The composition of bulk tank milk has a decisive influence on the production of safe, high-quality dairy products. One major factor responsible for the deterioration of the quality of the raw milk is mastitis, and its negative effect on milk composition is well established (Kitchen, Reference Kitchen1981; Munro et al. Reference Munro, Grieve and Kitchen1984). It is the subclinical form of mastitis that constitutes problems for the dairies, since these cases often go undetected and the milk is delivered to the dairy (Leitner et al. Reference Leitner, Silanikove, Jacobi, Weisblit, Bernstein and Merin2008). Studies show that during mastitis the casein content, valuable for the cheesemaking industry, decreases while the whey protein content increases (Barbano et al. Reference Barbano, Rasmussen and Lynch1991; Auldist et al. Reference Auldist, Coats, Sutherland, Mayes, McDowell and Rogers1996; Urech et al. Reference Urech, Puhan and Schallibaum1999). In addition, increased proteolysis is often observed in milk from cows with mastitis (Schaar, Reference Schaar1985; Auldist et al. Reference Auldist, Coats, Sutherland, Mayes, McDowell and Rogers1996). Proteolysis in milk is one of the major product-deteriorating factors with negative impact on the quality and stability of milk and dairy products (Mara et al. Reference Mara, Roupie, Duffy and Kelly1998; Kelly et al. Reference Kelly, O'Flaherty and Fox2006). Saeman et al. (Reference Saeman, Verdi, Galton and Barbano1988) found that after an udder infection the proteolytic activity may be sustained even though the somatic cell count (SCC) has returned to normal levels. Larsen et al. (Reference Larsen, Rasmussen, Bjerring and Nielsen2004) established that casein degradation not only occurred in the infected quarter but also in the neighbouring quarters, even though there was no effect on SCC. In the bulk tank, milk from healthy udder quarters will be commingled with milk from infected quarters, and thus the entire bulk tank may be affected by protein degradation. This is a problem, especially for cheesemaking, since curd formation properties will be impaired and yield reduced (Mara et al. Reference Mara, Roupie, Duffy and Kelly1998, Leitner et al. Reference Leitner, Silanikove, Jacobi, Weisblit, Bernstein and Merin2008). Likewise, proteolytic activity in UHT-milk may cause off-flavours and gelation, and consequently reduced shelf-life of the products (Ma et al. Reference Ma, Ryan, Barbano, Galton, Rudan and Boor2000; Santos et al. Reference Santos, Ma and Barbano2003; Barbano et al. Reference Barbano, Ma and Santos2006; De Noni et al. Reference De Noni, Pellegrino, Cattaneo and Resmini2007).
SCC in milk has been used extensively since the 1960s in the diagnosis of mastitis, and the bulk tank milk somatic cell count (BTMSCC) is widely used in the assessment of raw milk quality. In many EU countries milk payment systems favour a low BTMSCC. There are, however, no clear scientific data defining the level of BTMSCC that is associated with additional benefits in terms of milk quality. Several authors have reported that SCC is not a suitable indicator for proteolysis in quarter milk samples (Le Roux et al. Reference Le Roux, Colin and Laurent1995; Urech et al. Reference Urech, Puhan and Schallibaum1999) and recent studies have also shown that BTMSCC gives a poor prediction of raw milk quality for cheese production (Leitner et al. Reference Leitner, Silanikove, Jacobi, Weisblit, Bernstein and Merin2008). Research to find new sensitive and specific markers for disadvantageous changes in raw milk composition due to udder health disturbances is therefore warranted.
The acute phase proteins (APP) have become important diagnostic markers of disease in human medicine and are also being evaluated in veterinary diagnostics (Eckersall, Reference Eckersall2004). The major bovine APP are haptoglobin (Hp) and serum amyloid A (SAA), which both increase dramatically upon infection, inflammation or trauma. Hp and SAA are mainly produced by the liver but are also produced locally in the mammary gland (McDonald et al. Reference McDonald, Larson, Mack and Weber2001; Hiss et al. Reference Hiss, Mielenz, Bruckmaier and Sauerwein2004). Some studies have found that Hp and SAA have antibacterial effects (Eaton et al. Reference Eaton, Brandt, Mahoney and Lee1982; Hari-Dass et al. Reference Hari-Dass, Shah, Meyer and Raynes2005; Larson et al. Reference Larson, Weber, Weber and McDonald2005) and considering that they are locally produced, their role in the inflammatory defence is interesting. In several studies Hp and SAA in milk have been evaluated as markers for mastitis (Horadagoda et al. Reference Horadagoda, Knox, Gibbs, Reid, Horadagoda, Edwards and Eckersall1999; Eckersall et al. Reference Eckersall, Young, McComb, Hogarth, Safi, Weber, McDonald, Nolan and Fitzpatrick2001; Grönlund et al. Reference Grönlund, Hulten, Eckersall, Hogarth and Persson Waller2003; Nielsen et al. Reference Nielsen, Jacobsen, Andersen, Niewold and Heegaard2004; Grönlund et al. Reference Grönlund, Hallén Sandgren and Persson Waller2005; Eckersall et al. Reference Eckersall, Young, Nolan, Knight, McComb, Waterston, Hogarth, Scott and Fitzpatrick2006; Hiss et al. Reference Hiss, Mueller, Neu-Zahren and Sauerwein2007) but so far little attention has been paid to their potential for predicting changes in milk composition and technological properties of the raw milk. In a previous study we reported that detectable levels of Hp and SAA could be found in bulk tank milk samples (Åkerstedt et al. Reference Åkerstedt, Persson Waller and Sternesjö2007). In a more recent study we also investigated APP in relation to raw milk quality parameters in cow composite milk samples (Åkerstedt et al. Reference Åkerstedt, Persson Waller, Bach Larsen, Forsbäck and Sternesjö2008). To our knowledge, these papers are the only studies examining APP in bovine bulk tank milk or applying APP research to the field of product quality. However, no studies have been reported on Hp and SAA in relation to the quality of the raw bulk tank milk. Quality programmes for milk payment and advisory measures to improve raw milk quality are mostly based on analysis of bulk tank milk samples. For APP to be a potential candidate as an indicator for unfavourable changes in milk composition due to udder health disturbances in the herd, it is important that levels of APP in the bulk tank milk are related to important quality traits of the raw milk.
The aim of this study was to investigate relationships between the presence of Hp and SAA, and various raw milk quality parameters (total protein, casein, whey protein, proteolysis, fat, lactose and SCC) in bulk tank milk samples. In addition, APP in relation to the coagulating properties of the bulk tank milk samples were evaluated.
Materials and Methods
Bulk tank milk samples
The study included 91 bulk tank milk samples collected from different dairy farms in cooperation with the Milko dairy cooperative (Grådö, Hedemora, Sweden). One representative sample from each farm was taken by the tanker driver just before emptying the bulk tank, in connection with the ordinary milk collection, which occurred every second day. At the sampling occasion the farms delivered 90–13 025 kg milk (average 1610 kg) indicating that the herd size of the participating farms varied markedly. The average BTMSCC for the samples was 195 000 cells/ml, ranging from 33 000 to 1 365 000 cells/ml (median 146 000 cells/ml). The bulk tank milk samples were collected at the dairy plant for further transportation to the university laboratory the same day. Sample aliquots for the analyses of Hp, SAA and proteolysis were frozen and stored at −70°C until analysis, whereas the other parameters were analysed using fresh milk samples.
Assay of haptoglobin and serum amyloid A
Hp was analysed by an optical biosensor assay described earlier (Åkerstedt et al. Reference Åkerstedt, Björck, Persson Waller and Sternesjö2006; Åkerstedt et al. Reference Åkerstedt, Persson Waller, Bach Larsen, Forsbäck and Sternesjö2008) with some additional modifications. In this study, the Hp surface was prepared by using a solution containing Hp in 0·01 m-acetate buffer at 20 mg/l instead of 500 mg/l, and the activation of the surface during immobilization was reduced from 7 min to 3 min. For regeneration of the sensor surface the concentration of sodium dodecyl sulphate (SDS) was increased from 2 mm to 3 mm. An extra reconditioning step was added, in which 50 mm-glycine (pH 9·5) was injected over the sensor surface for 30 s, after the ordinary regeneration step. Bovine Hp (Life Diagnostics, Clarkston GA, USA) was used for immobilization and standards, and the limit of detection (LOD) of the modified assay was 0·3 mg/l.
SAA was determined using a commercial ELISA with a LOD of 0·3 mg/l (Phase™ Serum Amyloid A Assay, Tridelta Development Ltd, Wicklow, Ireland).
Measurement of SCC, total protein, whey protein and casein content, casein number, fat and lactose content
SCC in the bulk tank milk samples was measured by an electronic fluorescence-based cell counting technique (Fossomatic 5000, Foss, Hillerød, Denmark). Total protein, fat and lactose contents were measured on fresh milk using mid-infrared spectroscopy (Fourier Transform Instrument, FT 120, Foss). Casein content was determined by an indirect method which was described earlier (Åkerstedt et al. Reference Åkerstedt, Persson Waller, Bach Larsen, Forsbäck and Sternesjö2008).
Measurement of proteolysis
The extent of proteolysis in the milk sample was measured according to a fluorescamine method as previously described (Wiking et al. Reference Wiking, Frost, Larsen and Qvist2002).
Measurement of coagulating properties
Coagulating properties of the milk were measured with a Bohlin VOR Rheometer (Malvern Instruments Nordic AB, Uppsala, Sweden) according to Hallén et al. (Reference Hallén, Allmere, Näslund, Andrén and Lundén2007) with one minor modification, i.e. Chymax Plus, strength 200 IMCU per gram (Christian Hansen A/S, DK-2970, Hørsholm, Denmark) was used instead of pure chymosin. Coagulation time was measured, i.e. the time (s) elapsed from chymosin addition until a weak coagulum corresponding to 5 Pa was formed. In addition, curd firmness (Pa) was measured 25 min after chymosin addition.
Statistical analyses
Parametric t test using SAS (Version 9.1, SAS Institute Inc., Cary NC, USA) was used to evaluate the relationships between APP and the various raw milk quality parameters analysed. Bulk tank milk samples were categorized into two groups; detectable or non-detectable levels of Hp or SAA, based on the detection limits of the assays used to determine the proteins in milk. SCC and curd firmness were logarithmically transformed before statistical analysis to obtain normally distributed data. Differences between groups were considered significant if P⩽0·05.
Results
Descriptive statistics for the milk quality parameters analysed in the study are presented in Table 1. Detectable levels of Hp were found in 19 (21%) of the 91 bulk tank milk samples. Average Hp concentration in these 19 samples was 1·02±0·99 mg/l and average SCC in these samples was 387 000±266 000 cells/ml. Detectable levels of SAA were found in 68 (75%) of the 91 bulk tank milk samples. Average SAA concentration in these 68 samples was 1·12±1·16 mg/l and average SCC in these samples was 218 000±179 000 cells/ml. Samples not containing detectable levels of Hp had an average SCC of 144 000±78 000 cells/ml while samples not containing detectable levels of SAA had an average SCC of 127 000±116 000 cells/ml.
Table 1. Milk composition, including contents of haptoglobin (Hp) and serum amyloid A (SAA), and coagulating properties of 91 bulk tank milk samples

† ND=not determined. Since many of the samples did not contain detectable levels of Hp or SAA, i.e. levels were below 0·3 mg/l, it was not considered appropriate to calculate a mean value
‡ eq leu=equivalent mm-leucine
Tables 2 and 3 present differences in milk composition between samples with and without detectable levels of Hp and SAA, respectively. Bulk tank milk samples in which Hp could be detected had a lower casein number, contained less casein, had increased proteolysis, a lower lactose content and higher SCC compared with samples without detectable levels of Hp. Bulk tank milk samples in which SAA could be detected had a lower casein number, increased whey protein, lower lactose content and higher SCC compared with samples not containing detectable levels of SAA.
Table 2. Differences in milk composition between bulk tank milk samples with (Hp+) and without (Hp−) detectable levels of Hp. Differences between Hp+ and Hp− samples were evaluated by parametric t test and were considered significant if P⩽0·05
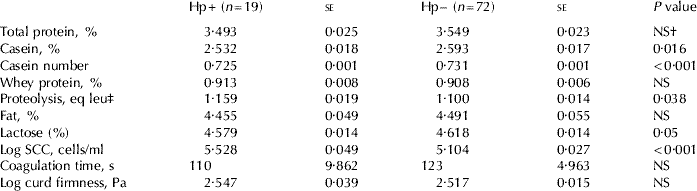
† NS=not significant
‡ eq leu=equivalent mm-leucine
Table 3. Differences in milk composition between bulk tank milk samples with (SAA+) and without (SAA−) detectable levels of serum amyloid A. Differences between SAA+ and SAA− samples were evaluated by parametric t test and were considered significant if P⩽0·05

† NS=not significant
‡ eq leu=equivalent mm-leucine
In Table 4, results obtained for differences in milk composition between samples containing high-BTMSCC (>195 000 cells/ml) and low-BTMSCC (<195 000 cells/ml) are presented. Samples with high-BTMSCC had lower casein number than samples with low-BTMSCC. In the high-BTMSCC group, 16 of the 29 samples (55%) contained Hp, while 26 of the 29 (90%) samples contained SAA. In the low-BTMSCC group, 3 of the 62 samples (5%) contained Hp, while 42 of the 62 (68%) contained SAA.
Table 4. Differences in milk composition between bulk tank milk samples with high SCC (>195 000 cells/ml) and low SCC (<195 000 cells/ml). Differences between high-SCC and low-SCC samples were evaluated by parametric t test and were considered significant if P⩽0·05

† NS=not significant
‡ eq leu=equivalent mm-leucine
Discussion
To our knowledge, the present study is the first investigating relationships between the quality of raw bulk tank milk and the presence of APP as an indicator of mastitis. When studying markers for udder health disturbances and effects on milk composition it is important to have in mind that the type of milk sample, i.e. quarter, cow composite or bulk tank milk, may affect the results. Owing to the dilution effect from quarter milk to composite and bulk tank milk, significant relationships found at quarter level may not be present at cow or bulk tank level and vice versa. However, the main findings of the present study, i.e. unfavourable changes in protein composition in bulk tank milk samples with detectable levels of APP, are largely in agreement with the results of our earlier study in cow composite milk (Åkerstedt et al. Reference Åkerstedt, Persson Waller, Bach Larsen, Forsbäck and Sternesjö2008).
In this study, bulk tank milk samples with detectable levels of Hp had lower casein content as well as lower casein number. This was most likely due to increased proteolytic activity, since these samples also had significantly higher proteolysis than samples without detectable levels of Hp. Bulk tank milk samples containing detectable levels of SAA also had a lower casein number than samples without detectable levels of SAA. In this case, however, the effect was most likely due to the observed increased whey protein content in the samples. It is thus likely that decreased synthesis, proteolysis and influx of components from the blood will occur simultaneously but to evaluate to what extent was outside the scope of this paper.
In the present study, detectable levels of Hp in bulk tank milk was related to increased proteolytic activity, which is in contrast to the results of our previous study on cow composite milk samples (Åkerstedt et al. Reference Åkerstedt, Persson Waller, Bach Larsen, Forsbäck and Sternesjö2008). One possible explanation for not observing proteolysis in cow composite milk but in bulk tank milk may be related to the fact that bulk tank milk consists of commingled milk from different milkings, as well as milk from non-infected and subclinically infected glands (Leitner et al. Reference Leitner, Silanikove, Jacobi, Weisblit, Bernstein and Merin2008). Moreover, the storage time before collection and freezing was longer for bulk tank, than for cow composite milk samples, allowing proteolysis to proceed for a longer time. The bulk tank milk samples were collected at the dairy plant and were kept at +4°C during the entire chain from farm to laboratory. Since the milk is collected every second day, the oldest batch of milk in the tank was stored for approximately 2·5 d before it was frozen. Refrigerated storage of raw milk is known to favour the growth of psychrotropic bacteria, which may produce heat-resistant extracellular proteinases and lipases. Proteinases are mainly secreted at the end of the log phase of the bacterial growth, at numbers in the order of 107 cfu/ml, indicating that very high numbers of bacteria are required to result in proteolysis (Sørhaug & Stepaniak, Reference Sørhaug and Stepaniak1997). In a study by Haryani et al. (Reference Haryani, Datta, Elliott and Deeth2003), such high numbers were reached first after 7 d at 4°C, and proteolysis, as measured by the fluorescamine method, was observed on day 6. Considering the very high numbers of bacteria needed for proteolysis to become a problem, it is unlikely that microbial contamination of milk samples would explain the observed relationships between the presence of Hp and increased proteolysis. An influence of microbial proteinases cannot, however, be excluded at this stage.
Bulk tank milk samples with detectable levels of SAA contained less lactose, in agreement with earlier studies on udder quarter and cow composite milk samples (Lindmark-Månsson et al. Reference Lindmark-Månsson, Bränning, Aldén and Paulsson2006; Åkerstedt et al. Reference Åkerstedt, Persson Waller, Bach Larsen, Forsbäck and Sternesjö2008). In the present study, bulk tank milk samples with detectable levels of Hp also contained reduced levels of lactose. The most common explanation for decreased lactose content is reduced synthesis owing to damaged epithelial cells. Another explanation, suggested by Silanikove et al. (Reference Silanikove, Shamay, Shinder and Moran2000), is that proteolysis of β-casein might result in release of peptides with a regulatory effect on lactose secretion.
Bulk tank milk samples with detectable levels of Hp and SAA had significantly higher SCC than samples without Hp and SAA, respectively. In our previous study, bulk tank milk samples with detectable levels of SAA, but not samples with detectable levels of Hp, had higher SCC (Åkerstedt et al. Reference Åkerstedt, Persson Waller and Sternesjö2007). The discrepancy between the studies might be explained by the use of different statistical methods, differences in the categorization of the samples and the selection of milk samples. To assess the potential of APP as markers for milk quality in comparison with the commonly applied SCC, we also investigated relationships between SCC and the different quality traits in the same samples. In this study, bulk tank milk samples with elevated SCC (>195 000 cells/ml) had a lower casein number than samples with a lower SCC (<195 000 cells/ml). In our previous study (Åkerstedt et al. Reference Åkerstedt, Persson Waller, Bach Larsen, Forsbäck and Sternesjö2008) with cow composite milk, samples with elevated SCC (>83 000 cells/ml) had reduced lactose and increased whey protein content compared with samples with a lower SCC (<83 000 cells/ml). The threshold values used in those studies (195 000 cells/ml and 83 000 cells/ml) are median and mean values, respectively. These threshold values are relevant since several studies demonstrate that the composition is deteriorated on quarter and cow level between 50 000 and 100 000 cells/ml, while the Swedish milk payment system give additional bonus payment when the bulk tank contains less than 175 000–200 000 cells/ml. Our studies suggest that the presence of APP in milk is related to disadvantageous changes in several milk quality parameters and that these relationships are valid in both cow composite and bulk tank milk. SCC, on the other hand, is not related to milk quality traits to the same extent as APP and the type of relationships observed differs between cow composite and bulk tank milk.
No significant relationships between APP and coagulation properties, i.e. coagulation time and curd firmness, were found in this study. Since there are no other studies published investigating APP in relation to the coagulating properties of milk, it is difficult to evaluate the results obtained. In earlier studies, significant correlations between mastitis and impaired coagulating properties were observed (for review see Munro et al. Reference Munro, Grieve and Kitchen1984). In contrast, Leitner et al. (Reference Leitner, Silanikove, Jacobi, Weisblit, Bernstein and Merin2008) found no correlation between coagulation time and SCC at bulk tank or silo level. In general, most published research on coagulating properties is based on studies with milk from a small number of animals (Barbano et al. Reference Barbano, Rasmussen and Lynch1991; Mazal et al. Reference Mazal, Vianna, Santos and Gigante2007). It is also common that batches of milk with a specific SCC are constructed by pooling milk with very high SCC, often originating from cows with clinical mastitis, and milk with a low SCC. Such a procedure is not ideal, as the composition of milk from cases of clinical mastitis is very deviant from milk originating from cows without clinical signs. Consequently, this type of constructed milk samples are not representative for real bulk tank milk samples, representing commingled milk from a large number of clinically healthy cows.
Casein content is an important quality parameter in cheese production and decreased casein content implies large losses for the dairy industry. At present, total protein content is used as a major quality parameter, largely affecting the milk price to the producer. Total protein content, however, also includes the whey proteins, of which those originating from blood, and of no interest for the dairies, will increase during mastitis. The extent of proteolysis is another important factor influencing milk quality, although not presently assessed. There are thus no techniques in place allowing reliable, large-scale analyses of the protein quality of milk, although research and development in this field is ongoing. In a future perspective, the dairies might have the possibility to differentiate raw milk based on quality to be used for different purposes. SCC is a good marker for udder health disturbances at udder quarter level but several studies have demonstrated that SCC is not a strong candidate for predicting the processing quality of the bulk tank milk (Le Roux et al. Reference Le Roux, Colin and Laurent1995; Urech et al. Reference Urech, Puhan and Schallibaum1999; Leitner et al. Reference Leitner, Krifucks, Merin, Lavi and Silanikove2006; Leitner et al. Reference Leitner, Silanikove, Jacobi, Weisblit, Bernstein and Merin2008). Since many dairy products such as cheese and fermented products require high protein quality it should be of great importance to have a sensitive and specific marker for disadvantageous changes in milk composition, e.g. those associated to poor udder health. Changes in levels of such a marker should preferably be associated with the protein composition of the raw milk. In this study, Hp and SAA have been shown to be potential candidates for predicting the raw bulk tank milk quality, specifically in relation to protein quality. This study and our previous studies therefore suggest that APP may be used as indicators for changes in milk composition as a consequence of udder health disturbances, in quarter or cow composite milk samples at the farm, as well as in bulk tank milk at the dairy plant or milk grading laboratory.
The authors thank the Swedish Farmers' Foundation for Agricultural Research for financial support. We are also grateful to Malin Thors and Lotta Wall at the Department of Food Science for their technical assistance, the dairy cooperative MILKO for collaboration, and their helpful tanker drivers for assistance during milk sampling.






