During heat treatment of milk, numerous reactions occur that influence the nutritional, physical and functional properties of the milk. The nature and extent of these reactions depend on the temperature, the duration of the heat treatment and milk composition (total solids, mineral composition, protein content and pH) (Parris & Baginski, Reference Parris and Baginski1991; Xiong et al. Reference Xiong, Dawson and Wan1993; Oldfield et al. Reference Oldfield, Singh and Taylor2005).
The mineral equilibria between the colloidal and aqueous phases of milk, particularly the calcium and phosphate equilibria have an important role in maintaining the integrity of casein micelles (Aoki et al. Reference Aoki, Yamada and Kako1990). There is ~29 mm of calcium in milk, of which ~19 mm is colloidal and ~10 mm is in the serum phase. Of the latter, free ionic calcium is about ~2 mm and ~7 mm is coordinated to citrate and ~0·7 mm to phosphate. There is ~21 mm of phosphate in milk. Of this, about 8 mm is colloidal, 0·7 mm is bound to calcium in the serum phase as CaHPO4 and 10 mm is free inorganic phosphate present as HPO42− and H2PO4− (Holt, Reference Holt2004). Changes in composition of milk with respect to calcium, phosphate and pH result in changes in the chemical equilibria, the structure, processability and the functional properties of milk such as solubility, gelation, viscosity and emulsification (Zhang & Aoki, Reference Zhang and Aoki1996; Augustin & Udabage, Reference Augustin and Udabage2007). Some of the most important calcium equilibria are shown below:
The solubility of calcium phosphate decreases markedly with increase in temperature (Morr, Reference Morr1985; Castro et al. Reference Castro, Corzo and Olano1986; O'Connell & Fox, Reference O'Connell and Fox2001; de la Fuente et al. Reference De La Fuente, Olano and Juarez2002; Singh, Reference Singh2004). On increasing the temperature, precipitation of calcium phosphate occurs until the equilibrium is re-established (Eq 1). Removal of HPO42− and PO43− from the serum phase requires re-equilibration of equation 2 & 3, which results in a decrease in pH. In addition, calcium forms complexes with phosphates and citrates in the serum phase as illustrated in equation 4, 5 & 6.
On cooling milks that have been heated (<100°C), there is largely a reversal of these pH and calcium activity changes (Augustin & Clark, Reference Augustin and Clarke1991; Pouliot et al. Reference Pouliot, Boulet and Paquin1989b). The extent of the reversibility of these changes depends on the severity of the heat treatment (Pouliot et al. Reference Pouliot, Boulet and Paquin1989b). For example, Zhang & Aoki, (Reference Zhang and Aoki1996) & Zhang et al. (Reference Zhang, Foegeding and Hardin2004) stated that approximately 60% of calcium and 40% of phosphate in the serum phase transferred to the colloidal phase on heating to 90°C for 40 min and after cooling to 4°C for 20 hrs, 75–90% of the heat precipitated calcium phosphate was resolubilized. These findings indicate that the composition of calcium phosphate, its dissolution behaviour, and the amount of calcium bound directly to casein remained largely unaffected by heating below 90°C, provided cooling was followed by prolonged storage. The slow re-equilibration in the colloidal calcium phosphate composition, which takes place after heating, was attributed to a complex equilibrium by Pouliot et al. (Reference Pouliot, Boulet and Paquin1989b).
With few notable exceptions (Chaplin & Lyster, Reference Chaplin and Lyster1988; Ma & Barbano, Reference Ma and Barbano2003), most of the studies on pH and calcium activity of milks to date have been carried out by heating the milk and determining the changes that have occurred after the milk is cooled. The current study focuses on the heat-induced changes that occur in milk as measured directly in real time during heating. Heat treatment has a significant effect on the functionality of milk. Heat treatment is applied prior to yoghurt fermentation to improve the texture of yoghurt products. However, heat treatment may cause undesirable effects such as gel formation during sterilisation of milk. Understanding the changes in pH and calcium activity in response to temperature change from 25 to 90°C is important as they influence the heat-induced changes to protein which have effects on milk functionality. The pH and calcium activity changes in situ have been examined as a function of initial pH (pH 6·2–7·2), milk concentration (9–21% w/w MSNF) and the addition of calcium complexing agents (10–30 mm phosphate (Pin) & EDTA). While pH measurements were carried out at 90°C, the limitations of the calcium electrode used in this study only allowed reliable measurements of calcium activity up to 60°C. Measurements of pH and calcium activity were also done on cooling the milks after heat treatment to assess the extent of reversibility of the changes that occur during heating.
Materials and Methods
Materials
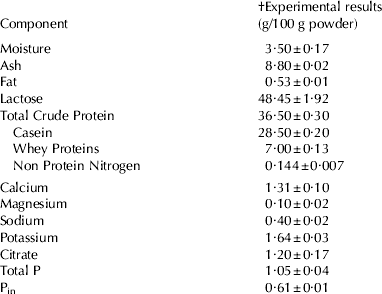
A commercial low heat (72°C/20 s) skim milk powder (SMP) was obtained from Tatura Milk Industries (PO Box 213, Tatura, VIC 3616, Australia). The compositional analysis of the powder was carried out using standard methods (International FIL-IDF methods, 1964, 1987, 1990 and 1993; Standards Association of Australia, 1988, 1991 & 1994). The composition is given in Table 1. All other chemicals were analytical grade and they were obtained from BDH Chemicals (Kilsyth, VIC 3137, Australia) and Sigma-Aldrich Pty Ltd (Castle Hill, NSW 1765, Australia). Ultra pure (MilliQ) water was used at all times.
Table 1. The composition of the skim milk powder used throughout the study
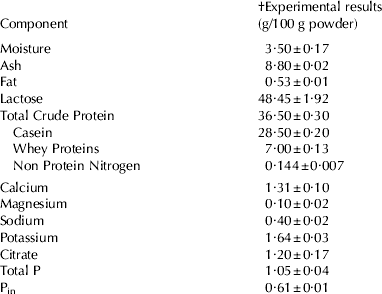
† Mean values and s.d. of (n⩾3) analyses
Preparation of reconstituted skim milk solutions
SMP was reconstituted in MilliQ water to obtain 180 g/kg or 300 g/kg MSNF solution. The skim milk solution was continuously stirred for one hour at room temperature (~25±3°C) to ensure complete mixing. MilliQ water was added to dilute the milk solution. The pH adjustment was carried out by drop wise addition of 0·1m-NaOH or 0·1m-HCl. Further addition of MilliQ water was made such that at the end of the final pH adjustment, only a small amount of additional MilliQ water was required. The skim milk solutions were kept overnight at 4°C. On the next day, the milk solutions were equilibrated at 25°C for 1 hour. Minor pH adjustments were carried out as necessary and very small amounts (~0·5 g) of MilliQ water was added to obtain milk solutions at the final desired concentration (9–21% w/w MSNF) and pH (6·2–7·2).
For the preparation of milk solutions with added salts (Pin or EDTA), the salt solutions were added drop wise with continuous stirring. Calculated amounts of MilliQ water were added, before the addition of the respective salt solution such that at the end of the addition and final pH adjustment, only a small amount of MilliQ water was required to obtain the 9 or 18% w/w MSNF solution with the respective amounts of the additive (10–30 mm added Pin or EDTA). All amounts were measured by mass. The salt solutions used were 100 mmol Pin (equi molar mixture of Na2HPO4 and NaH2PO4)/kg and 200 mmol Na2H2EDTA/kg.
Heat treatment of reconstituted skim milk solutions
Aliquots (50 g) of milk were transferred to stainless steel tubes and heated in a water bath at 90°C. The time to reach 90°C was 8 min. The skim milk solutions were held for a further 10 min at 90°C. After the heat treatment, the samples were cooled by immersion in a water bath held at 25°C for 5–10 min. The cooled solution was stored overnight at 4°C. The next day, the milk solution was equilibrated at 25°C for a minimum of 1 hour prior to measurements.
pH measurements
The pH of the skim milk solutions at 25 and at 90°C was measured by an InPro 2000 liquid electrolyte pH electrode with an integrated temperature sensor (Mettler Toledo, Australia) connected to a Metrohm pH meter (Metrohm AG, Oberdorfatrasse 68, 9101 Herisau, Switzerland). The pH probe was calibrated at 25°C using phosphate buffer at pH 6·86 and phthalate buffer at pH 4·01. The temperature compensation of the pH electrode was checked against the buffer systems used in the present study (CRC Handbook of Chemistry and Physics, 1974–1975). The actual pH measured by the meter was compared with the calculated values and the compensation of the pH meter was accurate within pH unit ±0·018 for the buffer solutions in the pH range of 4 to 8.
Measurement of calcium activity
Ca2+ activity was measured at 25 and at 60°C using a Ca Ion Selective electrode connected to a pH meter (Metrohm, AG CH-9101, Hensau, Switzerland) fitted with a reference Ag/AgCl electrode. Calibrations were carried out using a range of CaCl2 solutions from 0·0003 to 0·025 m, with an ionic strength of 0·08 m, adjusted with KCl. A standard curve of potential vs ln ![]() was used. The Ca2+ activity (
was used. The Ca2+ activity (![]() ) of these standard solutions was calculated as:
) of these standard solutions was calculated as:
Where, c 0=1 mmol/L and ![]() and 0·403 are the activity coefficients of the Ca2+ as given by Debye-Huckel approximation at 25 and at 60°C respectively. The calibrations were performed every time prior to measurements of each batch. The standard curve was reproducible. Although the manufacturers stated that the upper limit for the electrode was 80°C, it was found that the practical upper limit was 60°C.
and 0·403 are the activity coefficients of the Ca2+ as given by Debye-Huckel approximation at 25 and at 60°C respectively. The calibrations were performed every time prior to measurements of each batch. The standard curve was reproducible. Although the manufacturers stated that the upper limit for the electrode was 80°C, it was found that the practical upper limit was 60°C.
Results and Discussion
Milk solutions without added calcium complexing agents
The pH and calcium activity at 25°C, at 90/60°C and at 25°C after re-cooling of a series of skim milk solutions with concentrations ranging from 9–21% w/w MSNF with initial pH values at 25°C before heating of 6·2 to 7·2 are listed in Table 2 & 3 respectively.
Table 2. The pH at 90 and at 25°C after re-cooling of 9–21% w/w MSNF milk solutions as a function of initial pH values at 25°C prior to heating of 6·2 to 7·2

† Mean values and s.d. of (n=3) analyses
‡ The skim milk solutions at their unadjusted pH values
Table 3. The calcium activity at 60 and at 25°C after re-cooling of 9–21% w/w MSNF milk solutions as a function of initial pH values at 25°C prior to heating of 6·2 to 7·2

† Mean values of (n=3) analyses. Pooled s.d.=0·024
‡ The skim milk solutions at their unadjusted pH values
The decrease in pH on heating from 25 to 90°C (ΔpH (25–90°C)) was defined as the difference between the pH at 25°C before heating and pH at 90°C (measured at 90°C). In the present study, the magnitude of the pH change from heating 25 to 90°C was greater than previously reported by Chaplin & Lyster, (Reference Chaplin and Lyster1988) where the pH measurements were limited to temperature up to 80°C. This is expected because of the higher temperature used in our work. The change in pH in situ was linearly related to temperature during heating. The slope of plots of pH at temperature versus temperature for 9% w/w MSNF milk at pH 6·65 was found to be −0·0079 in this study. This value is close that obtained (−0·0073) by Chaplin & Lyster (Reference Chaplin and Lyster1988) for pH 6·65 milk solutions. To our knowledge, this is the first time that pH at 90°C for concentrated milk systems have been reported.
For unheated milks, the calcium activity decreased with increasing milk concentration and with increasing initial pH at 25°C. These results are in accordance with others (Nieuwenhuijse et al. Reference Nieuwenhuijse, Timmermans and Walstra1988; Van Boekel et al. Reference Van Boekel, Nieuwenhuijse and Walstra1989; Augustin & Clark, Reference Augustin and Clarke1990, Reference Augustin and Clarke1991; De la Fuente, Reference De La Fuente1998; Lin et al. Reference Lin, Lewis and Grandison2006). As pH decreases, the dissolution of calcium phosphate from the colloidal phase to the aqueous phase occurs (Van Hooydonk et al. Reference Van Hooydonk, Hagedoorn and Boerrigter1986; Dalgleish & Law, Reference Dalgleish and Law1989; Goddard & Augustin, Reference Goddard and Augustin1995). This leads to an increase in the amount of Ca2+ ions in the serum, which results in an overall increase in calcium activity. The calcium activity decreased on heating from 25 to 60°C in accord with the results of other authors (Geertz et al. Reference Geertz, Bekhof and Scherjon1983; Nieuwenhuijse et al. Reference Nieuwenhuijse, Timmermans and Walstra1988; Van Boekel et al. Reference Van Boekel, Nieuwenhuijse and Walstra1989; Augustin & Clark, Reference Augustin and Clarke1990; De la Fuente et al. Reference De La Fuente, Olano and Juarez2002; Singh, Reference Singh2004; Lin et al. Reference Lin, Lewis and Grandison2006).
The decrease in pH and calcium activity during heating may be related to the changes that occur to calcium and phosphate components of the milk (Chaplin & Lyster, Reference Chaplin and Lyster1988; Pouliot et al. Reference Pouliot, Boulet and Paquin1989a; Singh, Reference Singh2004). The solubility of calcium phosphate in milk solutions decreases with increasing temperature leading to the precipitation of calcium phosphate (Eq 1). This process is in accord with the observation of progressive transfer of calcium and Pin from the aqueous phase to the colloidal phase during thermal processing (Hardy et al. Reference Hardy, Muir, Sweetsur and West1984; Nieuwenhuijse et al. Reference Nieuwenhuijse, Timmermans and Walstra1988; Pouliot et al. Reference Pouliot, Boulet and Paquin1989a; Zhang & Aoki, Reference Zhang and Aoki1996; De la Fuente et al. Reference De La Fuente, Olano and Juarez2002; Singh, Reference Singh2004). During heating, precipitation of calcium phosphate results in a re-equilibration between the HPO42− and H2PO4−/PO43− in the serum phase (Eq 2 & 3), which produces H+ ions and results in a decrease in pH. It has been found by others that there was no change in pH when reconstituted (in water) casein micelle dispersions (CMD) were heated at 95°C in the absence of serum (Le Ray et al. Reference Le Ray, Maubois, Gaucheron, Brule, Pronnier and Garnier1998). Van Boekel et al. (Reference Van Boekel, Nieuwenhuijse and Walstra1989) also observed that the whey proteins present in the aqueous phase did not contribute to the change in pH during heating. This suggests that changes in pH on heating milks are primarily influenced by components that are inherent in the serum phase of milk other than the protein components of milk.
The magnitude of the decrease in pH upon heating from 25 to 90°C increased both as a function of increasing initial pH at 25°C and increasing milk concentration from 9 to 21% w/w MSNF. Similarly, the decrease in calcium activity on heating from 25 to 60°C (Δcalcium activity(25–60°C)) was defined as the difference between the calcium activity at 25°C before heating and calcium activity at 60°C (measured at 60°C). The magnitude of the decrease in calcium activity upon heating from 25 to 60°C was lower with increase in milk concentration and increase in initial pH at 25°C. This trend is opposite to the decrease in pH during heating where a larger decrease with increasing milk concentration and increasing initial pH was observed. At an equivalent pH, the concentration of free calcium ions present in the aqueous phase of more concentrated systems is lower due to the increased concentration of serum phosphate. The lower free calcium results in less transfer of calcium ions from the serum phase to the colloidal phase during heating. This explains both the smaller decrease in calcium activity on heating as milk concentration increases and the smaller decrease in calcium activity with increasing pH at 25°C of the more concentrated milks.
The pH and calcium activity changes that occurred during heating (90°C/10 min) were largely restored after overnight equilibration at 4°C. Table 2 shows, that at lower initial pH values at 25°C (pH<7), pH at 25°C on cooling was restored to the original pH of the milk prior to heating. At higher initial pH values at 25°C (pH>7), the pH on cooling was slightly lower than that of the starting pH, with the degree of irreversibility being greater as milk concentration decreased. Other authors (Pouliot et al. Reference Pouliot, Boulet and Paquin1989b) showed that 90–95% recovery of changes in partitioning of calcium and Pin of single strength milks heated to 85°C for 40 min after subsequent cooling at 4°C for 24 hours. A high recovery of changes in calcium and Pin after cooling is consistent with a high degree of reversibility of pH values on cooling heated milks. Augustin & Clark (Reference Augustin and Clarke1991) found that heat induced changes in calcium activity are reversible upon cold storage of milk that has been heated at temperature up to 85°C provided enough time for equilibration. Thus, the reversibility of calcium activity changes observed in the present study is in line with those by Augustin & Clark (Reference Augustin and Clarke1991).
Milk solutions with added Pin or EDTA
The decrease in pH on heating of 9% w/w MSNF solutions with or without addition of (a) Pin or (b) EDTA from 25 to 90°C as a function of initial pH at 25°C are shown in Fig 1. The decrease in pH on heating from 25 to 90°C was less for skim milk solutions with the addition of calcium complexing agents (Pin and EDTA) in comparison with skim milk solutions without the additives at a particular pH. For an example, the decrease in pH was 0·43 and 0·45 for milk solutions with added 10 mm-Pin and EDTA respectively at pH 7·2, whereas the decrease in pH was 0·61 for milk solution without the addition at that particular pH. Chaplin & Lyster, (Reference Chaplin and Lyster1988) also showed a smaller decrease in pH on heating milk systems with added EDTA to 80°C compared with systems without added EDTA.

Fig. 1. The decrease in pH on heating from 25 to 90°C as a function of initial pH at 25°C with different amounts of (a) Pin (b) EDTA added 9% w/w MSNF milk solutions.
The calcium activity changes on heating from 25 to 60°C and the reversibility for 9% w/w MSNF milk solutions with/without addition of Pin and EDTA as a function of initial pH of 6·2–7·2 at 25°C are given in Table 4. The data show that the addition of calcium complexing agents lead to a reduction in calcium activity due to the complexation of calcium by the added calcium complexing agents, as also observed by others (Van Hooydonk et al. Reference Van Hooydonk, Hagedoorn and Boerrigter1986; Nieuwenhuijse et al. Reference Nieuwenhuijse, Timmermans and Walstra1988; Udabage et al. Reference Udabage, McKinnon and Augustin2000). Most of the added Pin and EDTA remains in the serum fraction (Udabage, Reference Udabage1999; Udabage et al. Reference Udabage, McKinnon and Augustin2000; McKinnon & Chandrapala, Reference McKinnon and Chandrapala2006). The calcium activity decreased at 60°C. However, this decrease in calcium activity on heating from 25 to 60°C was lower for systems with added calcium complexing agents in comparison with systems without the added calcium chelating agents for a given pH value. The decrease in calcium activity upon heating from 25 to 60°C was lower for systems with EDTA addition than Pin addition at a particular pH. Increasing amounts of added calcium chelating agents resulted in a lower decrease in calcium activity.
Table 4. The changes in calcium activity on heating at 60°C as a function of pH at 25°C prior to heating for 9% w/w MSNF milk solutions with/without addition of Pin & EDTA

† Mean values of (n=3) analyses. Pooled s.d.=±0·019
The addition of calcium complexing agents reduces the level of calcium ions available for calcium phosphate precipitation during heating as most of the calcium ions are already complexed with the added agents (equation 4, 5 & 6). This leads to a lower pH and calcium activity decrease during heating, as less phosphate is precipitated hence less phosphate re-equilibration (equation 2) and less H+ (aq) produced. The increased buffering capacity due to the additives also contributes to the lower decrease in pH during heating. In milk solutions containing additional calcium complexing agents, the distribution of ions between serum and micellar phases is modified. As an example, the calcium concentration in the serum phase decreased linearly from about 9·7 mm in the absence of added phosphate to 2·7 mm in the presence of 126 mm-phosphate at pH 6·80 (Gaucher et al. Reference Gaucher, Piot, Beacher and Gaucheron2007). These mineral transfers are quantitatively dependent on pH (range of 5·8–6·8) and are also related to the state of ionization of the phosphate ions (preferentially H2PO4− at acid pH compared with HPO42− at neutral pH (Gaucher et al. Reference Gaucher, Piot, Beacher and Gaucheron2007) or EDTA. Hence, the addition of phosphate or EDTA to the newly established thermodynamic state of the milk systems results in different magnitudes of pH decrease during heating.
The decrease in pH upon heating from 25 to 90°C was not markedly different up to 20 mm additions of Pin or EDTA at a given pH value at 25°C (Fig. 1). However, further increase in concentration of added EDTA (>20 mm), resulted in a smaller decrease in pH upon heating from 25 to 90°C, than the same amount of added Pin to skim milk solution for a given pH at 25°C. EDTA is a much stronger chelating agent than Pin. Hence, the calcium available for the precipitation of calcium phosphate is less in milk solutions with added EDTA, thereby resulting in a smaller pH decrease on heating compared with milk solutions with added Pin. Also, the disruption of the casein micelles is greater with the addition of EDTA. The present study found that the casein micelles did not reform after addition of ⩾20 mm-EDTA, which was in line with Udabage, (Reference Udabage1999). Although the micelles were not restored, the pH change was largely reversible indicating that the state of aggregation of the proteins did not influence the reversibility of the pH.
It was found that these pH and calcium activity values were largely restored back to the starting value when enough time was given for equilibration after cooling of the heated milk (data not shown). In the presence of calcium complexing agents, the reversibility of pH was lower for samples at higher initial pH at 25°C (pH >7) than those for milk solutions with lower starting pH at 25°C (pH <7), as was also observed in the absence of calcium complexing agents. Our results suggest that the lower pH reversibility is characteristic for higher pH skim milk solutions. Heating causes precipitation of calcium phosphate as its solubility is lowered at high temperature. Also on heating milk solutions with a lower initial pH at 25°C, there will be more dissolution of calcium phosphate compared to that of milk solutions with a higher starting pH – an effect that is due to increased dissociation of calcium phosphate complexes as pH is decreased. Hence it may be expected that net increase in colloidal calcium phosphate at the time of heating will be greater in milk solutions with higher starting pH. This may have contributed to the slightly lower reversibility of heat-induced changes at the higher pH.
The present work showed that the change in pH and calcium activity on heating from 25 to 90°C is not entirely predictable from the initial pH of the milk as the composition of the milk changes. Specifically the addition of mineral salts to milk has a marked influence on the changes in pH and calcium activity during heating.
This work was supported by Dairy Innovation Australia Limited and the Australian Research Council. Jayani Chandrapala would like to thank Dairy Innovation Australia and Monash University for the PhD scholarship.