In human nutrition, increased consumption of flaxseed is associated with lower incidence of cardiovascular disease, breast and prostate cancers, osteoporosis, and postmenopausal symptoms (Murkies et al. Reference Murkies, Wilcox and Davis1998). The beneficial effects of flax are thought to be partly mediated by its high concentration of a mammalian lignan precursor secoisolariciresinol diglucoside. Upon ingestion, microbial enzymes convert secoisolariciresinol diglucoside to mammalian lignans, mainly enterodiol (ED) and enterolactone (EL; Setchell et al. Reference Setchell, Lawson, Mitchell, Adlercreutz, Kirk and Axelson1980) under the action of colonic microflora, and ED and EL are subsequently absorbed into blood (Borriello et al. Reference Borriello, Setchell, Axelson and Lawson1985). In addition to their health effects, secoisolariciresinol diglucoside and its mammalian lignan metabolites have a high antioxidant activity (Kitts et al. Reference Kitts, Yuan, Wijewickreme and Thompson1999), which is an evidence of a great potential anticarcinogenic mechanism. Unfortunately, mammalian lignan precursors are not yet commercially available on the market and flaxseed is not commonly consumed in North America. However, the intake of lignans can be achieved through the consumption of milk from dairy cows fed flaxseed products. We (Petit & Gagnon, Reference Petit and Gagnon2009) have previously reported a linear increase in milk concentration of EL in cows fed greater amounts of flaxseed but no ED was detected. Lignans in grain are concentrated in the outer fibre-containing layers (Adlercreutz & Mazur, Reference Adlercreutz and Mazur1997), which would lead to higher concentration of secoisolariciresinol diglucoside in hulls than in seeds. Recent in vitro results have shown that the main mammalian lignan metabolite produced from flaxseed hulls by ruminal microbiota was EL while faecal microbiota led mainly to the net production of ED (Côrtes et al. Reference Côrtes, Gagnon, Benchaar, da Silva, Santos and Petit2008). Flaxseed hull is a co-product obtained from flax processing and plant lignans in flax hulls are found in greater concentrations than in whole seeds (32·0 and 9·2 nmol/mg, respectively; Côrtes et al. Reference Côrtes, Gagnon, Benchaar, da Silva, Santos and Petit2008). Feeding flaxseed hulls to dairy cows may then contribute to increase concentration of the mammalian lignan EL in milk.
Monensin, which is an ionophore, has been used extensively in the diet of dairy cows, and its effects on milk production and composition are well documented (Phipps et al. Reference Phipps, Wilkinson, Jonker, Tarrant, Jones and Hodge2000; Duffield et al. Reference Duffield, LeBlanc, Bagg, Leslie, Ten Hag and Dick2003). However, monensin is known to decrease the growth of Gram positive bacteria and some strains of bacteria involved in plant lignan metabolism such as those with β-glucuronidase activity (e.g. Ruminococcus and Eubacterium), which plays important role in the absorption of lignans (Jenab & Thompson, Reference Jenab and Thompson1996) are Gram positive bacteria (Beaud et al. Reference Beaud, Tailliez and Anba-Mondoloni2005). Recent results have shown that the main site for metabolism of flax lignans in dairy cows is the rumen (Gagnon et al. Reference Gagnon, Côrtes, da Silva, Kazama, dos Santos, Zeoula, Benchaar and Petit2009). Supplementation with monensin could then affect ruminal β-glucuronidase activity and the conversion of plant lignans in EL by rumen microbes. Therefore, the main objective of the experiment was to determine the effect of feeding a combination of monensin and flaxseed hulls on ruminal and milk concentration of EL and ruminal and faecal activity of β-glucuronidase. The hypothesis was that monensin supplementation has no effect on the incorporation of EL into milk when cows are fed flaxseed hulls.
Materials and Methods
Cows and diets
Four ruminally fistulated multiparous Holstein cows averaging 665±21 kg of body weight and 190±5 d in milk were assigned to a 4×4 Latin square design balanced for residual effect to determine the effects of monensin and flaxseed hulls supplementation on ruminal and milk concentration of the mammalian lignan EL and ruminal and faecal activity of β-glucuronidase. The experimental diets (Table 1) consisted of four different total mixed diets with a 2×2 factorial arrangement of treatments: 1) control (no flaxseed hulls and no monensin; CO), 2) diet containing 20% (dry matter basis) flaxseed hulls (FH), 3) diet with monensin (16 mg/kg of dry matter; MO), and 4) diet containing 20% (dry matter basis) flaxseed hulls and 16 mg/kg monensin (HM). Flaxseed hull is a product commercially available (Natunola Health Inc; Nepean, ON, Canada). All diets provided equal amounts of crude protein and energy and were formulated to meet nutrient requirements of 615 kg cow producing 29 kg/d of milk containing 3·9% fat (NRC, 2001). Cows were housed in tie stalls, fed individually for ad libitum intake (10% refusals) twice a day (0830 and 1530 h), and milked twice daily at 0800 and 1900 h. Milk production was recorded at every milking. Yield of 4% fat-corrected milk (FCM) was calculated according to the equation of Tyrrell & Reid (Reference Tyrrell and Reid1965). Cows were weighed on the first and last day of each experiment period. Cows were cared for in accordance with the guidelines of the Canadian Council on Animal Care (CCAC, Reference Olfert, Cross and McWilliam1993).
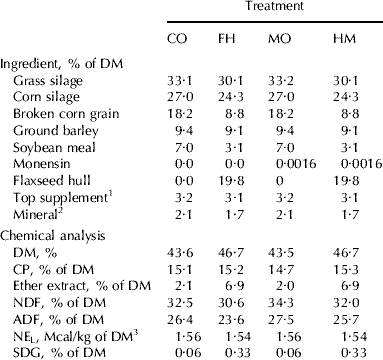
Table 1. Ingredient and chemical composition of total mixed diets of Holstein cows fed no flaxseed hulls and no monensin (CO), flaxseed hulls and no monensin (FH), no flaxseed hulls with monensin (MO) or a mixture of flaxseed hulls and monensin (HM)
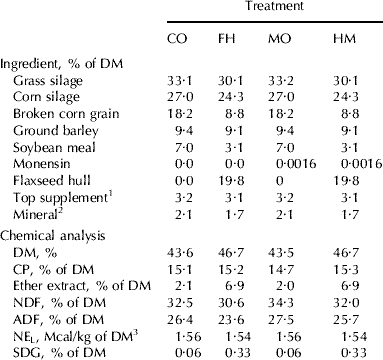
1 Contained 20% of canola meal, 30% of corn gluten meal, 20% of soybean meal, and 30% of brewer's corn
2 Contained 9·02% Ca, 4·90% P, 4·89% Mg, 1·76% S, 14% Na, 1·43% K, 2068 mg/kg Fe, 2718 mg/kg Zn, 447 mg/kg Cu, 1814 mg Mn, 69 mg/kg I, 7 mg/kg Co, 20 mg/kg Se, 452,000 IU/kg of vitamin A, 58,000 UI/kg of vitamin D3, and 2692 IU/kg of vitamin E
3 Calculated using published values of feed ingredients (NRC, 2001)
Experimental procedures
Each experimental period consisted of 28 d. Adaptation to experimental treatments was from d 1 to 20, sampling of ruminal fluid and faeces on d 21, and total milk collection from d 22 to 28. Feed intake and milk yield were measured daily. Samples of the total mixed diets were taken daily from d 22 to d 28 and pooled within period for each cow. Samples of flaxseed hulls were taken once a week and pooled for the whole experiment. All samples were frozen at −20°C for subsequent drying at 55°C. Milk samples were obtained from each cow for 14 consecutive milkings from d 22 to d 28 and pooled on a yield basis. One sample was kept frozen at −80°C without preservative for further analyses of milk fat and the mammalian lignan EL. Another sample was stored at +4°C with a preservative (bronopol-B2; DNF Company, Dublin, CA, USA) until analyzed for protein, urea N, lactose, and total solids.
On d 21, ruminal fluid (about 1 l) was collected from the anterior dorsal, anterior ventral, medium ventral, posterior dorsal, and posterior ventral locations within the rumen before feeding (0 h) and at 1, 2, 4, and 6 h after the am feeding. Ruminal fluid was strained through 2 layers of cheesecloth to separate the liquid and solid fractions. A 350 ml sample of strained ruminal fluid was immediately mixed with 26 g ruminal solid contents and stored at −80°C for further determination of β-glucuronidase activity. One sample of strained ruminal fluid was kept frozen at −80°C for determination of EL. On day 21, faeces (250 g) were collected 2, 4, 6 and 8 h after the am feeding and pooled on a fresh basis for β-glucuronidase activity analysis. All samples were frozen at −20°C until subsequently analyzed.
Chemical analyses
Analytical dry matter of the diets and flaxseed hulls was determined in a forced-air oven according to the procedure 934.01 (AOAC, 1990). Samples of the total mixed diets and flaxseed hulls were ground to pass a 1-mm screen in a Wiley mill before chemical analyses. Total N content of total mixed diets and flaxseed hulls was determined by thermal conductivity (LECO model FP-428 Nitrogen Determinator, LECO, St. Joseph, MI, USA) and crude protein was calculated as N×6·25. The concentration of neutral detergent fibre (NDF) in diets and flaxseed hulls was determined as described by Van Soest et al. (Reference Van Soest, Robertson and Lewis1991) without the use of sodium sulphite and with the inclusion of heat stable α-amylase. The acid detergent fibre (ADF) content in diets and flaxseed hulls was determined according to AOAC (1990; Method 973.18). The NDF and ADF procedures were adapted for use in an ANKOM200 Fibre Analyzer (ANKOM Technology Corp., Fairport, NY, USA). Ether extraction in diets and flaxseed hulls was conducted with Tecnal TE–044/1 (Maringá, Paraná, Brazil) according to the method No. 7.060 (AOAC, 1990). Fatty acid profile of flaxseed hulls was determined as described by Petit & Gagnon (Reference Petit and Gagnon2009).
Milk fat concentration was determined by the method of Roese-Goettlib (AOAC, 1990). Protein, lactose, total solids, and urea N concentrations in milk samples were analyzed by infrared spectrophotometer (System 4000 Milkoscan; Foss Electric of Hillerod, Denmark). Total antioxidant capacity of milk (Ferric Reducing Antioxidant Power – FRAP) was determined according to Benzie & Strain (Reference Benzie, Strain and Packer1999) adapted for milk after deproteinization with 100% alcohol before the assay. The FRAP method is based on a redox reaction in which an easily reduced oxidant (Fe3+) is reduced to the ferrous (Fe2+) form with an intense blue colour. The potential of antioxidants in milk to reduce Fe3+ to Fe2+ was expressed in μm-Fe2+. It is assumed that the higher the measured FRAP value, the higher the content of antioxidants in milk which could reduce the ferric ion to the ferrous ion.
Extraction and analysis of plant secoisolariciresinol diglucoside in diets and flaxseed hulls were performed according to the procedures described by Muir & Westcott (Reference Muir and Westcott2000). Lignans in ruminal fluid and milk were hydrolysed and extracted according to the procedures described by Gagnon et al. (Reference Gagnon, Côrtes, da Silva, Kazama, dos Santos, Zeoula, Benchaar and Petit2009). Only EL was analyzed in milk and rumen fluid as other studies have shown that the mammalian lignan ED is below detection level in milk (Petit & Gagnon, Reference Petit and Gagnon2009) and that EL is the main mammalian metabolite produced by ruminal microbiota (Côrtes et al. Reference Côrtes, Gagnon, Benchaar, da Silva, Santos and Petit2008). The determination of β-glucuronidase activity in faecal samples and ruminal fluid was based on a modified method of Jenab & Thompson (Reference Jenab and Thompson1996) as described by Gagnon et al. (Reference Gagnon, Côrtes, da Silva, Kazama, dos Santos, Zeoula, Benchaar and Petit2009).
Statistical analysis
All results were analyzed using the MIXED procedure of SAS (2000) as a 4×4 Latin square design balanced for residual effect within a 2×2 factorial arrangement of treatments. Data collected on the last week of each experimental period for milk production, milk composition, and feed intake, were analyzed using the following general model:
where: Yijk=the dependent variable, μ=overall mean, Ci=random effect of cow (i=1 to 4), Pj=fixed effect of period (k=1 to 4), Tk=fixed effect of treatment (k=1 to 4), and eijk=random residual error. Treatments were compared to provide factorial contrasts: 1) with vs. without monensin, 2) with vs. without flaxseed hulls, and 3) the interaction between monensin and flaxseed hulls. The residual effect was initially included in the model but was removed because it was not significant. Data on ruminal β-glucuronidase activity and EL concentration were analyzed as repeated measurements. Results are reported as least squares means±sem. Data on EL concentration in milk were transformed (log) as previously performed by Côrtes et al. (Reference Côrtes, Gagnon, Benchaar, da Silva, Santos and Petit2008) but results in the Figure are reported as the adjusted mean value (with confidence interval) on the original scale of measurements. Significance was declared at P⩽0·05 and a trend at 0·05<P⩽0·10 unless otherwise stated.
Results
Flaxseed hulls contained, expressed as a percentage of dry matter, 23·5% crude protein, 19·4% neutral detergent fibre, 14·3% acid detergent fibre, 29·8% total lipids, and 1·15% secoisolariciresinol diglucoside (SDG), and 6·86 kJ per gram of dry matter. Flaxseed hulls contained, expressed as a percentage of total fatty acids, 6% 16:0, 2% C18:0, 20% C18:1, 18% C18:2, 53% C18:3, and 1% others.
There was no interaction (P>0·10) between flaxseed hulls and monensin supplementation for dry matter intake and body weight measurements (Table 2). Intake of dry matter was significantly higher for CO and MO than for FH and HM. Intake of dry matter was similar (P>0·10) for cows supplemented or not with monensin. Initial body weight and body weight change were similar among treatments. Final body weight tended (P=0·07) to increase with monensin supplementation.
Table 2. Intake, initial and final body weight (BW) of Holstein cows fed total mixed diets containing no flaxseed hulls and no monensin (CO), flaxseed hulls and no monensin (FH), no flaxseed hulls with monensin (MO) or a mixture of flaxseed hulls and monensin (HM)

1 Least squares means with pooled standard error (SE)
2 Somatic cell score=log10(somatic cell count/mL)
There was no interaction (P>0·10) between flaxseed hulls and monensin supplementation for milk production, 4% fat-corrected milk yield, milk composition, milk yield of components, and somatic cell score (Table 2) although protein concentration tended (P=0·10) to be lower for cows fed FH. Milk production was decreased (P=0·03) for cows fed FH and HM. Moreover, supplementation with monensin tended (P=0·09) to decrease milk production. Production of 4% fat-corrected milk, somatic cell score and concentrations of milk components were similar (P>0·05) among treatments. Milk protein and lactose yields were significantly decreased (P=0·04 and P=0·03, respectively) for cows fed FH and HM and there was a trend (P=0·08) for a decrease in yield of total solids.
There was no interaction (P>0·10) between time and treatment for EL concentration in ruminal fluid and between flaxseed hulls and monensin supplementation for concentrations of EL in ruminal fluid and milk. Concentration of EL in ruminal fluid increased (P=0·001) postfeeding (Fig. 1) and remained higher for cows fed FH and HM compared with those fed CO and MO. Monensin had no effect on EL ruminal concentration. Supplementation with flaxseed hulls increased (P=0·001) EL concentration in milk and monensin had no effect (Fig. 2). Concentration of FRAP averaged 241:mol/l milk and was similar (P=0·10) among treatments.

Fig. 1. Concentration of enterolactone (nmol/L) in ruminal fluid of Holstein cows fed a total mixed diet containing no flaxseed hulls and no monensin (CO-□), flaxseed hulls (FH-⋄), monensin (MO-□), or a mixture of flaxseed hulls and monensin (HM-Δ). Data are means±SEM.

Fig. 2. Concentration of enterolactone (nmol/L) in milk of Holstein cows fed a total mixed diet containing no flaxseed hulls and no monensin (CO), flaxseed hulls (FH), monensin (MO), or a mixture of flaxseed hulls and monensin (HM). Data are the mean values with confidence intervals represented by vertical bars. Mean values with unlike superscript letters were significantly different (P<0·05).
There was no significant (P>0·10) interaction between hour and treatment for specific β-glucuronidase activity in ruminal fluid but there was a difference (P=0·001) among hours. Ruminal β-glucuronidase activity was lower before feeding and 1 h postfeeding to increase and reach a plateau from 2 h postfeeding (Fig. 3a). Cows fed FH and HM had lower (P=0·002) ruminal β-glucuronidase activity than those fed CO and MO. Moreover, cows fed FH had lower (P=0·05) β-glucuronidase activity in faeces than those fed CO (Fig. 3b) as shown by the interaction between flaxseed hulls and monensin supplementation. Faecal β-glucuronidase activity tended (P=0·08) to be lower for cows fed HM compared with those fed CO. Monensin supplementation tended (P=0·09) to decrease β-glucuronidase activity in ruminal fluid.

Fig. 3. Specific activity of β-glucuronidase in ruminal fluid (a) and fecal samples (b) of Holstein cows fed a total mixed diet containing no flaxseed hulls and no monensin (CO), flaxseed hulls (FH), monensin (MO), or a mixture of flaxseed hulls and monensin (HM). Data are means±SEM. Mean values with unlike superscript letters were significantly different (P<0·05).
Discussion
Milk production was decreased for cows fed flaxseed hulls probably as a result of lower dry matter intake. Diets with flaxseed hulls averaged 6·9% ether extract (dry matter basis), which may limit dry matter intake of late-lactating cows due to lower energy requirements compared with those of early-lactating cows. The effects of level and type of fat supplement on dry matter intake are negligible when total fat concentration is below 6% of the dry matter (Dhiman et al. Reference Dhiman, Satter, Pariza, Galli, Albridht and Tolosa2000; Petit et al. Reference Petit, Dewhurst, Scollan, Proulx, Khalid, Haresign, Twagiramungu and Mann2002). Declines in dry matter intake with fat-supplemented diets appear to be related to ruminal effects of fats as no depression in dry matter intake occurred in studies where ruminal effects of fat were not observed (Petit et al. Reference Petit, Dewhurst, Scollan, Proulx, Khalid, Haresign, Twagiramungu and Mann2002). Benson et al. (Reference Benson, Reynolds, Humphries, Rutter and Beever2001) hypothesized that long chain fatty acids are utilized differently in early compared with mid-lactation, suggesting that the negative effect of lipid supplementation on dry matter intake becomes more important as lactation progressed. As cows were in late lactation in the present experiment, they may have been negatively affected by the level of fat in the diet.
Monensin had no effect on intake of dry matter and milk production. Although the effects of including monensin as premix or controlled release capsule in dairy cattle rations have been extensively investigated, results have been variable in terms of dry matter intake. The addition of monensin did not influence (Ramanzin et al. Reference Ramanzin, Bailoni, Schiavon and Bittante1997; Broderick, Reference Broderick2004) or decreased (Sauer et al. Reference Sauer, Fellner, Kinsman, Kramer, Jackson, Lee and Chen1998) dry matter intake of lactating dairy cows. Moreover, feeding monensin at 24 and 22 mg/kg of the dry matter, respectively, for 15- (Bell et al. Reference Bell, Griinari and Kennelly2006) and 35-d (Osborne et al. Reference Osborne, Mutsvangwa, Alzahal, Duffield, Bagg, Dick, Vessie and McBride2004) periods had no effect on dry matter intake and milk yield of dairy cows. Similarly, monensin supplementation had no effect on milk production (Ramanzin et al. Reference Ramanzin, Bailoni, Schiavon and Bittante1997; Broderick, Reference Broderick2004). However, this disagrees with earlier trials establishing that the inclusion of 300 mg/d of monensin in dairy cow diets for the first 25 weeks of lactation would increase milk yield (Phipps et al. Reference Phipps, Wilkinson, Jonker, Tarrant, Jones and Hodge2000). According to Duffield et al. (Reference Duffield, Rabiee and Lean2008), the failure to report consistent responses in previous studies most probably reflects inadequate sample size and, consequently, insufficient statistical power to detect an approximate increase of 0·7 l in milk yield and decrease of 0·3 kg in dry matter intake. Similar estimates of the effect of monensin on feed intake and milk yield have been observed by Ipharraguerre and Clark (Reference Ipharraguerre and Clark2003) who reported a decrease of 0·3 kg of dry matter intake from 14 ionophore experiments and an increase in milk yield of 0·7 and 1·5 kg/d in low- and high-forage diets, respectively.
Milk composition was similar (P>0·05) among treatments. In general, milk protein concentration is little affected by monensin supplementation (Sauer et al. Reference Sauer, Fellner, Kinsman, Kramer, Jackson, Lee and Chen1998; Bell et al. Reference Bell, Griinari and Kennelly2006) although it decreased (Phipps et al. Reference Phipps, Wilkinson, Jonker, Tarrant, Jones and Hodge2000) or increased (Duffield et al. Reference Duffield, LeBlanc, Bagg, Leslie, Ten Hag and Dick2003) in some cases. In many of the studies where monensin reduced milk fat and protein concentrations, a parallel increase in milk production was observed, thus suggesting that a dilution effect was partly responsible for changes in milk composition (Phipps et al. Reference Phipps, Wilkinson, Jonker, Tarrant, Jones and Hodge2000) although this was not the case in the current experiment.
Supplementation with flaxseed hulls increased EL concentration in ruminal fluid and milk, which agrees with recent in vitro results showing that EL is the main metabolite produced when flaxseed hulls are incubated with ruminal microbiota (Côrtes et al. Reference Côrtes, Gagnon, Benchaar, da Silva, Santos and Petit2008). Different studies have confirmed the presence of polyphenolic compounds such as equol, daidzein, and genistein (Bannwart et al. Reference Bannwart, Adlercreutz, Wähälä, Kotiaho, Hesso, Brunow and Hase1988) and mammalian lignan EL (Steinshamn et al. Reference Steinshamn, Purup, Thuen and Hansen-Møller2008; Petit & Gagnon, Reference Petit and Gagnon2009) in milk. This may suggest that contrary to what has been previously reported for monogastric mammals such as humans (Heinonen et al. Reference Heinonen, Nurmi, Kiukkonen, Poutanen, Wähälä, Deyama, Nishibe and Adlercreutz2001) and pigs (Knudsen et al. Reference Knudsen, Serena, Kjær, Tetens, Heinonen, Nurmi and Adlercreutz2003), absorption of EL may occur either in the rumen or in the small intestine of ruminants before further conversion of ED to EL by colon microbes. Similarly, it has been shown that other phytoestrogens of the isoflavones family such as formononetin are metabolized to equol by bovine ruminal microbiota and that equol is available for absorption from the gastrointestinal tract (Dickinson et al. Reference Dickinson, Smith, Rander and Pemberton1988).
Concentration of EL in the milk of cows increased from 10·5×10−6 to 49·1×10−6 mg/ml with dietary supplementation of flaxseed hulls, which may contribute to improve human health. Indeed, it has been shown that people with higher blood concentrations of EL have lower incidence of cardiovascular diseases (Vanharanta et al. Reference Vanharanta, Voutilainen, Lakka, Van der Lee, Adlercreutz and Salonen1999). Moreover, bioactive components in bovine milk or colostrum such as growth factors have been identified with bioactivity observed for concentrations lower than 20×10−6 mg/ml (see review of Rowan et al. Reference Rowan, Haggarty and Ram2005), which is within the range of milk EL concentrations observed in the present experiment.
Concentration of FRAP in milk was similar among treatments. Although the FRAP assay is a recently developed test used for measuring total antioxidant capacity in blood serum (Benzie & Strain, Reference Benzie, Strain and Packer1999), Smet et al. (Reference Smet, Raes, De Block, Herman, Dewettinck and Coudijzer2008) reported that FRAP can provide a good measurement of milk oxidation as the ferric reducing antioxidant power of the product. It is known that lignan metabolites have a great antioxidant activity (Kitts et al. Reference Kitts, Yuan, Wijewickreme and Thompson1999). Flaxseed hulls is a rich source of plant precursors to mammalian lignans (Côrtes et al. Reference Côrtes, Gagnon, Benchaar, da Silva, Santos and Petit2008) and Petit & Gagnon (Reference Petit and Gagnon2009) already reported that the mammalian lignan EL can be transferred into milk when dairy cows were fed flaxseed. Although cows fed flaxseed hulls had higher EL concentration in milk than those fed no flaxseed hulls, the total antioxidant power FRAP test did not detect any difference between diets, thus suggesting that the concentration of EL in milk of cows supplemented with flaxseed hulls or the lignan, EL, was not efficient to change the ferric reducing antioxidant potential of milk. The FRAP assay monitors only one aspect (non–enzymatic reducing ability) of antioxidant status of biological fluids. Further antioxidant assays should be examined to evaluate the effect of EL on antioxidant status of milk.
Supplementation with flaxseed hulls decreased β-glucuronidase activity in both ruminal fluid and faeces of dairy cows. Flaxseed hulls contain 53% omega-3 FA, expressed as a percentage of total FA, and results from a recent in vitro study (Maia et al. Reference Maia, Chaudhary, Figueres and Wallace2007) reported that growth of ruminal bacteria can be affected by polyunsaturated FA such as omega-3 although sensitivity differs among species. The activity of β-glucuronidase has been attributed to bacteria belonging to the dominant human intestinal microbiota, such as Ruminococcus, Bacteroides, Bifidobacterium, and Eubacterium (Beaud et al. Reference Beaud, Tailliez and Anba-Mondoloni2005). Henderson (Reference Henderson1973) showed that the growth of some strains of some predominant rumen bacteria such as Butyrivibrio, Ruminococcus and Methanobrevibacter is strongly inhibited by the presence of long-chain FA, which may decrease the activity of β-glucuronidase in ruminal fluid. Although flaxseed hulls contain 1·15% secoisolariciresinol diglucoside in the dry matter, they are also rich in omega-3 FA. A similar decrease in β-glucuronidase activity in ruminal fluid has been reported by Gagnon et al. (Reference Gagnon, Côrtes, da Silva, Kazama, dos Santos, Zeoula, Benchaar and Petit2009) when cows received ruminal infusion of flax oil.
Monensin had no effect on ruminal microbiota involved in lignan metabolism as shown by similar EL concentrations for cows supplemented or not with monensin. On the other hand, the activity of β-glucuronidase in ruminal fluid tended to decrease with monensin supplementation. Monensin is known to decrease the growth of Gram positive bacteria and some strains of bacteria with β-glucuronidase activity such as Ruminococcus and Eubacterium are Gram positive bacteria. Therefore, supplementation with monensin may affect ruminal β-glucuronidase activity.
In conclusion, although there was a decrease in ruminal activity of β-glucuronidase when feeding flaxseed hulls, the metabolism of plant into mammalian lignans may be increased as shown by enhanced concentration of EL in the rumen and milk. Supplementation with flaxseed hulls then may contribute to change favourably milk composition for better human health by enhancing mammalian lignan EL concentration.
The authors would like to express their gratitude to the staff of the Dairy & Swine Research & Development Centre for their contribution to the present study. The authors thank V. Roy, S. Dallaire and L. Veilleux at Sherbrooke for technical assistance and Steve Méthot for his help in the statistical analyses. D. da Silva, R. Kazama and G.T. dos Santos received a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação Araucária do Paraná from Brazil and C Côrtes was a recipient of a fellowship from the National Science and Engineering Research Council of Canada. This project was sponsored by Elanco Division Eli Lilly Canada Inc. and the Matching Investment Initiative of Agriculture and Agri-Food Canada.