kefir-fermented products were determined by inoculating agar plates with serial dilutions in tryptone solution. A differential enumeration was performed on De Man–Rogosa–Sharpe agar (Difco, Detroit, MI 48232, USA) for LAB and on yeast extract–glucose–chloramphenicol agar (Merck, D-64271 Darmstadt, Germany) for yeasts. The results were expressed as the number of colony-forming units (CFU)/g in the kefir grains and CFU/ml in the kefir-fermented products.
DNA extraction from kefir grains
Five different procedures for kefir-grain disruption were evaluated in order to optimise the DNA extraction:
-
1. One gram of kefir grains in 20 ml sterile water were heated in a boiling-water bath for 20 min in order to dissolve the polysaccharide attached to the cells. The cells were recovered by centrifugation for 15 min at 15 000 g in a Sorvall RC-5B plus centrifuge (Sorvall Products, L.P. Newtown, CT, USA) and then resuspended in 10 ml sterile water, heated, and recentrifuged. The resulting pellet was finally resuspended in 1 ml sterile water.
-
2. After the above procedure was carried out, the final resuspended sample in 1 ml water was temperature-shocked by 3 successive alternate 30-s immersions in first liquid nitrogen and then water at 80°C.
-
3. One gram of kefir grains was frozen in liquid nitrogen, disrupted by grinding in a mortar, and collected in 1 ml water at room temperature. This procedure was then repeated.
-
4. One gram of kefir grains was frozen in liquid nitrogen, disrupted by grinding in a mortar, and then suspended in 10 ml water at 80°C.
All samples were next centrifuged for 15 min at 15 000 g in an Eppendorf centrifuge 5430 (Eppendorf, Hamburg, Germany) and the DNA purified by means of an AccuPrep Genomic DNA Extraction kit (BIONEER, Korea). The manufacturer's protocol was either performed directly or after a prior treatment with lytic enzymes. For the enzymatic treatment, samples were resuspended in 100 μl lyticase buffer (0·9 m Sorbitol, 0·1 m Tris-HCl, pH 8, 0·1 m EDTA), and 10 μl lyticase at 2·5 mg/ml (Sigma Chemical, St. Louis, USA) were added. After incubating the mixture for 1 h at 37°C, 40 μl TES buffer (50 mm Tris-HCl, 5 mm EDTA, 50 g sucrose/l, pH 8) and 40 μl lysozyme at 10 mg/ml (Sigma Chemical, St. Louis, USA) were added; and the resulting mixture was incubated for 15 min at 20°C.
The purity and concentration of DNA was determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc, Wilmington, DE, USA).
DNA extraction from fermentation products
Of the kefir-fermented whey or milk, 1·5 ml was centrifuged for 15 min at 10 000 g , the enzymic digestion described above was performed and the DNA extracted by means of the AccuPrep Genomic DNA Extraction kit.
DNA extraction from reference strains
For the DNA extraction of all microbial strains, the AccuPrep Genomic DNA Extraction kit was used according to the manufacturer's protocol. The commercial strains used were: Lactobacillus plantarum DSMZ 20174, Lb. parakefir DSMZ 1055, Lb. kefiranofaciens subsp. kefiranofaciens DSMZ 5016 and JCM 6985, Lb. kefiranofaciens subsp. kefirgranum JCM 8572, Lb. acidophilus DSMZ 20079, Lb. casei DSMZ 20011, Lb. brevis ATCC 8287 and JCM 1059, Lb. acidophilus ATCC 314, Lb. kefir ATCC 8007 and JCM 5818, Kluyveromyces lactis, and Lactococcus lactis subsp. cremoris NZ 9000. The ATCC strains were purchased from the American Type Culture Collection (Manassas, VA 20108, USA), the DSMZ strains from the Deutsche Sammlung von Mikroorganismen and Zellkulturen (Germany), the JCM strains from the Japanese Collection of Microorganisms (Japan), and the NZ strains from the New Zealand Dairy Research Institute Culture Collection (New Zealand). In addition, the following strains previously isolated from kefir grains and belonging to the CIDCA culture collection were used: Lb. plantarum CIDCA 83114 and 8321, Lb. parakefir CIDCA 8328, Lc. lactis subsp. lactis CIDCA 8213, Lb. kefir CIDCA 8348, Acetobacter sp. CIDCA 8431, K. marxianus CIDCA 8154, Sac. unisporus CIDCA 81107, and Sac. cerevisiae CIDCA 8112.
PCR amplification
The Eubacterial-community DNA was amplified with the primers 338fGC (5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCACGG GGG GAC TCC TAC GGG AGG CAG CAG-3′), with the GC clamp underlined, and 518r (5′-ATT ACC GCG GCT GCT GG-3′), spanning the V3 region of the 16S-rDNA gene (Muyzer et al. Reference Muyzer, Waal and Uitterlinden1993). The yeast-community DNA was amplified with the forward primer NL1GC (5′-GCG GGC CGC GCG ACC GCC GGG ACG CGC GAG CCG GCG GCG GGC CAT ATC AAT AAG CGG AGG AAA AG-3′, with the GC clamp underlined, and the reverse primer LS2 (5′-ATT CCC AAA CAA CTC GAC TC-3′), spanning the D1 region of the 26S-rRNA gene (Cocolin et al. Reference Cocolin, Aggio, Manzano, Cantoni and Comi2002).
The PCR reactions were performed in a total reaction volume of 20 μl containing 0·5 μm each of the primer, 2·5 U of Taq DNA polymerase (Inbio Highway, Tandil, Argentina), 2 μl of the buffer supplied with the enzyme (100 mm Tris-HCl, 500 mm KCl, pH 9), 2·5 mm MgCl2, 0·2 mm of each dNTP, and 3 μl of the isolated DNA. The PCR reactions were performed in a MyCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA). For the LAB an initial 5-min denaturation at 94°C was followed by 35 cycles of: a 30-s denaturation at 94°C, a 60-s annealing at 54°C, and a 30-s chain elongation at 72°C; with a final 5-min elongation at 72°C. After an initial 5-min denaturation at 95°C, the DNA from yeasts was amplified over 35 cycles: a 60-s denaturation at 95°C, a 45-s annealing at 60°C, and a 60-s elongation at 72°C, followed by a final 7-min chain elongation at 72°C. Aliquots of the amplification products were analysed by electrophoresis in 10 g/l agarose gels containing ethidium bromide and finally visualised under U.V. light.
Denaturing gradient-gel-electrophoresis analysis
The PCR products were analysed by denaturing-gradient-gel electrophoresis (DGGE) through the use of a DGGE-2401 analyzer (C.B.S. Scientific Co., Del Mar, CA, USA) with gels of 15×20×0·075 cm. The samples were applied to 80 g/l polyacrylamide gels in TAE buffer. An optimal separation was achieved with a 40–60% urea-formamide denaturing-gradient gel (with 100% corresponding to 7 m urea and 40% [v/v] formamide) for Eubacterial and 30–70% for yeast PCR products. Electrophoresis was performed at a constant voltage of 100 mV for 16 h at 60°C. Gels were stained with Syber Gold (Invitrogen, Oregon, USA) and visualised under U.V. light.
Identification of DGGE bands
The DGGE profiles were compared with those of reference strains and sequenced. Each band was excised with a sterile scalpel, eluted in 50 μl TE buffer (50 mm Tris-HCl, 5 mm EDTA, pH 8), and placed at 4°C overnight for DNA diffusion. The DNA was then amplified with the original pair of primers but without the GC clamp. The PCR products were analysed by electrophoresis in 10 g/l agarose gels. The direct sequencing of PCR products was performed on a 3730XLs 23 ABI DNA sequencer and the resulting sequences finally compared with those in the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST) through the BLAST program. Sequences with an identity of 97% or greater were considered to belong to the same species (Stackebrandt & Goebel, Reference Stackebrandt and Goebel1994; Palys et al. Reference Palys, Nakamura and Cohan1997).
Statistical analysis
For statistical comparisons of the microbiologic composition and the biomass increment of the kefir grains, the Student t test was performed at a P-value significance level of <0·05. The results were expressed as the means±standard deviations (sd) of at least three separate duplicate experiments.
Results and Discussion
Suitability of kefir grains as a starter for whey fermentation at different incubation temperatures
In order to determine if kefir grains are a suitable starter for whey fermentation at different temperatures, the weight increment, acidification capacity, and chemical and microbiologic composition of the grains after 20 subcultures in whey were compared with the corresponding properties of the original kefir grains coming directly from milk.
The acidification rate increased with the incubation temperature when the kefir grains were cultured for the first time in whey (Fig. 1a). When the incubation was performed at 30°C and at 37°C, a pH of 4·0 was reached after 2 h incubation. In contrast, when the incubation temperature was 20°C, 4 h were necessary to reach the same pH.

Fig. 1. Acidification capacity of kefir grains at the first subculture (a) and after 20 subcultures (b) in whey. Incubation temperature: 20°C (◯), 30°C (Δ), and 37°C (□)
Twenty subcultures were conducted at each temperature in order to determine if changes occurred in the kefir grains under the new growth conditions. When the fermentation was conducted at 20 and 30°C, the grains maintained their acidification capacity with respect to that of the first culture in whey, but at 37°C this property was notably reduced (Fig. 1a, b). The weight increment at 20°C was constant throughout the subcultures, and the grains furthermore did not change their appearance (Fig. 2). At 20 and 30°C the growth of the grains was similar during the first nine subcultures, but thereafter the grains at 30°C began to grow more slowly and after 20 subcultures had a less granular appearance, a looser consistency, and a greater adhesivity than the original grains. At 37°C the kefir grains failed to increase their biomass, had a smaller size, and were more compact than the grains at the beginning of the trial (Fig. 2).
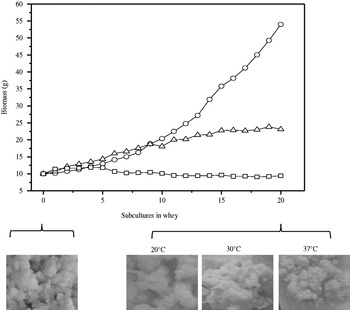
Fig. 2. Biomass increment and visual appearance of kefir grains at the beginning of the assay (original kefir grains) and after 20 subcultures in whey at 20°C (◯), 30°C (Δ), and 37°C (□)
Table 1 shows the microbiologic and chemical composition of the original kefir grains and the same grains after 20 subcultures in whey at the three temperatures. At 20 and 30 °C the microbiologic composition of the grains did not change significantly (P>0·05) from that of the traditional kefir grains, whereas at 37°C the grains contained a significantly (P<0·05) lower concentration of yeasts. Furthermore, significant changes in the chemical composition were observed at 30 and at 37°C: the grains had a lower water content and a higher protein-to-polysaccharide ratio than the original ones. These changes agreed with the macroscopic appearances and weight increments of the grains under the different conditions as indicated in Fig. 2. The kefir grains at 20°C, besides increasing their biomass at a higher rate, retained a protein-to-polysaccharide ratio similar to that present in the original grains from milk A variation in the synthesis of matrix components—mainly the polysaccharide—apparently occurs that is accompanied by a loss in the ability of the grains to increase their weight.
Table 1. Microbiological and chemical composition of kefir grains after 20 subcultures in whey at 3 different temperatures.

Within a given column different superscript letters indicate statistical differences among the data (P<0·05) according to the Student t test.
Kefir grains are conventionally cultured in milk at room temperature, usually between 20 and 25°C. Previous reports had indicated that at higher incubation temperatures the increase in biomass was greater, the ethanol production began earlier (Goršek & Zajsek, Reference Goršek and Zajsek2010), the fermentation was faster, and the lactose consumption increased (Golfinopoulos et al. Reference Golfinopoulos, Kopsahelis, Tsaousi, Koutinas and Soupioni2011). Our results, however, demonstrate that kefir grains do not maintain their chemical and microbiologic composition if they are repeatedly subcultured at 30°C or at 37°C. Thus, to employ kefir grains as a starter in whey fermentation, a temperature of 20°C is highly recommended for their preservation over time.
Microbial diversity of kefir grains grown in whey
Since DGGE fingerprinting and sequencing of 16S rDNA have been described as valuable culture-independent approaches for the analysis of the microbial consortium present in kefir grains (Garbers et al. Reference Garbers, Britz and Witthuhn2004; Wang et al. Reference Wang, Li, Jia, Wu and Guo2006), this method was applied to compare the microbial diversity of the original kefir grains and the grains after 20 subcultures in whey at 20, 30, or 37°C.
The DGGE analysis of the kefir-grain microorganisms was performed with DNA obtained by different DNA-extraction procedures. For bacteria, all the procedures showed the same high-quality DGGE profile, but for yeasts only the procedures with a hot-water step (e.g., 1, 2, and 4; cf. Materials and Methods) followed by lytic-enzyme digestion were successful. Since all three of these procedures showed a satisfactory quality and quantity of DNA, with a 260/280-nm–absorbance ratio at greater than 1·8 and a DNA concentration higher than 70 ng/μl, procedure 1 was selected because of its simplicity.
The bacterial DGGE profile of the kefir grains did not change after successive subcultures in whey at different temperatures (Fig. 3a). For band identification several LAB that had been described as present in kefir were included in the analysis. Bands at the same positions in the DGGE profiles were found for the different strains of the same species with respect to Lb. plantarum (strains CIDCA 83114 and DSMZ 20174), Lb. kefir (strains CIDCA 8348, ATCC 8007 and JCM 5818), and Lb. brevis (strains JCM 1059 and ATCC 8287). Three different species, however, had similar patterns: Lb. plantarum, Acetobacter sp. and Lc. lactis subsp. cremoris (data not shown). Kefir grains shared bands only in the positions of Lb. kefiranofaciens, Lb. kefir, and Lb. parakefir. Therefore, these three strains were included in a separate lane in each run as a reference (Fig. 3, lane RB).

Fig. 3. PCR-based DGGE fingerprints of the original kefir grains (OG) and the kefir grains after 20 subcultures in whey at different incubation temperatures (20, 30 and 37°C) with Eubacteria- (a) and yeast- (b) specific primers. RB: reference strains of lactic-acid bacteria. RY: yeast reference strains
For further identification of DGGE bands a sequence analysis was made. Table 2 shows the results of the search for similar sequences in GenBank, whose findings confirmed the presence of Lb. kefiranofaciens (bands A to G), Lb. parakefir (band K), and Lb. kefir (bands I and J)—along with Lc. lactis (band H) as well—in all the kefir grains.
Table 2. Percent (%) similarity of partial 16S-rDNA sequences to their closest relatives in the NCBI nucleotide-sequence database

The yeast DGGE profile of the kefir grains subcultured in whey differed depending on the incubation temperature (Fig. 3b). In order to identify yeast bands, four reference strains were included in the gel (Fig. 3, lane RY): K. lactis, Sac. unisporus, K. marxianus, and Sac. cerevisiae. In the original kefir grains and after their culture in whey at 20°C the presence of Sac. unisporus (also named Kazachstania unispora), K. marxianus, and Sac. cerevisiae was indicated by band position in comparison with the reference strains (Fig. 3b) and confirmed by band-DNA sequencing (Table 3). A loss or reduction of certain yeast populations was detected after 20 subcultures in whey at 20°C, since the bands a and h, corresponding to K. exigua or K. turciensis, and band c—whose DNA sequences did not align with any available in the GenBank database—were absent in that DGGE profile. A further reduction in the diversity of yeasts was found in grains grown in whey at the higher incubation temperatures. Bands corresponding to Sac. unisporus and K. marxianus were absent from kefir grains when fermentation was conducted at 30°C and at 37°C (Fig. 3b).
Table 3. Percent (%) of similarity of partial 26S-rDNA sequences to their closest relatives in the NCBI nucleotide-sequence database

In agreement with this study, Magalhães et al. (Reference Magalhães, Pereira, Nicolau, Dragone, Domingues, Teixeira, de Almeida Silva and Schwan2010) found the presence of Lb. kefiranofaciens, Sac. cerevisiae, Sac. unisporus, and K. marxianus in kefir grains cultured in whey at temperatures below 30°C. In addition to those species the present experiments detected Lb. kefir, Lb. parakefir, and Lc. lactis.
Although different methods of DNA extraction were assessed here, the microbial diversity found by DGGE analysis was lower than that described through the use of culture methods (Garrote et al. Reference Garrote, Abraham and De Antoni2001; Simova et al. Reference Simova, Beshkova, Angelov, Hristozova, Frengova and Spasov2002; Loretan et al. Reference Loretan, Mostert and Viljoen2003; Witthuhn et al. Reference Witthuhn, Schoeman and Britz2004). For example, bands corresponding to Lb. plantarum and acetic-acid bacteria previously detected in CIDCA kefir grains (Garrote et al. Reference Garrote, Abraham and De Antoni2001) did not appear in the present DGGE profiles. A plausible explanation could be the low concentration of these microorganisms (less than 1% of the total community) or a sufficient resistance to the lytic agent employed in the DNA extraction (Muyzer et al. Reference Muyzer, Waal and Uitterlinden1993; Heuer et al. Reference Heuer, Krsek, Baker, Smalla and Wellington1997). Moreover, the differential amplification of competitor templates from microorganisms that are present in high concentration could disadvantage the detection of certain species. Consistent with our work, Chen et al. (Reference Chen, Wang and Chen2008) showed that several LAB from kefir that had been previously identified by cultivation were not detected by PCR-DGGE and furthermore that bacteria that had not been isolated in culture were, in fact, revealed by DGGE.
Microbiological composition of whey fermented with kefir grains
The beneficial health properties of kefir have been attributed to the presence of probiotic microorganisms and their metabolic products, mainly organic acids, that inhibit pathogenic and food-spoilage microorganisms (Garrote et al. Reference Garrote, Abraham, De Antoni, Mozzi, Raya and Vignolo2010; Dobson et al. Reference Dobson, O'Sullivan, Cotter, Ross and Hill2011). Since, based on the results presented above, the fermentation temperature of 20°C proved to be necessary in order to preserve kefir grains as a continuous starter, we compared the microbiologic composition of whey fermented at this temperature for 24 h with that of the original milk kefir. In fermented whey compared with fermented milk, the number of LAB was significantly lower (1·0±0·7×107 vs. 3·9±3·1×108, respectively; P<0·05) and the number of yeast significantly higher (4·2±1·7×106 vs. 8·8±5·4×105, respectively; P<0·05). The species composition was similar in both fermentation products; the main difference in the DGGE profiles was in the intensity of specific bands, indicating possible variations in the relative amount of certain populations (Fig. 4).
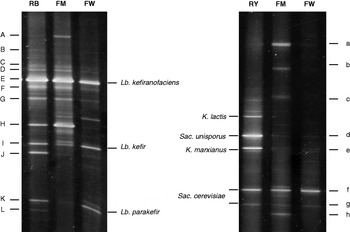
Fig. 4. PCR-based DGGE fingerprints of milk (FM) and whey (FW) fermented with kefir grains at 20°C with Eubacteria- (a) and yeast- (b) specific primers. RB: reference strains of lactic-acid bacteria. RY: yeast reference strains
In both bacterial DGGE profiles, the presence of Lb. kefiranofaciens, Lb. kefir, Lb. parakefir, and Lc. lactis was detected (Table 2). The bands corresponding to Lb. kefir (bands I and J) and Lb. parakefir (band K), however, had less intensity in fermented milk than in fermented whey.
With respect to the yeast DGGE profiles, the presence of Sac. unisporus, K. marxianus, and Sac. cerevisiae was detected in fermented whey and in fermented milk. Just as in the DGGE profiles of the grains, the bands corresponding to K. exigua or K. turicensis (bands a and h) were present in fermented milk but not in fermented whey; while the band corresponding to K. marxianus (band e) had a higher intensity in the latter than in the former.
The greater intensity of bands corresponding to Lb. kefir, Lb. parakefir, and K. marxianus in fermented whey than in fermented milk could be ascribed to a higher level of those species in the whey product since the intensity of an individual band is usually considered to be a semi-quantitative measure of a particular species within the community. Nevertheless, the relationship between the intensity of the DGGE bands and the amount of original DNA template is not always linear. In multi-template PCR certain mechanisms can favour the amplification of specific templates as a result of the properties of those genes, of their flanking sequences, or of the overall genome. This kind of an effect may skew the template-to-product ratios with the result that certain numerically prevalent organisms in the environment are not, in fact, represented by strong bands in DGGE gels (Suzuki & Giovannoni, Reference Suzuki and Giovannoni1996; Polz & Cavanaugh, Reference Polz and Cavanaugh1998).
The Lb. kefiranofaciens identified in fermented whey is considered the main producer of kefiran—with that polysaccharide having, in turn, health-promoting properties (Abraham et al. Reference Abraham, Medrano, Piermaria, Mozzi and Hollingworth2010). In addition, species with probiotic potential such as Lb. kefir, K. marxianus, and Sac. cereviciae were identified here in the fermented whey by DGGE. Certain strains of those species have a potential for abolishing the intestinal inflammatory response and preventing pathogen adhesion and invasion into intestinal cells (Golowczyc et al. Reference Golowczyc, Mobili, Garrote, Abraham and De Antoni2007, Romanin et al. Reference Romanin, Serradell, González, Lausada, Garrote and Rumbo2010). The presence of these microorganisms in whey fermented with kefir grains suggests the potential of this fermentation product as a probiotic.
With respect to organic acids, we found that the production of lactic and acetic acid in whey was similar to that in milk (data not shown). In addition to the presence of potentially probiotic microorganisms, the metabolites produced by those microbes—such as the organic acids with strong inhibitory activity against pathogenic bacteria (Londero et al. Reference Londero, Quinta, Abraham, Sereno, De Antoni and Garrote2011) or the bioactive peptides produced by the bacterial proteolytic activities (Ferreira et al. Reference Ferreira, Pinho, Monteiro, Faria, Cruz, Perreira, Roque and Tavares2010)—could all contribute to the beneficial effects of whey fermented with kefir grains. Nevertheless, more studies will be needed to determine to what extent this product can be utilised for that purpose.
Conclusions
Incubation temperature markedly affects the preservation of kefir grains in whey. When fermentation is carried out repeatedly at 30 or 37°C, the appearance and chemical composition both become altered, a misbalance occurs among the microflora, and the growth of the grains is reduced; all these findings indicating that those higher incubation temperatures are counterproductive.
Only at 20°C are the acidification capacity, the growth, the appearance, and the chemical composition of the kefir grains maintained after 20 successive subcultures in whey. In addition, the bacterial diversity of the grains does not change after those passages, though variations in the relative amounts of certain yeast populations do occur. At this lower temperature the microbiologic composition of the fermented whey is similar to that of fermented milk. A temperature of 20°C is therefore strongly suggested in order to preserve kefir grains as a starter for whey fermentation.
A knowledge of the correct incubation conditions is seen to be relevant in order to achieve a consistent product for the appropriate commercial valorisation of whey fermented by kefir grains.
The authors acknowledge the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina), and the Universidad Nacional de La Plata (UNLP, Argentina) for financial support. A. Londero and M. F. Hamet are doctoral fellows from CONICET, A. G. Abraham and G. L. Garrote are CONICET researchers, and G. L. De Antoni is a researcher of the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC-PBA). The authors wish to thank Dr Donald F. Haggerty, a retired career investigator and native English speaker, for editing the final version of the manuscript.









