Small Ruminant Lentiviruses (SRLV) are a group of closely related Retroviruses responsible for caprine arthritis-encephalitis (CAE) in goats. SRLV infection is life-long and may result in several clinical manifestations. As CAE is widespread all around the world, especially in dairy goats, it is believed to pose a substantial threat to goat population (Adams et al. Reference Adams, Oliver, Ameghino, DeMartini, Verwoerd, Houwers, Waghela, Gorham, Hyllseth and Dawson1984). Moreover, many studies have indicated that CAE has considerable impact on milk yield and milk properties although size of this effect is disputable (Smith & Cutlip, Reference Smith and Cutlip1988; Greenwood, Reference Greenwood1995; Nord & Adnøy, Reference Nord and Adnøy1997; Turin et al. Reference Turin, Pisoni, Giannino, Antonioni, Rosati, Ruffo and Moroni2005; Leitner et al. Reference Leitner, Krifucks, Weisblit, Lavi, Bernstein and Merin2010; Kaba et al. Reference Kaba, Strzałkowska, Jóźwik, Krzyżewski and Bagnicka2012; Martínez-Navalón et al. Reference Martínez-Navalón, Peris, Gómez, Peris, Roche, Caballero, Goyena and Berriatua2013).
Cheese is a main product of dairy goats (Lejaouen & Toussaint, Reference Lejaouen and Toussaint1993). Although it may be assumed that influence of CAE on milk yield and composition would indirectly lead to the decrease in amount of cheese obtained from an infected goat, no studies have so far investigated direct relationship between SRLV infection and cheese production.
Therefore the goal of this study was to determine an exclusive influence of SRLV infection in goats on cheese yield.
Materials and methods
Animals, serological testing and records
The observational cohort study was carried out in a dairy goat herd. The goats were of two breeds – Polish White Improved (PWI, 56%) and Polish Fawn Improved (PFI, 44%). They were kept in a concrete barn with efficient gravitational ventilation and adequate amount of dry straw bedding. From the late spring to the early autumn they were kept on a high-quality pasture for 4–6 h a day. The goats were fed according to the INRA feeding norms (Jarrige, Reference Jarrige2002), mainly with hay in the summer (additionally to the pasture) and haylage or silage in the winter. Mineral supplements were available at will. Goats were machine milked twice a day (at 6 a.m. and 3 p.m.). Their average milk yield per 280-d lactation was approximately 800 kg, with 3·35% fat and 3·20% total protein (data from previous 10 years). Goat were mated for the first time when they were 7–8 month-old so they used to start their first lactation when they were roughly 1 year-old. Therefore, parity and age are highly correlated in this herd.
The herd had been infected with SRLV for at least 10 years before the onset of the study. The infection was confirmed in the herd with virus isolation (Kaba et al. Reference Kaba, Rola, Materniak, Kuźmak and Nowicki2009). All goats in the herd used to be tested serologically at the age of 4–6 months and then twice a year (in the spring and autumn) using immunoenzymatic test (indirect Pourquier ELISA Maedi-Visna/CAEV Serum Verification, Institut Pourquier, France). The test was performed according to the manufacturer's manual using ELISA reading device ICN Flow Titertek Multiscan Plus Mk11 (Labsystems, Espoo, Finland). Sensitivity and specificity of the ELISA tests were 84 and 100%, respectively, basing on the study of Brinkhof & van Maanen (Reference Brinkhof and van Maanen2007).
The observation lasted 3 years and included 63 dairy goats in all. Twenty one (33%) of the goats tested seropositive for SRLV at least twice during a year preceding the first record collection. They were 5·7 ± 1·6 year-old. Given the aforementioned sensitivity of the ELISA test serial testing of a goat kept in the SRLV-infected herd with prevalence of infection of at least 20% gives positive predictive value of almost 100%. These goats provided SRLV-positive records and formed a cohort exposed to SRLV. The remaining 42 goats tested seronegative for SRLV at least twice during a year preceding the first record collection as well as for the entire observation period and constituted a cohort unexposed to SRLV. They were 2·8 ± 1·1 year-old. Goats which seroconverted either during the observation period or during a year preceding the onset of the study were excluded from the analysis.
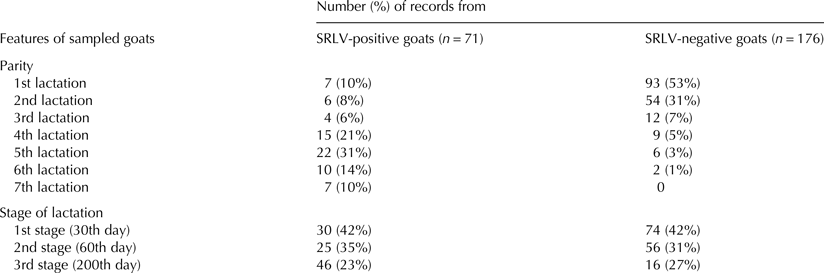
In total 247 records of milk yield (kg), milk acidity (pH), protein (g/kg), and fat milk contents (g/kg), somatic cell count (SCC), number of bacteria as well as cheese yield (g/kg) were gathered on the 30th, 60th and 200th day of lactation. Seventy one records came from 42 seropositive goats (3 goats were recorded 5 times, 5–4 times and 12–3 times) and 176 from 21 seronegative goats (15 recorded 5 times, 20 – four times and 7 – three times). Records were collected only from clinically healthy goats. Goats which had mastitis during the observation period were excluded from the further analysis. Distribution of records with respect to the parity and stage of lactation is presented in Table 1.
Table 1. Distribution of parity and stage of lactation from which records used in the analysis were collected

Determining milk properties
The total protein and fat contents were determined in milk samples using MilkoScan FT2 (FOSS, Denmark) and pH was measured with manual pH meter.
SCC was measured by an automated fluorescent microscopic somatic-cell counter Bactocount (Bentley IBCm, Bentley Instrument, USA) which counts only DNA-containing cells stained by ethidium bromide.
Every milk sample was tested for the presence of bacteria. Bacteria were cultured on the Columbia agar supplemented with 5% sheep blood and the MacConkey agar (bioMérieux, France). Both media were inoculated with 100 and 10 μl of milk samples. Then plates were incubated at 37 °C for 48 h. Standard microbiological techniques were used to identify and quantify isolated bacteria.
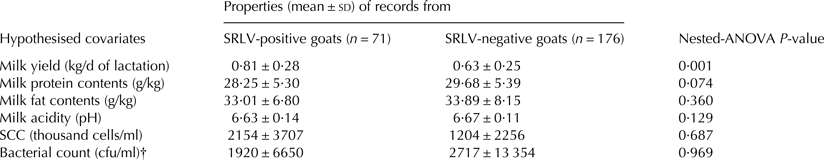
Milk properties of goats from both groups are presented in Table 2.
Table 2. Milk properties in records from goats with different SRLV-infection status

† cfu stands for colony forming units.
Determining cheese yield
To measure cheese yield, 6 g sodium chloride was mixed with 0·2 g rennet (mainly containing chymosin) and filled up with water to 200 ml. Then 1 ml of this solution was added to 20 ml unpasteurised milk and incubated at 37 °C for 2 h. Subsequently the clot which had formed was drained off on a filter and weighed for next 24 h. The result was multiplied by 50 so that cheese yield was expressed as a number of fresh cheese grams obtained from 1 kg of milk.
Statistical analysis
Nested analysis of variance (ANOVA) with SRLV-infection as a fixed-effects factor and the goat as a random-effects factor was used to compare milk properties between records from SRLV-infected and SRLV-free goats.
The following four-level hierarchical linear model was developed to assess the influence of SRLV infection adjusted by important covariates on cheese yield in dairy goats:
where Y – cheese yield, B 0 – intercept, B goat – regression coefficient for repeated measurements, X goat – repeated measurements, B parity – regression coefficient for age, X parity – age (years), B n – regression coefficients for confounding variables, X n – confounding variables, B SRLV – regression coefficient for SRLV infection, X SRLV – SRLV infection (1-yes, 0-no).
Numerical variables were expressed as arithmetic mean ± standard deviation (sd). SCC was subject to natural logarithmic transformation to normalise the distribution. Confidence intervals (CI) of 95% were computed for all parameters. Normality of distribution of all numerical variables was assessed with Shapiro–Wilk test (α = 0·05).
To control for possible confounding effect covariates were selected on the basis of current knowledge of the animal-associated factors influencing cheese production as well as of the changes in milk caused by SRLV infection. Amount of cheese produced directly depends on milk yield as well as concentration of protein and fat in milk (Guo et al. Reference Guo, Park, Dixon, Gilmore and Kindstedt2004). On the other hand, these properties seem to be affected by SRLV infection (Kaba et al. Reference Kaba, Strzałkowska, Jóźwik, Krzyżewski and Bagnicka2012; Martinez-Navalon et al. Reference Martínez-Navalón, Peris, Gómez, Peris, Roche, Caballero, Goyena and Berriatua2013) and their decrease could be an actual direct cause of the cheese yield reduction. Milk acidity, SCC and bacterial count were offered to the model as indicators of milk freshness and subclinical mastitis as these qualities of milk also directly influence cheese production (Leitner et al. Reference Leitner, Merin and Silanikove2004; DeMarchi et al. Reference De Marchi, Fagan, O'Donnell, Cecchinato, Dal Zotto, Cassandro, Penasa and Bittante2009; Le Maréchal et al. Reference Le Maréchal, Thiéry, Vautor and Le Loir2011). Given the fact that milk composition changes along lactation, stage of lactation is considered an important confounding factor in every milk and cheese production analysis and thus was also included in the final model (Lopez et al. Reference Lopez, Luna, Laencina and Falaga1999; Strzałkowska et al. Reference Strzałkowska, Joźwik, Bagnicka, Krzyżewski, Horbańczuk, Pyzel and Horbańczuk2009).
Independent variables were introduced into the model in four steps: Firstly, variable denoting each goat was entered to include variability resulting from the unbalanced study design (different number of repeated measurements for each goat). Secondly, goat age was added to overcome a considerable difference between compared groups as well as to control for age effect on cheese yield. Furthermore, as CAE is a chronic progressive disease and goats usually acquire it when they are young (Ellis et al. Reference Ellis, Carman, Robinson and Wilcox1986), the influence of the infection on goat production is expected to be age-related with older goats manifesting more severe symptoms. In the third step, all the hypothesised confounders were introduced as either numerical (milk yield, protein milk contents, fat milk contents, milk acidity, SCC, bacterial count) or dichotomous (the 2nd and the 3rd stage of lactation) variables. At the very end, SRLV-infection status was entered as a dichotomous variable.
The hierarchical linear model with P-value of 0·05 for variable inclusion based on maximum likelihood method and backward elimination of hypothesised covariates was developed (Raudenbush & Bryk, Reference Raudenbush and Bryk2002). Goat-effect and goat's age were forced into the model. Adjusted coefficient of multiple correlations (R 2) was a goodness-of-fit measure for the final model. Statistical analyses were performed in IBM SPSS Statistics 21.
Results
Cheese yield was 62·53 ± 12·86 and 68·43 ± 14·04 g cheese per 1 liter of milk in SRLV-positive and SRLV-negative goats, respectively. Milk properties were similar in compared groups of records apart from milk yield which was significantly higher in SRLV-infected goats (Table 1). Bacteria were isolated from 53% (131/247) of milk samples. Fifty nine per cent (42/71) of milk samples from SRLV-infected goats and 51% (89/176) of milk samples from SRLV-free goats were positive for bacteria. Coagulase-negative Staphylococci were isolated in 92% (120/131) of bacteria-positive samples, Enterococci in 8% (10/131) of samples and Corynebacterium sp. in one sample.
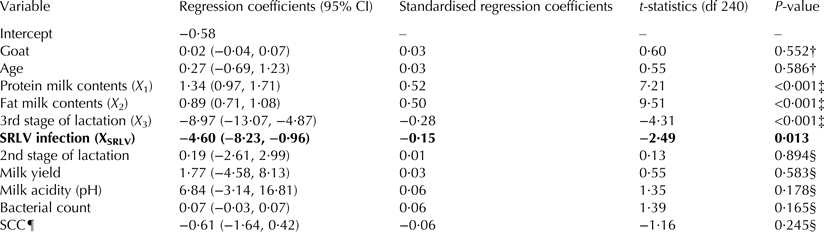
Hierarchical analysis identified four variables statistically significantly linked to the cheese yield (Table 3). Controlling for the goat-effect, goat's age, stage of lactation (X 3) as well as protein (X 1) and fat (X 2) milk concentration, SRLV infection proved to be an independent factor decreasing the cheese yield. However, SRLV infection accounted for only 1% of the total variability of the cheese yield (Table 4). The final model had an adjusted-R 2 of 0·58 (F(6,240) = 58·51, random error = 81·13, P < 0·001) and could be expressed with the equation:
Table 3. Results of hierarchical linear multivariable analysis

† Variables retained in the final model although statistically insignificant to control for the unbalanced study design and age effect.
‡ Potential confounders included in the final model.
§ Variables excluded from the final model in order of elimination.
¶ Offered to the model after natural logarithmic transformation.
Table 4. Change of coefficient of multiple correlations (R 2) associated with addition of subsequent variables to the model

† includes only intercept and goat.
‡ Age added.
§ 3rd stage of lactation, protein milk contents and fat milk contents added.
¶ SRLV infection added.
Discussion
The study results indicate that SRLV infection may reduce the amount of cheese obtained from a goat. Moreover, this seems to occur not only through reduction of milk component concentration but also via some other mechanism deteriorating cheese-forming properties of milk. Given that dairy goats outnumber meat goats in most of regions where goat breeding is popular (Castel et al. Reference Castel, Ruiz, Mena and Sánchez-Rodríguez2010; Mahmoud, Reference Mahmoud2010) and that cheese is the fastest growing market for goat milk in many countries e.g. the United States (Anonymous, 2010) this information may be of economic importance. Nevertheless, the extent of cheese yield reduction, although statistically significant, seems to be very small. Provided that stage of lactation, protein and fat milk contents are held constant the mean cheese yield will be lowered by 4·6 g cheese per 1 kg of milk on average) in a seropositive goat compared with a seronegative one.
Insignificance of milk acidity, SCC and bacterial count as covariates in the multivariable analysis can be explained by the fact that none of the sampled goats had clinical mastitis. Moreover, amount of bacteria was fairly low and never approached legal limits of the European Union (Anonymous, 2004). Given that SRLV-infection status remained significant irrespective of the presence or absence of these covariates in the backward-elimination analysis we decided to drop them from the final model.
Milk yield was significantly higher in SRLV-infected goats. In our opinion this can be attributed to the fact that these goats were significantly older (Carnicella et al. Reference Carnicella, Dario, Ayres, Laudadio and Dario2008) – more than 75% of them were in at least 4th lactation whereas more than 80% of SRLV-free goats were in the 1st or 2nd lactation. Therefore, we chose not to force milk yield into the model as a distinct covariate.
Interestingly multivariable model showed that when SRLV-infection status as well as protein and fat milk contents were held constant the mean cheese yield would be reduced in the 3rd stage of lactation. This phenomenon called ‘positive net suppression’ implies that relationship between lactation phase and cheese yield is mediated not only by concentration of milk components but there is also some unknown background mechanism which independently lowers cheese yield in the last stage of lactation (Messick & Van de Geer, Reference Messick and Van de Geer1981). This negative relationship is however weak enough to be masked by the positive influence of elevated protein and fat contents.
The study has several possible shortcomings. First, it was carried out on a quite small sample of animals kept in a single location and good environmental condition which may render it difficult to generalise the results to other goat populations. Secondly, age distributions substantially differ between SRLV-positive and negative goats and although we did our utmost to handle this problem with statistical analysis some influence on parameter estimations may have remained. Furthermore, the model explains only 58% of the variability of the cheese yield which means that there are still some explanatory factors which we did not manage to identify and investigate. Their introduction might modify the conclusion about the role of SRLV infection which makes our model only a tentative solution to the problem. Therefore, the results of our study should be considered rather as qualitative information that SRLV infection worsens cheese yield in goats than quantitative analysis of the effect size.
Nevertheless, even though several papers have stated the relationship between SRLV infection and milk production and composition (Smith & Cutlip, Reference Smith and Cutlip1988; Greenwood, Reference Greenwood1995; Nord & Adnøy, Reference Nord and Adnøy1997; Turin et al. Reference Turin, Pisoni, Giannino, Antonioni, Rosati, Ruffo and Moroni2005; Leitner et al. Reference Leitner, Krifucks, Weisblit, Lavi, Bernstein and Merin2010; Kaba et al. Reference Kaba, Strzałkowska, Jóźwik, Krzyżewski and Bagnicka2012; Martinez-Navalon et al. Reference Martínez-Navalón, Peris, Gómez, Peris, Roche, Caballero, Goyena and Berriatua2013), this is so far the only one which investigates the direct link between the infection and cheese yield.
This study was supported by grant from the Ministry of Science and Higher Education, no. 2P06Z 01330.