Colostrum is the first lacteal secretion from the mammary gland and contains a combination of immunoglobulins, antimicrobial molecules, growth factors and other biologically active molecules (Singh et al. Reference Singh, Pandita, Vaidya, Singh, Chandra, Pampoori, Huozha, Pathan, Kushwaha and Sharma2011). Bovine colostrum differs markedly in composition from bovine milk, containing more protein (approximately 4 fold more), less lactose (approximately 2·5 fold less) but a similar fat content (approximately 4 % by weight) (Klimes et al. Reference Klimes, Jagos, Bounda and Gajdusek1986; Shah, Reference Shah2000; Yamada et al. Reference Yamada and Murakami2002; Darewicz et al. Reference Darewicz, Dziuba, Mickiewicz and Dziuba2011). The high protein concentration largely reflects the provision of immunoglobulins required for passive immunity and other critical biologically active peptides and proteins responsible for neonatal development. Many of these active peptides reside within the amino acid sequences of the casein and whey proteins in colostrum and are only released after enzymatic processing. The biological activities of these peptides include opioid, antihypertensive, immunostimulating, antimicrobial and antithrombotic activities (Darewicz et al. Reference Darewicz, Dziuba, Mickiewicz and Dziuba2011). Some of these activities are exhibited by intact peptides expressed directly from the mammary gland into colostrum (Fiat et al. Reference Fiat, Migliore-Samour and Jolles1993; Ferranti et al. Reference Ferranti, Traisci, Picariello, Nasi, Boschi, Siervo, Falconi, Chianese and Addeo2004). Short peptide sequences are known to be a source of potent pharmacologically active peptides: one such example is the opiate peptide bovine β-casomorphin which is a product of the cleavage of bovine β-casein at amino acids 60-YPFPG-64, (Schanbacher et al. Reference Schanbacher, Talhouk and Murray1997).
Previously, undigested bovine colostrum has been shown to increase cell proliferation in mouse hybridomas (Ramirez et al. Reference Ramirez, Sureshkumar and Mutharasan1990), balb/c 3T3 cells (Klagsbrun & Neumann Reference Klagsbrun and Neumann1979), bovine calf and embryonic kidney cells, canine kidney epithelial cells (Klagsbrun, Reference Klagsbrun1980) and in a human foetal small intestinal cell line (Purup et al. Reference Purup, Vestergaard, Pedersen and Sejrsen2007).
The provision of nutrients in colostrum is vital for the development and function of the GIT in neonatal mammals. Enhancing enterocyte cell proliferation in the GIT is important for decreasing the incidence of GIT diseases of humans and animals such as inflammatory bowel disease, necrotising enterocolitis in young infants, non-steroidal anti-inflammatory drug-induced gut injury and oesophagitis and Helicobacter pylori-related disease (Playford et al. Reference Playford, MacDonald and Johnson2000; Diehl-Jones & Askin Reference Diehl-Jones and Askin2004). Screening hydrolysed colostrum protein samples for proliferative activity in a gastrointestinal epithelial cell line in vitro has the potential to identify fractions capable of gastrointestinal epithelial repair to prevent or reduce the effects of GIT syndromes and diseases.
The aim of this study was to identify potential sources of such proteins and peptides from bovine colostral digests collected from various regions of the bovine GIT or generated from enzymatic digests in vitro and assess their impact on human intestinal epithelial cell proliferation. These peptides may lead to new therapeutic products which will provide commercial opportunities for the dairy industry. The development of GIT experimental models is limited by the innate complexity of the GIT and our inability to mimic normal intestinal epithelial cell function in vitro (Abud et al. Reference Abud, Watson and Heath2005). The proliferative capacity of a human colonic epithelial cell line, T84, was employed as a high throughput screen for the identification of novel proliferative proteins and peptides from these colostral digests. This cell line differentiates spontaneously to form polarised monolayers with well-formed tight junctions (Donato et al. Reference Donato, El-Merhibi, Gundsambuu, Mak, Formosa, Wang, Abbott and Powell2011). It is also commonly used in drug transport studies (Quaroni & Holchman Reference Quaroni and Holchman1996) and has been used in studies of the pathophysiology of enteric pathogens and immunity (McCormick et al. Reference McCormick, Colgan, Delp-Archer, Miller and Madara1993). These cells secrete specific cytokines in a similar manner to AB IPEC-J2 cells which are derived from the porcine intestinal columnar epithelium (Brosnahan & Brown, Reference Brosnahan and Brown2012). Therefore their proliferation was chosen as a net reflection of their multi-functional activity in response to colostral proteins and peptides.
Materials and methods
Digestion of colostrum in vitro
Three colostrum samples (C1, C2, C3), collected from Holstein-Friesian cows on day 1 of lactation, which expressed differences in β-casein and β-lactoglobulin protein variants, were selected for digestion. Native PAGE analysis (data not shown) for the β-lg and β-casein milk protein genotype consisted of two alleles for either A and/or B (Medrano & Sharrow, Reference Medrano and Sharrow1989). C1 expressed the B allele for β-lg, C2 expressed the A allele for β-lg while C3 expressed both A and B alleles for β-lg. The A allele for β-casein was expressed in all three cows and the B allele for β-casein was only expressed in C2. These samples were defatted by centrifuging at 12 000 g for 30 min (Beckman Avanti J-25 centrifuge, Gladesville, NSW, Australia). The supernatant was collected and the cell debris (pellet) and overlying fat layer were discarded. Initially a range of conditions for each enzyme was trialled for 1 mg/ml of a colostrum sample. Samples with pepsin were incubated at 37 °C for 0, 0·5, 1, 2 and 12 h at concentrations of 0·1, 1, 5 U samples with chymosin were incubated at 25 °C for 0, 0·5, 1, 2 and 12 h at concentrations of 0·1, 0·01, 0·001 U while samples with trypsin were incubated at 37 °C for 0, 0·5, 1, 2, 5 and 12 h at concentrations of 1, 0·1, 0·01 U (units; 1 U hydrolyses 1 μmol/min), according to manufacturer's instructions. The duration and temperature of digestions and enzyme concentrations were selected based on the extent of degradation of high molecular weight proteins and the appearance of low molecular weight proteins (LMWP) observed on SDS–PAGE gels. Samples were then digested at optimal conditions for production of the greatest abundance of LMWPs as follows; trypsin (Sigma-Aldrich, St Louis, USA [EC 3.4.21.4]) at 1 U for 5 h at 37 °C, chymosin (Sigma-Aldrich [EC 3.4.23.4]) at 0·001 U for 1 h at 25 °C or pepsin (Sigma-Aldrich [EC 3.4.23.1]) at 0·1 U for 2 h at 37 °C. Following digestion, samples were sterilised using 0·22 μm filters (Pall Life Sciences, Australia).
Digestion of colostrum in vivo
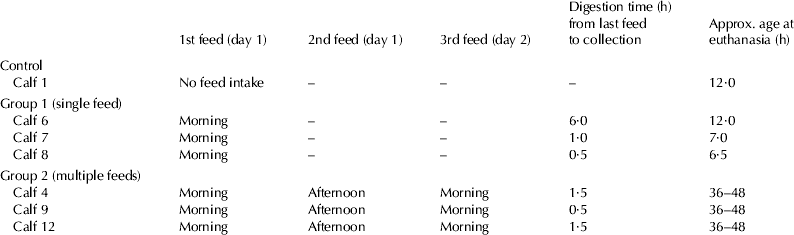
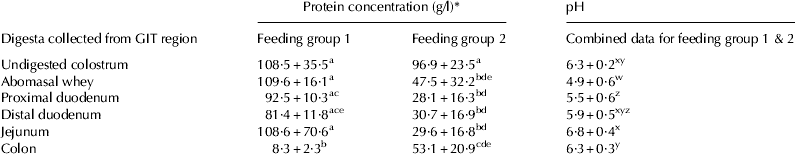
Seven newborn male Holstein-Friesian calves were used for in vivo digestions of colostrum. The experimental procedures complied with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Calves in group 1 (single fed calves; n=3) were fed 2 l of day 1 colostrum from their maternal source and calves in group 2 (multiple fed calves; n=3) were given three feeds, each of 2 l at intervals of 12 h over a 24 h period again from their maternal source. The duration of digestion relative to the time of the final feed prior to euthanasia is presented in Table 1. The control calf (n=1) did not consume any colostrum and contents of the GIT were collected 12 h after birth. All calves were euthanised with excess Lethabarb™ (Virbac Aus. Pty. Ltd., Peakhurst 2210, NSW, Australia). A laparotomy was performed and segments of the tract were ligated with nylon. Approximately 50 to 200 ml digesta were collected from the abomasum, proximal duodenum, distal duodenum, jejunum, and the spiral colon. All samples were centrifuged at 12 000 g at 4 °C for 30 min and diluted to 1 mg protein per ml for sterile filtration.
Table 1. Feeding and digestion times in calves
Protein concentration and pH analysis of digested samples
A BCA protein assay reagent kit (Pierce, Rockford, IL) was used to determine the protein concentration as per manufacturer's instructions. The pH of undigested colostrum and samples collected from the abomasum, proximal duodenum, distal duodenum, jejunum and spiral colon from calves in feeding group 1 (n=3) and 2 (n=3) were measured using a pH meter (ACTIVON model 210).
Gel electrophoresis
Protein samples (20 μg) were loaded in SDS polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (containing 10 g SDS/l, 0·03 ml glycerol/l, 0·005 ml mercaptoethanol/l, 0·025 g coomassie blue G/l, 12·5 mm Tris pH 6·8) on 15 % gels. Gels were run at 30 mA for 45 min on a Mini-PROTEAN II electrophoresis cell unit (Biorad, USA). Tris-Tricine SDS-PAGE as described by Schagger & von Jagow (Reference Schagger and Von Jagow1987) were run along with a protein MW marker; 2·5 to 200 kDa, Mark 12™ unstained standard (Invitrogen Life Technologies). Tris-tricine gels were run at 35 V for 20 min followed by 40 mA for 3 h. All gels were fixed for 1 h in 100 ml/l acetic acid and 500 ml/l methanol. They were then stained in 0·5 g/l coomassie brilliant blue G in 100 ml/l acetic acid, then destained in 100 ml/l acetic acid. All gels were scanned using ImageMaster Labscan (Amersham Biosciences, Australia).
Cell proliferation assay
Human epithelial adenocarcinoma cells (T84 cells) of colonic origin at passage 22 were grown in media (500 ml Ham's F12/l and 500 ml DMEM/l (JRH Biosciences, Inc) with 100 ml FCS/l, 20 ml penicillin/streptomycin/l, 0·2 M L-glutamine in saline, 0·2 ml Normocin/l (100 g/l) and 0·5 ml Fungin/l (50 g/l) (Invitrogen) and kept in an incubator at 5 % CO2 and 37 °C. Between passages 45–56, T84 cells were seeded onto 96 well plates at 5×103 cells/well and left overnight. The following day, media was replaced with keratinocyte serum free medium (SFM) containing human recombinant Epidermal Growth Factor (2·5 mg/l) and Bovine Pituitary Extract (25 g/l) (Gibco, Life Technologies) for a further overnight incubation. Initially, a number of protein concentrations (0·0001, 0·001, 0·01, 0·1 and 1 mg/ml were added to T84 cells. Protein samples (1 mg/ml) from colostrum digested in vitro and in vivo were then added to triplicate wells and incubated for 16 h. Controls consisted of SFM, 100 ml/l FCS and 100 μg/well of immunoglobulin G, a non-specific bovine protein digest (Polypep (PP): Sigma-Aldrich, St Louis, USA). MTT dye (15 μl) from a Celltiter 96 non-radioactive cell proliferation assay (Promega, Australia) was added to cells for 4 h. Absorbance was then read at 570 nm on a spectrophotometer (Titertek Multiskan MCC/340, Pathtech Diagnostic Pty Ltd, Victoria).
Statistical analysis
Absorbance data from cell proliferation experiments were analysed using a REML linear mixed model in GenStat (GenStat, VSN International Ltd, UK). These analyses were performed on all absorbance data obtained using Treatment/(Feeding Group×Treat GIT Region) as the fixed model and Experiment/Animal/Region for the random model. The protein concentration and pH readings were analysed using a two way ANOVA (GenStat 9th Edition) was performed to assess the effect of feeding strategy on the yield of peptides with proliferative activity from the different regions of the GIT.
Results
Optimisation of protein concentration for intestinal proliferation assay
Initially a protein concentration series was tested to identify the concentration giving optimal proliferative responses of T84 cells (Fig. 1). The concentration of 1 mg protein/ml yielded the most consistent proliferative response using the 3 intact colostrum samples relative to the concentrations of 0·0001 to 1 mg/ml. This protein concentration was then used for all subsequent proliferative assessments.

Fig. 1. Optimisation of sample concentration for application in the T84 cell proliferation assay. Cell proliferation of T84 cells in response to FCS (foetal calf serum; positive control), PP (non-specific protein control- Polypep), SFM (base media control) and three different colostrum samples (C1, C2 and C3) at various concentrations. Each bar represents the mean±sem from three replicated experiments each performed in triplicate.
In vitro digestion of colostrum
Conditions were optimised for production of LMWPs from in vitro digestion of colostrum. Digestion of first day colostrum with the enzyme pepsin at 0·1, 1 and 5 U for 1, 2 and 24 h of incubation resulted in a decrease in the number of protein bands present on the gel over time with the exception of the protein band at 15 kDa which most likely represented β-lg (Fig. 2a). β-lg remained relatively stable throughout all digestion time points and enzyme concentrations except with 5 U pepsin for 24 h after which protein bands were no longer observed. An abundance of LMWPs was seen below 15 kDa after incubation with 0·1 U pepsin for 2 h. At this time point (lane 5, Fig. 2a) the majority of high molecular weight proteins were degraded, compared with 0·1 U pepsin at 1 h (lane 2, Fig. 2a). Optimal conditions for chymosin and trypsin digestion of colostrum were selected based on the presence of LMWPs and degradation of high molecular weight proteins (data not shown).

Fig. 2. Protein profiles and proliferative activity of in vitro digested bovine colostrum. (a) Representative 15 % SDS-PAGE demonstrating different protein profiles of colostrum when digested with 0·1, 1 and 5 U of pepsin at pH 3 and 37 °C for 1, 2 and 24 h time-points. (b) Cell proliferation of T84 cells in response to FCS (foetal calf serum; positive control), PP (non-specific protein control- Polypep), SFM (base media control) and three different colostrum samples (C1, C2 and C3) in the absence (NE: No Enzyme) and presence of pepsin (P), chymosin (Ch) and trypsin (T). Each bar represents the mean±sem from three replicated experiments each performed in triplicate. Columns that do not share the same letter are significantly different from each other at P<0·05.
Cellular proliferation in response to in vitro digested colostrum
The proliferation of intestinal epithelial cells differed in the presence of colostrum samples digested with exogenous enzymes compared with the PP and SFM controls (Fig. 2b). PP was used as a non-specific protein negative control, FCS was used as a positive control and SFM was used as a base media control. Undigested colostrum samples (C1, C2, C3; Fig. 2b NE-no enzyme) induced a similar proliferative activity to that with the FCS positive control, which was significantly greater than that observed with the PP and SFM controls. No significant differences in the proliferative activity of cells were seen between colostrum samples (C1, C2 and C3) exhibiting different milk protein genotypes (Fig. 2b). Colostrum digested with chymosin retained its proliferative action on cells in comparison wit undigested colostrum as did C1 and C2 digested with pepsin. The retention of proliferative activity suggests that these enzymes may not have had an effect on cell viability. In contrast, trypsin digestion resulted in a significant reduction (P<0·05) in the proliferative activity of cells co-cultured with the digest products when compared with undigested colostral protein fractions: cell morphology on the other hand remained unchanged between different enzyme treatments suggesting cell viability was not directly affected and that trypsin may degrade the biologically active molecules responsible for the observed proliferative effect in other treatments.
It is unclear whether the same molecule(s) was responsible for the observed proliferative activity caused by treatment with undigested colostrum and chymosin or pepsin digested colostrum. The only protein that appeared to remain intact following chymosin and pepsin digestion was β-lg. However, it was also intact following trypsin digestion which resulted in a decrease in cellular proliferation suggesting that β-lg was not the biologically active agent responsible for the proliferative activity observed in both undigested and chymosin or pepsin digested colostrum. Thus different molecules may be responsible for the biological activity observed in the undigested and digested colostrum.
In vivo digestion of colostrum
Colostrum proteins digested in vivo from samples collected from specific regions of the GIT of calves fed once or multiple times were examined using tris-tricine gels. LMWPs were clearly evident at approximately 2·5–3·5 kDa, throughout the GIT of all calves fed multiple times prior to sacrifice irrespective of the timing of the last feed (Fig. 3b and Table 1). These LMWPs were only present in the abomasal casein precipitate of group 1 calves (Fig. 3a). In general, the abomasal casein precipitate showed the greatest difference in protein concentration between calves, relative to digests from other intestinal regions and abomasal whey proteins. Despite the variation, protein bands were expressed consistently between 15–30 kDa in all samples excluding the spiral colon in feeding group 1. In addition, high molecular weight (>100 kDa) immunoglobulins were present on tris-tricine gels in all samples as far as the spiral colon (data not shown). The abomasal and intestinal contents of a control calf which consumed no colostrum were used to distinguish between milk proteins and those derived from endogenous secretions. Similarities were seen in digesta from the distal duodenum and jejunum of the control calf when compared with all other calves in that two prominent protein bands were present at approximately 12 and 55 kDa in all samples (data not shown).

Fig. 3. Representative tris-tricine gels showing protein profiles of in vivo digested colostrum and respective proliferative activities. (a) Protein profile of a feeding group 1 calf given only one feed 30 min prior to collection. (b) In vivo digested colostrum from a feeding group 2 calf given three feeds for 24 h with the last feed at 1·5 h prior to collection. (c) Cell proliferation of epithelial cells in response to FCS (positive control), PP (non-specific protein control- Polypep), SFM (base media) and colostrum digesta from different regions of the GIT from feeding group 1 and 2 (1 g/l). Each bar represents the mean±SEM from three replicated experiments. The rate of replication within the experiment varies from 18 to 27 wells. Columns that do not share the same letter are significantly different from each other at P<0·05. FCS: foetal calf serum; PP: polypeptide; SFM: serum free medium; C: undigested colostrum; AC: abomasal casein precipitate; AW: abomasal whey; PD: proximal duodenum; DD: distal duodenum; J: jejunum; SC: spiral colon.
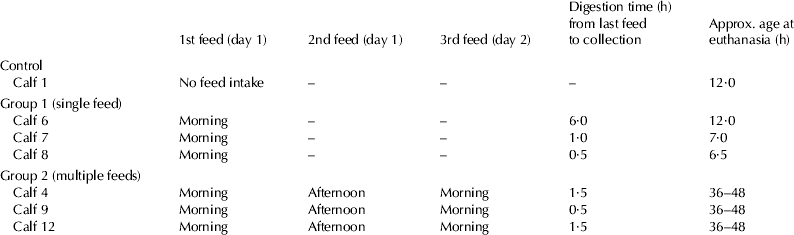
The protein concentrations of whole colostrum given to the three calves within each of the two feeding groups were not significantly different (Table 2). The protein concentration of samples collected from the abomasum through to the jejunum in group 1 calves remained significantly higher than in the comparable samples collected from group 2 calves. Interestingly, the colonic samples showed the reverse to the latter where the group 2 colon samples were significantly higher in protein concentration than in samples collected from colon of group 1 calves which only received a single feed. The pH of samples collected from the abomasum, proximal duodenum, distal duodenum, jejunum and spiral colon of group 1 calves (n=3) and group 2 calves (n=3) was not significantly different between feeding groups, however, pH differed significantly between GIT region in all calves analysed (Table 2). Before ingestion, the pH of colostrum was 6·30±0·21, which decreased significantly to 4·86±0·56 in the abomasum. This value was significantly lower than observed in all other regions of the GIT. A steady increase in pH continued through the duodenum until it reached 6·85±0·42 in the jejunum. This was significantly higher than samples collected from the abomasum, proximal duodenum and colon. The colonic digesta pH was 6·26±0·27 which was not significantly different from the pH of the colostrum originally consumed by the calves.
Table 2. Protein concentration (g/l) and pH of in vivo digested samples from group 1 calves (6, 7 & 8) and group 2 calves (4, 9, 12)
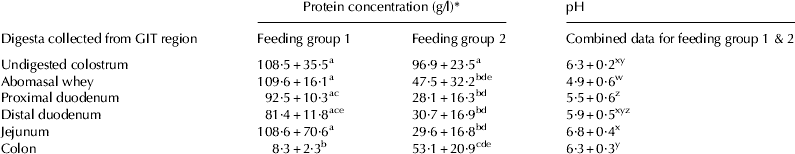
* Data expressed as means±sd. Letters a,b,c,d and e denote protein concentration and w, x, y, and z denote pH that are the statistically similar or different (P<0·05).
Cellular proliferation in response to in vivo digested colostrum
The proliferative activity of abomasal whey protein remaining after precipitation of caseins to form a curd, from both feeding groups, did not significantly differ from that of whole colostrum protein (Fig. 3c). Once colostrum protein entered the more distal regions of the intestinal tract, differences in the proliferative activity of the protein fractions became apparent. Peptide samples collected from the proximal and distal duodenum as well as the jejunum in calves from feeding group 1 did not promote observable proliferation of cells above that observed with control samples (PP and SFM), whereas samples collected from the distal duodenum and jejunum in calves from feeding group 2 did not retain their proliferative activity when co-incubated with cells. Significant differences were also seen in the proliferative response to protein samples collected from the colon of calves from the two different feeding groups. The colonic samples in group 2 fed calves receiving multiple feeds did not promote observable proliferative activity above control levels in the gastrointestinal epithelial cells whereas when calves were fed once daily (Group 1) the colonic peptides/proteins promoted proliferation of epithelial cells comparable to the whole colostrum and abomasal whey protein samples.
Calves receiving a single feed exhibited an absence of LMWPs in all digesta with the exception of the abomasal casein curd. All digests collected along the tract from these calves displayed a higher protein concentration excluding the colon sample and a similar if not higher proliferative activity per unit of peptide added compared with comparable samples collected from calves receiving multiple colostrum feeds (group 2).
Discussion
The effect of bovine colostrum on human intestinal epithelial cell growth was investigated to identify a source of growth promoting agents. Whole colostrum clearly had growth promoting properties that were not just due to a non-specific protein effect. Similarities between FCS and colostrum on the proliferation of epithelial cells were evident from this study. FCS is known to contain growth-promoting peptides such as EGF, IGF-1, HGF and TGF-β. These peptides can promote growth and proliferation by stimulating the ERK1/2 MAP kinase pathway (Rauch et al. Reference Rauch, Feifel, Amann, Spotl, Schennach, Pfaller and Gstraunthaler2011). Undigested bovine colostrum is well known to contain growth-promoting peptides including those present in FCS; transforming growth factors, epidermal growth factors and insulin-like growth factors (Rauch et al. Reference Rauch, Feifel, Amann, Spotl, Schennach, Pfaller and Gstraunthaler2011). These growth factors are most likely acting as the mitogens for the epithelial cells in the present study in both digested and whole colostrum fractions. To our knowledge, the proliferative effect of colostrum digested in vivo on gastrointestinal epithelial cells has not been reported previously.
The sample preparation procedures for colostrum and digests adopted in the current study involving defatting and filtration through a 0·22 μm filter were mild in comparison with those used in other studies: this makes it difficult to make direct comparisons with previously published results. It is well established that proteins and their biological activity may be altered by heat (Chatterton et al. Reference Chatterton, Smithers, Roupas and Brodkorb2006), pH changes, high pressure and chemical treatments to induce glycosylation and deglycosylation, phosphorylation and dephosphorylation (Korhonen et al. Reference Korhonen, Pihlanto-Leppala, Rantamaki and Tupasela1998). Sample preparation in this study aimed to maintain the biological activity of the colostral digests. Importantly, all of the protein fractions tested in this study contain enzymes that may affect cell viability. Coste et al. (Reference Coste, Rochet, Leonil, Molle, Bouballab and Tome1992) compared a heat inactivated and a control preparation of chymosin digested bovine β-casein. Both preparations resulted in a proliferative response for lymph nodes in comparison to co-incubation with native bovine β-casein suggesting that chymosin did not influence cell survival. In a study by Jovani et al. (Reference Jovani, Viadel, Laparra, Barber and Farre2004) heat inactivation of pepsin, pancreatin and bile extract was necessary to prevent cell detachment during mineral uptake assays using Caco2 cells. However, there was no observable cell death or cell detachment in the present study. Trypan blue or a cell viability assay could have been performed to confirm this.
Pancreatic enzymes such as chymotrypsin, trypsin, lipase and amylase are secreted in areas of the proximal duodenum through to the jejunum (Guilloteau et al. Reference Guilloteau, Corring, Toullec and Robelin1984; Yvon et al. Reference Yvon and Levieux1993) and may be involved in the decrease in proliferation as seen with the trypsin digested samples and in fractions obtained from the proximal duodenum, distal duodenum and jejunum, particularly for group 2. Further studies with individual pancreatic enzymes such as chymotrypsin could be conducted to assess if similar decreases in proliferation are seen. Similarly, a control with enzymes alone (without protein fractions) could be included in future studies.
Day one colostral protein enhanced the proliferative activity of intestinal epithelial cells which was also found in a study by Purup et al. (Reference Purup, Vestergaard, Pedersen and Sejrsen2007) where the whey fraction from undigested bovine milk from different stages of lactation stimulated the growth of a human foetal small intestinal cell line. In the present study, enzymes were selected to represent the secretion that would be present in the digesta collected in vivo. Similarities in proliferative activity were seen in digesta samples collected from digestion both in vivo and in vitro.
Colostrum digested with chymosin and pepsin in vitro promoted intestinal cell proliferation in a similar way to the abomasal whey fraction from the in vivo digested samples from single and multiple fed calves, where both chymosin and pepsin are active. Previously, pepsin-chymosin digestion of caseins has been shown to yield peptides that enhance the lymph node and spleen cell proliferative response in rats (Coste et al. Reference Coste, Rochet, Leonil, Molle, Bouballab and Tome1992). These peptides may also act as an agent responsible for promotion of gastrointestinal cell growth observed in the current study. In contrast, in vitro trypsin digestion of colostrum, decreased proliferative activity in comparison to whole colostrum and chymosin digested samples in the present study. Interestingly, trypsin is predicted to internally cleave, and hence inactivate, the biologically active casein peptide reported by Coste et al. (Reference Coste, Rochet, Leonil, Molle, Bouballab and Tome1992) suggesting that further investigation of this peptide as the agent responsible for promotion of gastrointestinal cell growth may be warranted. In addition, trypsin is typically released from the pancreas where it is secreted into the small intestine (Guilloteau et al. Reference Guilloteau, Zabielski and Blum2009). Hence, the failure of the digesta collected from the distal duodenum and jejunum to stimulate cell proliferation may be due to this enzyme.
The enhanced proliferative activity of colonic digesta from once fed calves (group 1) may be due to the lack of priming of enzyme secretions in the calves receiving a single feed in comparison to the older multiple fed calves. Thus biological activity is conserved throughout the GIT following the first initial feed to promote early intestinal growth and development. This enhanced activity could also possibly be related to the colonic origin of the T84 cell line which resembles human colonic crypt cells (Burleigh et al. Reference Burleigh, Fernandes and Perrit2000). Digesta isolated from a comparable site of the GIT influencing the cells derived originally from this same area of the GIT. Yet there are also several reports of milk proteins or milk product derived constituent peptides, in particular α-la, inhibiting colon derived neoplastic cells (Ganjam et al. Reference Ganjam, Thornton, Marshall and MacDonald1997; Sternhagen & Allen Reference Sternhagen and Allen2001).
The absence of LMWPs from the digesta, excluding the abomasal casein precipitate of newborn calves given a single feed of colostrum following digesta collection, was most likely due to rapid absorption from the GIT within 6 h following consumption. From 7 to 36 h post-partum the intestinal epithelium facilitates absorption of peptides and proteins up to the size of immunoglobulins to provide effective passive immunity and initiate developmental processes (Andrews, Reference Andrews2004). The absence of caseins from tris-tricine protein profiles of abomasal whey protein from group 1 calves suggests the precipitation of casein below a pH of 4·5. In this lower pH range at 3·5–3·7 chymosin will reach peak activity (Starovoitova et al. Reference Starovoitova and Velichko2006) while the optimal pH for pepsin activity is 1·4 (Dee et al. Reference Dee, Pencer, Nieh, Krueger, Katsaras and Yada2006). The pH of the abomasal whey in this study was 4·86±0·56 indicating appropriate conditions for enzymatic hydrolysis even though it was seemingly lower than the pH value of 5·63±0·11 reported by Gregory (Reference Gregory2003) in samples collected from three calves. These workers reported that colostrum with a pH of 6·29±0·2 had a strong buffering capacity within the abomasum: this was not apparent in the present study. Many reports have shown that intestinal chymosin is present in high concentrations at birth and declines with age (Guilloteau et al. Reference Guilloteau, Corring, Toullec and Robelin1984; Le Huerou-Luron et al. Reference Le Huerou-Luron, Guilloteau, Wicker Planquart, Chayvialle, Burton, Mouats, Toullec and Puigerserver1992; Davis & Drackley Reference Davis and Drackley1998). However, in the study of Gregory (Reference Gregory2003) it was suggested that some calves possess a deficiency in chymosin activity within the abomasal fluid which was not apparent in this study based on the patterns of protein degradation found in the casein curd on tris-tricine gels. The use of only three calves in the single fed group 1 however, may have limited our ability to evaluate chymosin activity in newborn calves. The presence of enzymes such as pepsin in the abomasum may have aided the abomasal precipitation of casein (Guilloteau et al. Reference Guilloteau, Corring, Toullec and Robelin1984; Davis & Drackley Reference Davis and Drackley1998). Calves receiving multiple feeds with digesta collected 0·5 to 1·5 h following a final feed were 36 to 48 h old at the time of collection of intestinal contents. A combination of gut closure and the presence of residual peptides from the first two colostrum feeds provides the likely explanation for the presence of LMWPs in group 2 multiple fed calves.
Furthermore, differences in peptide and protein composition between regions of the calf GIT is possibly related to the extent of proteolysis that occurred as digesta passed through the tract. The stability of β-lg, α-la, and immunoglobulins to hydrolysis in the GIT in all samples was consistent with previous observations (Yvon et al. Reference Yvon and Van Hille1984, Reference Yvon and Levieux1993; Toullec et al. Reference Toullec and Lalles2001). In some samples, however, β-lg and α-la were not present in the contents collected from the jejunum and spiral colon: they are known to be digested by pancreatic elastase II secreted into the duodenum (Guilloteau et al. Reference Guilloteau, Zabielski and Blum2009). Interestingly, a previous study (Toullec et al. Reference Toullec and Lalles2001) found that colostral IgG can resist digestion by proteolytic enzymes along the GIT up to the ileum which is consistent with results from this study in which high molecular weight (>100 kg/mol) immunoglobulins were present on Tris-tricine gels in all samples as far as the spiral colon. Small peptides found in the abomasum of lambs (Yvon et al. Reference Yvon and Levieux1993) and calves (Yvon et al. Reference Yvon and Pelissier1985) fed bovine milk and colostrum were found to originate from casein as whey proteins were processed and absorbed rapidly from the abomasum. Gut closure is an important factor when assessing the protein components of digesta from a newborn calf. In this study, the use of 36 to 48 h old calves for feeding and then recovery of gut contents after a short period of digestion increased the yields of LMWPs as gut closure was likely to be complete by this time. These LMWPs are of great interest as it is likely that they may contain a vast array of biological activities. They may act locally by interacting with the luminal surface of the gastrointestinal cells or through translocation through tight junctions or absorption (Meisel & Bockelmann Reference Meisel and Bockelmann1999).
Conclusion
These data suggest that the protein component of bovine colostrum when subjected to exogenous and endogenous proteolyic enzymes may influence GIT epithelial cell proliferation in human intestinal epithelial cells. In particular, protein fractions generated after the first calf feeding following birth may yield peptides that influence the proliferation of the GIT. Chymosin digestion in vitro yields similar bioactivity. A chymosin-pepsin generated casein peptide has previously been associated with promoting growth of other cell types and is a candidate as a proliferative peptide in the present study. The relevant fractions identified in this study should be further characterised to identify the biologically active peptides responsible for promoting human gastrointestinal cell growth. These will have applications for both animal and human health.
The authors would like to thank Dr Guy Saunders at the University of Adelaide, South Australia for providing T84 cells and Mr Kim McKean for assisting with colostrum and digesta collection. This work was supported by CRC for Innovative Dairy Products and the Geoffrey Gardiner Foundation.