Pecorino cheese is an Italian cheese made only from ewe milk that is generally produced in Central and Southern Italy (Cevoli et al. Reference Cevoli, Cerretani, Gori, Caboni, Gallina Toschi and Fabbri2011). The manufacture of many ewe milk cheeses is regulated by a Protected Designation of Origin (PDO), according to which a product has a designated origin and its production, processing and preparation has to take place in a specific geographical area (Pirisi et al. Reference Pirisi, Comunian, Urgeghe and Scintu2011). Several types of Pecorino without PDO are produced in Italy and are characterised by a shorter ripening time (60 d) and semi-hard consistency (Caridi et al. Reference Caridi, Micari, Caparra, Curari and Sarullo2003). They can be made from raw, thermised or pasteurised milk (Pirisi et al. Reference Pirisi, Comunian, Urgeghe and Scintu2011). Thermised milk involves bringing milk up to 55◦C for 2 to 16 s and is commonly used to produce artisanal Pecorino cheese in small-scale dairy plants. These artisanal cheeses are technically classified as unpasteurised (i.e., in the same category as raw milk cheese) (Fox et al. Reference Fox, McSweeney, Cogan and Guinee2004). Today there is a renewed interest among consumers in artisanal cheeses used to carry an image of foods tasting good (Cayot, Reference Cayot2007). Furthermore there is a trend in dairy production towards the enhancement of health-promoting properties in cheeses (Mele et al. Reference Mele, Contarini, Cercaci, Serra, Buccioni, Povolo, Conte, Funaro, Banni and Lercker2011; Mughetti et al. Reference Mughetti, Sinesio, Acuti, Antonini, Moneta, Peparaio and Trabalza Marinucci2012). In particular, research has focused on altering the fatty acid (FA) profile of ewe milk for the production of n-3 FA and conjugated linoleic acid (CLA)-enriched cheeses consistent with health recommendations (Gómez-Cortés et al. Reference Gómez-Cortés, Bach, Luna, Juárez and de la Fuente2009). The n-3 FA and CLA content in milk and cheese can be enhanced by feeding ruminants diets supplemented with oilseeds, such as linseed (Zangh et al. Reference Zangh, Mustafa and Zhao2006; Gómez-Cortés et al. Reference Gómez-Cortés, Bach, Luna, Juárez and de la Fuente2009; Mele et al. Reference Mele, Contarini, Cercaci, Serra, Buccioni, Povolo, Conte, Funaro, Banni and Lercker2011). Results concerning the effects of linseed-supplemented diets on the sensory properties of milk products are not consistent (Chilliard & Ferlay, Reference Chilliard and Ferlay2004; Branciari et al. Reference Branciari, Valiani, Trabalza Marinucci, Miraglia, Ranucci, Acuti, Esposto and Mughetti2012; Mughetti et al. Reference Mughetti, Sinesio, Acuti, Antonini, Moneta, Peparaio and Trabalza Marinucci2012).
The objective of this experiment was to demonstrate whether the effects of this type of diet on the sensory and nutritional qualities of artisanal Pecorino cheese can be influenced by the manufacturing procedure (use of raw milk vs. thermised milk and addition of selected lactic acid bacteria).
Materials and methods
Animals and experimental design
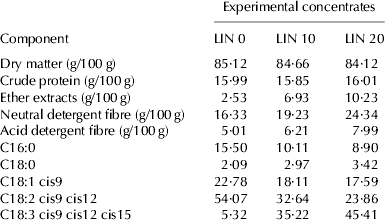
This study was performed at the experimental farm of the University of Perugia, Italy, and was conducted in accordance with the European recommendations for the protection of animals used for scientific purposes (EU Directive 2010/63/EU, September 22, 2010). Sixty-nine Sarda ewes were subjected to oestrus synchronisation and natural mating. At 20 d before the expected date of parturition, the ewes were randomly divided into three groups of equal size, balanced for body condition score and assigned to one of the following isoenergetic and isonitrogenous concentrates: a control concentrate (LIN 0) and two experimental concentrates containing 100 g/kg (LIN 10) and 200 g/kg (LIN 20) of extruded linseed, respectively (Table 1). The animals received 400 g concentrate/d during late pregnancy and 800 g/d during lactation, in two equal portions during milking. The forage base of the diet consisted of alfalfa hay (crude protein: 14·1 g/100 g; neutral detergent fibre (NDF): 46·2 g/100 g; acid detergent fibre (ADF): 34·0 g/100 g) administered ad libitum.
Table 1. Chemical analysis of the concentrates (LIN 0, control; LIN 10, 10 g extruded linseed/100 g; LIN 20, 20 g extruded linseed/100 g) used in the experimental diets. Fatty acid values are given as g/100 g total fatty acids
Lambs were weaned at 50 d of age. After weaning, a two-period and three-treatment crossover trial was conducted. Each period lasted 30 d, 15 d of which were for adaptation to the three experimental diets, and the remaining 15 d were for measurements and sample collections. The study ended at approximately 110 d of lactation. The sheep were housed in a stable with access to an outdoor paddock for the entire length of the experiment.
Cheesemaking
Bulk milk from 3 separate bulk tanks (one per dietary treatment), collected at the end of each experimental period, was used for manufacturing cheese. The ewe's milk used to produce cheeses had the following characteristics: pH of 6·62, 6·65, 6·68 for LIN 0, LIN 10 and LIN 20, respectively, containing, respectively, 4·55, 4·61 and 4·60% w/w lactose; 6·59, 6·53, 6·50% w/w fat; 5·35, 5·27 and 5·33% w/w protein (MilkoScan 6000, Foss Electric, Hillerød, Denmark) and 554 000, 632 000, 615 000 somatic cells/ml (Fossomatic 5000, Foss Electric, Hillerød, Denmark). The microbiological quality of raw milk was: total viable count 5·50, 5·62 and 5·34 log cfu/ml (EN/ISO 4833:2004); enterococci 3·04, 3·20 and 3·11 log cfu/ml (Slanetz-Bartley, Biokar Diagnostics, at 37 °C for 48 h); Enterobacteriaceae 1·82, 1·93 and 1·75 log cfu/ml (ISO 21528-2:2004); mesophilic lactic acid bacteria 4·54, 4·31 and 4·43 log cfu/ml (ISO 15214:1998).
Two different manufacturing procedures (raw milk cheese, RMC, and thermised milk cheese with addition of starter culture, TMC) were used to make Pecorino cheese from the milk of the three dietary groups (LIN 0, LIN 10, LIN 20) collected in three different refrigeration tanks.
To obtain the TMC, 50 l raw ewe milk was thermised (65 °C, 10 s), cooled to 37 °C and a lyophilised mixed-strain starter culture was added (Lactobacillus lactis subsp. helveticus and Streptococcus thermophylus – Fermenti lattici, Laboratorio Prodor, Bobbio, PC, Italy). The milk was ripened for 30 min, before adding a liquid calf rennet. Coagulation was allowed to take place over 30–45 min. When the coagulum had developed the desired firmness, evaluated subjectively, the curd was cut into small pieces (about 5 mm in size), cooked at 41 °C for 5 min and pressed into cylindrical forms. To facilitate whey draining, the forms were maintained at 30 °C, until the pH reached the value of 5.2. The curd was then dry salted and stored for ripening at 12±1 °C and 80±5% relative humidity for 60 d.
For raw milk cheeses, the cheesemaking was conducted using raw milk heated at 37 °C and a liquid calf rennet was added to curdle the milk. The following steps were the same as previously described for TMC. All samples were collected after 60 d ripening; half of each cheese was stored at −80 °C until analysis, while the other half was refrigerated and used for sensory analysis.
Approximately nine 1 kg cheeses were made from 50 l of whole ewe's milk. For each experimental group, a total of approximately 36 cheeses were obtained (9 cheeses×2 manufacturing processes×2 experimental periods).
Physicochemical analysis of feeds and cheese
All feeds were analysed for DM (AOAC, 2000), crude protein and crude fat (AOAC, 1990), NDF and ADF (Van Soest et al. Reference Van Soest, Robertson and Lewis1991). Sodium sulphite was used in the NDF procedure, and both the NDF and ADF are expressed inclusive of ash.
The physicochemical determinations of the cheese were conducted after 60 d on three samples for each set. The pH measurements were made using a puncture electrode probe connected to a portable pH meter (Mettler Toledo Inc., Columbus, OH, USA). The cheese samples were analysed for moisture, fat, protein, ash and salt content as described by Branciari et al. (Reference Branciari, Valiani, Trabalza Marinucci, Miraglia, Ranucci, Acuti, Esposto and Mughetti2012). The surface colour (CIE L*a*b* colour system, 1976) of the cheeses was measured using a Minolta Tristimulus Chromometer CR-400 (Minolta, Chromameter, Osaka, Japan- light source of D65, standard observer of 10°, 45°/0° geometry, in light surface, standard white tile). The colour measurements were performed after a 5 min bloom period at refrigeration temperature, and the results are expressed as lightness (L*), redness (a*), and yellowness (b*).
Cheese fatty acids were extracted and analysed according to Branciari et al. (Reference Branciari, Valiani, Trabalza Marinucci, Miraglia, Ranucci, Acuti, Esposto and Mughetti2012). Fatty acid methyl esters were separated and quantified using a VARIAN 3400 gas chromatograph equipped with a flame ionisation detector (FID) and a split-splitless injector. Analyses were performed using a CP-SIL column 88×50 m×0·22 mm i.d., with a 0·20-μm film thickness (Chrompack, Varian, Inc., Harbor City, CA, USA). The injection volume was 1 μl. The carrier gas was high purity helium with a flow rate of 1 ml/min. The injector and detector temperatures were maintained at 290 °C. The column oven temperature was programmed at 120 °C, increasing by 3·2 °C/min, up to 170 °C and then increasing by 2·1 °C/min from 170 to 225 °C. Fatty acids were identified by a comparison with a standard obtained by mixing a standard (37-Component FAME Mix) distributed by Supelco (Supelco Park, Bellefonte, PA, USA) and a CLA isomer (cis 9 trans 11 and trans 10 cis 12) standard obtained as described by Branciari et al. (Reference Branciari, Valiani, Trabalza Marinucci, Miraglia, Ranucci, Acuti, Esposto and Mughetti2012). Quantifications were performed using nonadecanoic acid as an internal standard (C19:0, Supelco Park, Bellefonte, PA, USA) added to the cheese samples at the time of extraction.
Sensory evaluation of the cheeses
A descriptive sensory analysis was performed using an eight-member panel, which was trained following the criteria of the ISO 8586-1:1993, on a cheese cube of 1·5×1·5×1·5 cm size. The descriptors were generated as described by Mughetti et al. (Reference Mughetti, Sinesio, Acuti, Antonini, Moneta, Peparaio and Trabalza Marinucci2012). The descriptive analysis of the cheeses was replicated twice (Lawless & Heymann, Reference Lawless and Heymann1998). For quantification of the intensity of each attribute, 9-point scales were employed, in which 0 referred to the minimum intensity and 9 to the maximum of each parameter according to ISO 13299:2003.
A preference ranking test was performed with a consumer panel consisting of 60 people recruited from the staff of the University of Perugia, with an equal male/female and age distribution, which ranged between 21 and 60 years (ISO 8587:2006 Sensory analysis- Methodology-Ranking). Each judge was asked to rank samples in decreasing order of preference (6 corresponded to the highest preference, 1 corresponded to the lowest preference).
Statistical analysis
Data concerning the chemical composition of the cheeses were analysed using SAS JMP (2001). The ANOVA model included the diet (LIN 0, LIN 10 and LIN 20) and processing technology (raw milk and thermised milk) as fixed factors, as well as their interaction. The experimental period (1 or 2) of the crossover trial was not considered in the model because it was not found to be significant (P>0·05). P value less than 0·05 was considered to be statistically significant.
A PCA model was built to analyse the fatty acids of the cheese and the sensory profile data. The chemiometric package ‘SIMCA v.13.0.0’ (Umetrics AB, Umeå, Sweden) was used. The raw data were normalised with the subtraction of the mean and autoscaled, dividing these results by the standard deviation (sd). The results of the PCA modelling are presented in graphical form (Fig. 1). The results of the ranking preference test were analysed by non-parametric Friedman's test (P<0·05).

Fig. 1. PCA. Bi-plot PC1 vs. PC2 for the 16 sensory attributes of all treatments in 60 d ripened cheese. ▲=cheese, ●=attribute, RMC, raw milk cheese; TMC, thermised milk cheese with addition of starter culture; LIN 0, control group; LIN 10, ewes fed 10 g extruded linseed/100 g concentrate; LIN 20, ewes fed 20 g extruded linseed/100 g concentrate.
Results
The chemical composition of the cheese is reported in Table 2. Differences were observed between the RMC and TMC with regard to moisture, ash, and salt. The moisture content was significantly higher in TMC compared with RMC, whereas salt and ash were lower in TMC. The cheese's chemical composition was not influenced by diet. The colour of the cheeses was affected by diet and manufacturing procedure. In particular, RMC-LIN 10 was darker than the others, and both RMC-LIN 20 and TMC-LIN 20 showed a trend towards a higher yellowness. The pH was influenced by dietary treatment and manufacturing procedure.
Table 2. Effect of linseed supplementation and manufacturing procedure on chemical composition and physical characteristics of cheeses after 60 d of ripening

Means within a row with different superscripts differ: *P<0·05. **P<0·01. ***P<0·001. NS, not significant
RMC, raw milk cheese; TMC, thermised milk cheese with addition of starter culture; LIN 0, ewes fed control concentrate without extruded linseed; LIN 10, ewes fed 10 g extruded linseed/100 g concentrate; LIN 20, ewes fed 20 g extruded linseed/100 g concentrate; M, manufacturing process effect; D, dietary effect
The fatty acid composition of the cheese was modified by the dietary treatment (Table 3), whereas only four fatty acids (C12:0, C14:1, C18:2 cis9, cis12, C21:0) were affected by the manufacturing procedure. The proportion of saturated fatty acids (SFA) from 6:0 to 17:0 decreased with the extruded linseed diets in both the RMC and TMC. Extruded linseed supplementation increased the level of most 18-carbons (18:0, 18:1, 18:2, 18:3 and isomers). The concentrations of stearic (C18:0) and oleic (cis 9 C18:1) acids were positively influenced by linseed supplementation. The vaccenic acid (VA, trans 11 C18:1) content increased almost 3-fold with the LIN 20 diet. Other trans C18:1 isomers also rose with lipid supplementation; however, the level of the increase was lower than with VA. With dietary supplementation, a remarkable increase of some C18:2 isomers was observed. In fact, the percentage of rumenic acid (RA C18:2 cis 9 trans 11) showed a similar trend to VA and an increase with linseed supplementation was also observed for the linoleic (C18:2 cis 9 cis 12) and the α-linolenic acid (C18:3 n3) values. The eicosanoid and docosahexaenoic n-3 PUFA (C 20:4 n6; C 20:5n3; C 22:6n3) percentages in the cheese were below 0·2%. The extruded linseed supplementation decreased the percentage of SFA but increased the MUFA and PUFA contents and the proportion of desirable fatty acids (DFA: PUFA+MUFA+C18:0; Rhee, Reference Rhee and Chow2000).
Table 3. Effect of linseed supplementation and manufacturing procedure on the fatty acid composition (g/100 g of total fatty acid methyl esters) of cheeses after 60 d of ripening

Means within a row with different superscripts differ: *P<0·05. **P<0·01. ***P<0·001. NS, not significant
RMC, raw milk cheese; TMC, thermised milk cheese with addition of starter culture; LIN 0, control group; LIN 10, ewes fed 10 g extruded linseed/100 g concentrate; LIN 20, ewes fed 20 g extruded linseed/100 g concentrate; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; DFA, desirable fatty acids (DFA: PUFA+MUFA+C18:0); M, manufacturing process effect; D, dietary effect
The results of the descriptive sensory profile performed on the cheese samples are reported in Table 4. The cheesemaking process influenced all descriptors, except for bitterness. The dietary treatment influenced the following descriptors: holes, sheep milk odour, ripe cheese odour, acid, ripe cheese flavour and toughness In the TMC, there were no significant differences between cheeses for any descriptors, except for the acid, whereas in the RMC group, differences among the cheeses were observed for: colour homogeneity, holes, overall odour, sheep milk odour, ripe cheese odour, ripe cheese flavour and toughness. The panel showed a good reproducibility for all the attributes except for overall odour, ripe cheese flavour and moisture. The results of the biplot for the sensory profile are shown in Fig. 1. The PCA model explained 95% of the total variance with five significant principal components. The first PC (PC1) explains 75% of the total variability, and the RMC and TMC are distinguished by this component. The TMC cheeses were distinguished from the RMC cheeses by their high loadings for moisture, acidity, colour homogeneity and screeching. PC 2, which shows approximately 10% of variability, evidenced a discrimination of objects predominantly related to differences among the RMC cheeses for several attributes, with the LIN 20 cheeses having more overall flavour, ripe cheese flavour and ripe cheese odour. In contrast, the LIN 0 cheeses were characterised by a higher score for ‘sheep milk odour’. The means of the results of the preference ranking test are 172, 130 and 89 for RMC LIN 0, RMC LIN 10 and RMC LIN 20, respectively and 274, 303 and 296 for TMC LIN 0, TMC LIN 10 and TMC LIN 20, respectively. These results indicate that cheeses obtained from thermised milk were preferred to those obtained from raw milk. In TMC, dietary treatments led to no differences in preference. In contrast, dietary treatment markedly influenced the preference of the RMC, with the RMC LIN 0 having the highest scores compared with the RMC LIN 10 and the RMC LIN 20.
Table 4. Effect of dietary and manufacturing procedure on the organoleptic properties of cheeses after 60 d of ripening

Means within a row with different superscripts differ: *P<0·05. **P<0·01. ***P<0·001. NS, not significant
RMC, raw milk cheese; TMC, thermised milk cheese with addition of starter culture; LIN 0, control group; LIN 10, ewes fed 10 g extruded linseed/100 g concentrate; LIN 20, ewes fed 20 g extruded linseed/100 g concentrate; M, manufacturing process effect; D, dietary effect; R, Replicate effect
Discussion
Significant variations were observed between RMC and TMC with regard to moisture, ash and salt content. The mild heat treatment of milk might contribute, together with other factors, to increase the protein hydrophilic properties. The denaturation of whey proteins may occur upon heating milk above 60 °C and these proteins can interact with the casein micelles by aggregation through hydrophobic interaction and disulphide-thiol interchanges (Guyomarc'h, Reference Guyomarc'h2006).
The FA profile of the cheese was markedly affected by linseed dietary supplementation, which is in agreement with other studies (Zangh et al. Reference Zangh, Mustafa and Zhao2006; Gómez-Cortés et al. Reference Gómez-Cortés, Bach, Luna, Juárez and de la Fuente2009), with the most pronounced impact being observed at the highest level (20%) of supplementation. The changes in C18:0, C18:1 and C18:2 levels in cheese fat due to linseed supplementation were similar to those obtained by Mughetti et al. (Reference Mughetti, Sinesio, Acuti, Antonini, Moneta, Peparaio and Trabalza Marinucci2012). However, in the present experiment, the modifications were more evident. This result is possibly related to the different forage base of the ration, which consisted entirely of hay, whereas in the work by Mughetti et al. (Reference Mughetti, Sinesio, Acuti, Antonini, Moneta, Peparaio and Trabalza Marinucci2012) ewes were allowed to graze natural pasture. Fresh forage-based regimens are known to enhance the content of PUFA in milk (Addis et al. Reference Addis, Cabiddu, Pinna, Decandia, Piredda, Pirisi and Molle2005).
No substantial changes in the fatty acid profiles of the cheeses were observed as a result of using raw or thermised milk with the addition of starter cultures. Our results are consistent with Gnädig et al. (Reference Gnädig, Chamba, Perreard, Chappaz, Chardigny, Rickert, Steinhart and Sébédio2004), who investigated different processing factors, such as raw or slightly heated milk (68 °C for 20 s), cooking/moulding temperatures and different strains of Propionibacterium freudenreichii, and found no effect on the CLA content in French Emmental cheese.
Heat-treating the milk and adding a starter apparently reduced the differences in the cheese's sensorial characteristics induced by diet and found in cheeses made with raw milk. These data are consistent with the results from previous research aimed at investigating the effects of the diet on consumer acceptability of Pecorino cheese made with heat-treated milk: neither a trained panel nor an electronic nose were able to discriminate among cheeses obtained from ewes fed either extruded linseed or a control feed (Branciari et al. Reference Branciari, Valiani, Trabalza Marinucci, Miraglia, Ranucci, Acuti, Esposto and Mughetti2012). As for the differences in flavour and odour attributes in RMC, they are most likely associated with specific attributes of milk induced by diet. It is known that PUFA can be responsible for modifications of milk and cheese sensory properties according to their concentration (Chilliard & Ferlay, Reference Chilliard and Ferlay2004). The lack of differences among TMC may be due to microbiota responsible for fermentation and ripening processes (Beresford et al. Reference Beresford, Fitzsimons, Brennan and Cogan2001), as natural microbiota of raw milk is affected by thermisation and starter culture development. Furthermore it is reported in literature (Kiernan et al. Reference Kiernan, Beresford, O'Cuinn and Jordan2000) that Lb. helveticus was able to influence the flavour of the cheese because it autolysed proteins very rapidly and resulted in high levels of free amino acids responsible for the flavour. In this case the flavour developed from Lb helveticus probably minimised the diet effect as long-chain FA (>12 carbon atoms) play a minor role due to their high perception thresholds (Molimard & Spinnler, Reference Molimard and Spinnler1996).
In addition, our data highlight the marked effect of the manufacturing process on the texture of cheese, since TMC had no variations in the texture. PUFA effects seemed evident in RMC only, higher PUFA levels being associated with cheeses of a softer consistency (Chilliard & Ferlay, Reference Chilliard and Ferlay2004). Sinesio et al. (Reference Sinesio, Trabalza Marinucci, Sabato, Moneta and Nardo2000) observed that the cheesemaking technique strongly affected the aspect and texture of cheeses.
Results from the present study confirm that a linseed-enriched diet, by altering the FA composition of cheese, offers the opportunity to respond to market forces on human health recommendations and to consumer demand for artisanal product. An appropriate manufacturing process (a mild milk heat treatment and the addition of specific starters) may be an acceptable strategy to obtain artisanal cheeses with improved health-promoting properties and an unaltered (or even increased) acceptance level among consumers.







