Bovine milk fat contains 70% saturated fatty acids (SFA), 25% monounsaturated fatty acids and 5% polyunsaturated fatty acids (PUFA) (Grummer, Reference Grummer1991). Milk fatty acid (FA) composition influences the technological properties of butter (MacGibbon & McLennan, Reference MacGibbon and McLennan1987; MacGibbon, Reference MacGibbon1996). For example, increasing the concentrations of 18 : 1 and 18 : 2 and reducing the concentrations of 8 : 0 to 14 : 0 results in softer milk fat, with improved spreadability.
Concentrations of FA in milk fat are affected by diet (Palmquist et al. Reference Palmquist, Beaulieu and Barbano1993; Dewhurst et al. Reference Dewhurst, Shingfield, Lee and Scollan2006), the natural genetic variation between cows (Soyeurt et al. Reference Soyeurt, Dardenne, Gillon, Croquet, Vanderick, Mayeres, Bertozzi and Gengler2006b; Stoop et al. Reference Stoop, Van Arendonk, Heck, Van Valenberg and Bovehuis2008) and by breed (Beaulieu & Palmquist, Reference Beaulieu and Palmquist1995; Auldist et al. Reference Auldist, Johnston, White, Fitzsimons and Boland2004; Soyeurt et al. Reference Soyeurt, Dardenne, Gillon, Croquet, Vanderick, Mayeres, Bertozzi and Gengler2006b; Palladino et al. Reference Palladino, Buckley, Prendiville, Murphy, Callan and Kenny2010; Maurice-Van Eijndhoven et al. Reference Maurice-Van Eijndhoven, Hiemstra and Calus2011). The most notable differences in the composition of FA are between Holstein (or Holstein-Friesian) and Jersey breeds. Milk from Jersey cows tends to have higher concentrations of some short- and medium-chain saturated FA, but lower concentrations of some unsaturated FA (UFA) relative to milk from Holstein cows (Arnould & Soyeurt, Reference Arnould and Soyeurt2009). This variation could be exploited in a crossbreeding programme to achieve a preferred FA profile.
Arnould & Soyeurt (Reference Arnould and Soyeurt2009) summarised estimates of heritability of individual FA of the bovine milk, heritability ranged from 0·00 to 0·54, depending on sample size, fatty acid and statistical model (sire or animal) used for the estimation of genetic variances. Interest in the genetic variation on FAs has been renewed recently, and several studies have reported genetic parameters for FAs using larger data sets with animal models and restricted maximum likelihood procedures (Soyeurt et al. Reference Soyeurt, Dardenne, Gillon, Croquet, Vanderick, Mayeres, Bertozzi and Gengler2006b, Reference Soyeurt, Gillon, Vanderick, Mayeres, Bertozzi and Gengler2007; Bobe et al. Reference Bobe, Bormann, Lindberg, Freeman and Beitz2008; Stoop et al. Reference Stoop, Van Arendonk, Heck, Van Valenberg and Bovehuis2008; Mele et al. Reference Mele, Dal Zotto, Cassandro, Conte, Serra, Buccioni, Bittante and Secchiari2009), and more recently Krag et al. (Reference Krag, Poulsen, Larsen, Larsen, Janss and Buitenhuis2013), used single nucleotide polymorphism markers instead of the traditional pedigree relationships and a Bayesian approach. With these new methods and larger data sets the estimates of heritability were moderate within the range of 0·07 to 0·40.
The estimates of heritability of FAs indicate that there is sufficient genetic variation to implement a successful selection programme to achieve preferred FA compositions. However, the implementation of such breeding programme has not been attempted because the determination of FA composition in individual samples through gas chromatographic analyses is expensive and time consuming. Recently, several authors (Soyeurt et al. Reference Soyeurt, Dardenne, Dehareng, Lognay, Veselko, Marlier, Bertozzi, Mayeres and Gengler2006a; Rutten et al. Reference Rutten, Bovenhuis, Hettinga, van Valenberg and Van Arendonk2009; Maurice-Van Eijndhoven et al. Reference Maurice-Van Eijndhoven, Soyeurt, Dehareng and Calus2013b) have shown that it is possible to estimate the FA concentrations using mid-infrared (MIR) spectrometry. This technology is faster and cheaper than the reference chemical analysis. Using the predicted concentrations of individual FAs by MIR spectrometry, Soyeurt et al. (Reference Soyeurt, Dardenne, Gillon, Croquet, Vanderick, Mayeres, Bertozzi and Gengler2006b) and Maurice-Van Eijndhoven et al. (Reference Maurice-Van Eijndhoven, Bovenhuis, Soyeurt and Calus2013a) studied the differences across dairy breeds and Soyeurt et al. (Reference Soyeurt, Gillon, Vanderick, Mayeres, Bertozzi and Gengler2007) estimated heritability and genetic correlations for the major fatty acids.
There are studies reporting genetic strain (Meier et al. Reference Meier, Verkerk, Kay, Macdonald and Roche2013) and breed (MacGibbon & McLennan, Reference MacGibbon and McLennan1987; MacGibbon, Reference MacGibbon1996) differences for fatty acids in New Zealand dairy cattle, but as far as is known by the authors, there is a lack of estimates of other genetic parameters such as heterosis effects and genetic variances and heritabilities for FA. Heterosis is defined as the superiority expressed from the first crossbred cows compared with the average of the parental breeds. The objective of this study was to estimate heritability and crossbreeding parameters (breed and heterosis effects) of FA concentrations in milk fat predicted by MIR spectroscopy in New Zealand dairy cattle.
Material and methods
Calibration equations
A total of 850 milk samples were collected during the season 2003–04 from 348 second-parity crossbred Holstein-Friesian x Jersey cows in peak lactation (35 d post calving), mid lactation (fixed date in mid-November) and late lactation (fixed date in late February). These cows were part of a crossbreeding experiment designed for the identification of quantitative trait loci determining traits of economic importance in New Zealand dairy cattle (Spelman et al. Reference Spelman, Miller, Hooper, Thielen and Garrick2001). The herd was managed as a conventional spring-calving herd grazing on rye grass/white clover pastures, milked twice a day on a rotary platform.
Concentrations of FA in the 850 milk samples were determined by fatty acid methyl ester analysis using gas chromatography (GC) (MacGibbon & Reynolds, Reference MacGibbon, Reynolds, Fuquay, Fox and McSweeney2011). The results were expressed as percentage fatty acid of total fatty acid. The same milk samples were analysed on a Foss MilkoScan FT6000 (Foss, Hillerød, Denmark) to provide the MIR spectra. The Foss MIR spectrum contained 1060 data points, which represented the absorption of infrared light through the milk sample at wave numbers in the 926 cm−1 to 5012 cm−1 region. The wave numbers 926 cm−1, 1069 cm−1, 1620 to 1698 cm−1, 3040 to 3665 cm−1 and 5000 to 5012 cm−1 were removed because these bands were found to only contribute noise. This yielded a spectrum consisting of 872 data points. Absorbance values at each wave length were standardised with mean of 0 and sd of 1 using a standard normal variance correction method. A principal component analysis using SAS (Statistical Analysis System, version 9.1; SAS Institute Inc., Cary, NC, USA) was undertaken across all FA and whole spectra to calculate the Mahalanobis distance for each sample. As described by Williams (Reference Williams2007), an outlier sample or spectrum is a sample or spectrum that differs from the mean of the population by 3 or more times the Mahalanobis distance. Using this threshold, 12 samples were considered as outliers and discarded.
The calibration equation for each FA was determined using partial least squares (PLS) (Haaland & Thomas, Reference Haaland and Thomas1988) using SAS. For each FA calibration equation, the 850 milk samples were split totally at random into two equally sized data sets, calibration and validation. The calibration data set was used to develop the calibration equation using split-sample cross-validation with the minimum standard error of calibration (SEC) calculated as:
 $${\rm SEC} = \sqrt {\displaystyle{1 \over {N - f - 1}}\sum\limits_{i = 1}^n {(A_i -} {\rm} P_i )^2} $$
$${\rm SEC} = \sqrt {\displaystyle{1 \over {N - f - 1}}\sum\limits_{i = 1}^n {(A_i -} {\rm} P_i )^2} $$where A i is the ith concentration of a FA in milk fat determined by GC, P i is the ith concentration of the FA in milk fat predicted by the calibration equation, and f is the number of PLS factors that produced the minimum SEC from a maximum of 30 PLS factors allowed in the model. The calibration coefficient of determination in the calibration data set was calculated as:
where
 $$\eqalign{ & S_{\rm A}^2 = \displaystyle{1 \over n}\sum\limits_{i = 1}^n {(A_i - \bar A)^2}, \;S_{\rm P}^2 = \displaystyle{1 \over n}\sum\limits_{i = 1}^n {(P_i - \bar P)^2} \quad {\rm \; and} \cr & S_{{\rm AP}} = \displaystyle{1 \over n}\sum\limits_{i = 1}^n {(A_i - \bar A)(P_i - \bar P)},} $$
$$\eqalign{ & S_{\rm A}^2 = \displaystyle{1 \over n}\sum\limits_{i = 1}^n {(A_i - \bar A)^2}, \;S_{\rm P}^2 = \displaystyle{1 \over n}\sum\limits_{i = 1}^n {(P_i - \bar P)^2} \quad {\rm \; and} \cr & S_{{\rm AP}} = \displaystyle{1 \over n}\sum\limits_{i = 1}^n {(A_i - \bar A)(P_i - \bar P)},} $$Measures of goodness of fit calculated in the validation data set were
Standard error of validation,
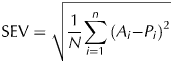 $${\rm SEV} = \sqrt {\displaystyle{1 \over {N{\rm}}} \sum\limits_{i = 1}^n {(A_i -} {\rm} P_i )^2} $$
$${\rm SEV} = \sqrt {\displaystyle{1 \over {N{\rm}}} \sum\limits_{i = 1}^n {(A_i -} {\rm} P_i )^2} $$Coefficient of determination,
Relative prediction error=
 ${\rm RPE} = ({\rm SEV}/\bar A) \times 100$
${\rm RPE} = ({\rm SEV}/\bar A) \times 100$Ratio performance deviation=RPD=S A/SEV
with
Fuentes-Pila et al. (Reference Fuentes-Pila, DeLorenzo, Beede, Staples and Holter1996) suggested that an RPE value lower than 10% is an indication of satisfactory prediction, whereas a RPE between 10 and 20% indicates a relatively acceptable prediction, and a RPE greater than 20% indicates poor prediction. Following Sinnaeve et al. (Reference Sinnaeve, Dardenne, Agneessens and Biston1994), a RPD value greater than 2 indicates that the calibration equation has good prediction and a RPD value lower than 2 indicates that predicted values are of poor quality and the equation cannot be used in practice.
Values corresponding to the CCC and their significance are as follow: from 0·21 to 0·40, fair prediction; from 0·41 to 0·60, moderate prediction; from 0·61 to 0·80, substantial prediction; and from 0·81 to 1·00, almost perfect prediction (McBride, Reference McBride2005).
Concentrations of fat, protein and lactose determined directly by the Foss MilkoScan FT6000 were validated with the calculated values using the MIR spectrum and PLS of SAS externally.
Estimation of breed effects and variance components
The calibration equations were used to predict the concentration of each FA in milk fat in cows participating in the sire proving scheme of Livestock Improvement Corporation (Newstead, Hamilton, New Zealand). The initial data set containing the MIR spectrum comprised 37 987 herd-day records from 10 072 cows. These milk samples were part of the herd-testing programme used for determination of concentrations of fat, protein, lactose and somatic cell counts using a Foss MilkoScan FT6000 instrument.
A total of 11 258 records were deleted for the following reasons: incomplete information on sire or dam identification, incomplete breed composition of cow sire or dam, herd-tests with less than 5 or greater than 300 d in milk, cows in parity 11 or higher, and herds with less than 40 cows herd-tested during the season. Absorbance values at each wave length were standardised with mean of 0 and sd of 1 using a standard normal variance correction method. Outlier samples were discarded using the Mahalanobis distance as described by Williams (Reference Williams2007). The final data set for estimation of genetic and crossbreeding parameters comprised of 26 769 milk samples from 2470 Holstein-Friesian (HF), 2115 Jersey (JE) and 2800 crossbred HF×JE cows sampled on average 3·62 times each cow during the 2007–08 season. There were 18 herds with HF and HF×JE cows, 4 herds with JE and HF×JE cows and 56 herds with HF, JE and HF×JE cows.
Variance components required for the estimation of heritability and repeatability and breed, heterosis and recombination effects were derived from a repeatability animal model across breeds using the statistical package ASReml (Gilmour et al. Reference Gilmour, Gogel, Cullis and Thompson2009). The model included the fixed effects of herd-test-day, lactation number, month of calving, and the regressions of days in milk using the Wilmink function (Wilmink, Reference Wilmink1987), proportion of HF, JE, heterosis HF×JE, recombination HF×JE, and the random effects of animal and cow.
Proportion of other breeds was not included in the model to avoid linear dependencies and was used as a base for comparison. Coefficients of specific heterosis and recombination were calculated between HF and JE breeds using the following identities (Dickerson, Reference Dickerson1973): h ij=αisαjd+αjsαid and r ij=αis+αjs+αjdαid where h ij and r ij are the coefficient of expected heterosis and recombination between fractions of breeds i and j in the progeny, αis and αjs are proportions of breeds i and j in the sire, respectively, and αid and αjd are proportions of breed i and j in the dam, respectively. The pedigree file included animal, parents and grandparents with complete breed information. Heritability was calculated as [σ2a/(σ2a+σ2c+σ2e)] and repeatability was calculated as [(σ2a+σ2c)/(σ2a+σ2c+σ2e)] where σ2a, σ2c, and σ2e are the animal, cow and residual variances, respectively. The animal variance is an estimate of the genetic variance and the cow variance is an estimate of the permanent environmental variance due to the cow affecting records during the lactation.
Concentrations for each of the FA of purebred HF and JE and first crossbred HF×JE cows were estimated by calculating the predicted means at an average lactation number of 3·7 and 125 d in milk. Predicted concentrations of FA for each of the breed groups and their standard errors were used for multiple means comparisons using a two-sample z statistic.
Results
Calibration equations
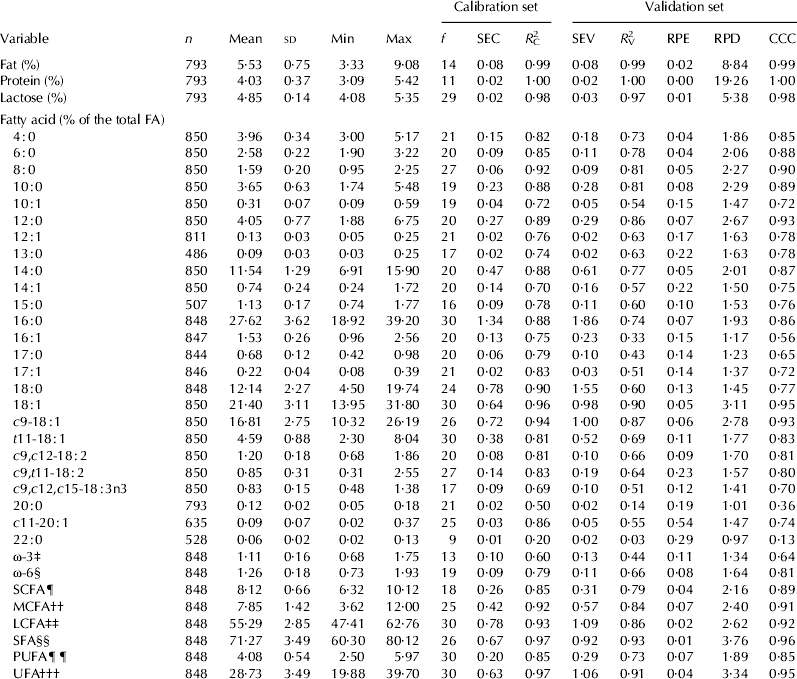
Descriptive statistics of concentration of fat, protein, lactose and concentrations of FA determined by GC are presented in Table 1. Measures of goodness of fit of the different prediction equations for routine herd-testing of fat, protein and lactose and concentrations of FA are shown in Table 1. The R 2V values of the PLS equations to predict fat, protein and lactose were 0·99, 1·00 and 0·98 respectively. The R 2V values of the PLS equations to predict concentrations of individual fatty acids, 8 : 0, 10 : 0, 12 : 0, c9-18 : 1 , and for the groups of FAs 18 : 1, Medium-chain fatty acids (MCFA), Long-chain fatty acids (LCFA), SFA and UFA were above 0·80 (Table 1). The R 2V values of the PLS equations for 4 : 0, 6 : 0, 14 : 0, 16 : 0 and PUFA were above 0·70.
Table 1. Descriptive statistics of the fatty acids determined by gas chromatography and goodness of fit measures of the calibration equations on the calibration and validation data sets†

Abbreviations: n=number of samples, sd=standard deviation, Min=minimum value, Max=maximum value, f=number of partial least squares factors, SEC=standard error of calibration using the calibration data set, R 2C=calibration coefficient of determination using the calibration data set, SEV=standard error of validation, R 2V=coefficient of determination using the validation data set, RPE=relative prediction error, RPD=ratio performance deviation, CCC=concordance correlation coefficient
† The total number of samples was split at random into two equally sized data sets, calibration and validation
‡ ω-3=Omega-3 fatty acids; sum of all omega-3 fatty acids
§ ω-6=Omega-6 fatty acids; sum of all omega-6 fatty acids
¶ SCFA=Short-chain fatty acids; sum of 4 : 0, 6 : 0 and 8 : 0
†† MCFA=Medium-chain fatty acids; sum of 10 : 0, 10 : 1, 12 : 0 and 12 : 1
‡‡ LCFA=Long-chain fatty acids; sum from 14 : 0 to 22 : 0
§§ SFA=Saturated fatty acids, sum of 4 : 0, 6 : 0, 8 : 0, 10 : 0, 12 : 0, 13 : 0, 14 : 0, 15 : 0, 16 : 0, 17 : 0, 18 : 0, 20 : 0 and 22 : 0
¶¶ PUFA=Polyunsaturated fatty acids, sum of c9,t11-18 : 2, c9,c12,c15-18 : 3n3, c11,c14-20 : 2n6, c8,c11,c14-20 : 3n6, c5,c8,c11,c14-20 : 4n6, c11,c14,c17-20 : 3n3, c8,c11,c14,c17-20 : 4n3, c5,c8,c11,c14,c17-20 : 5n3, c7,c10,c13,c16-22 : 4n6, c4,c7,c10,c13,c16-22 : 5n6, c7,c10,c13,c16,c19-22 : 5n3 and c4,c7,c10,c13,c16,c19-22 : 6n3
††† UFA=Unsaturated fatty acids, sum of PUFA and 10 : 1, 12 : 1, 14 : 1, 16 : 1, 17 : 1, c9-18 : 1, t11-18 : 1, c11-20 : 1 and 24 : 1
The goodness of fit of the PLS equations evaluated based on the RPE values in the validation data set are shown in Table 1. For FA which account for more than 2% of the total FA the PLS equations can be classified as providing satisfactory prediction (RPE less than 10%). The notable exception is 18 : 0 (RPE 13%). Values of RPD ranged from 0·97 for 22 : 0 to 3·76 for SFA which confirm the goodness of fit of the prediction equations in terms of RPE. The estimated values of CCCs in the validation data set re-affirmed the R 2V, RPE and RPD values suggesting that some prediction equations can be considered to have substantial predictive ability.
Breed, heterosis and recombination effects
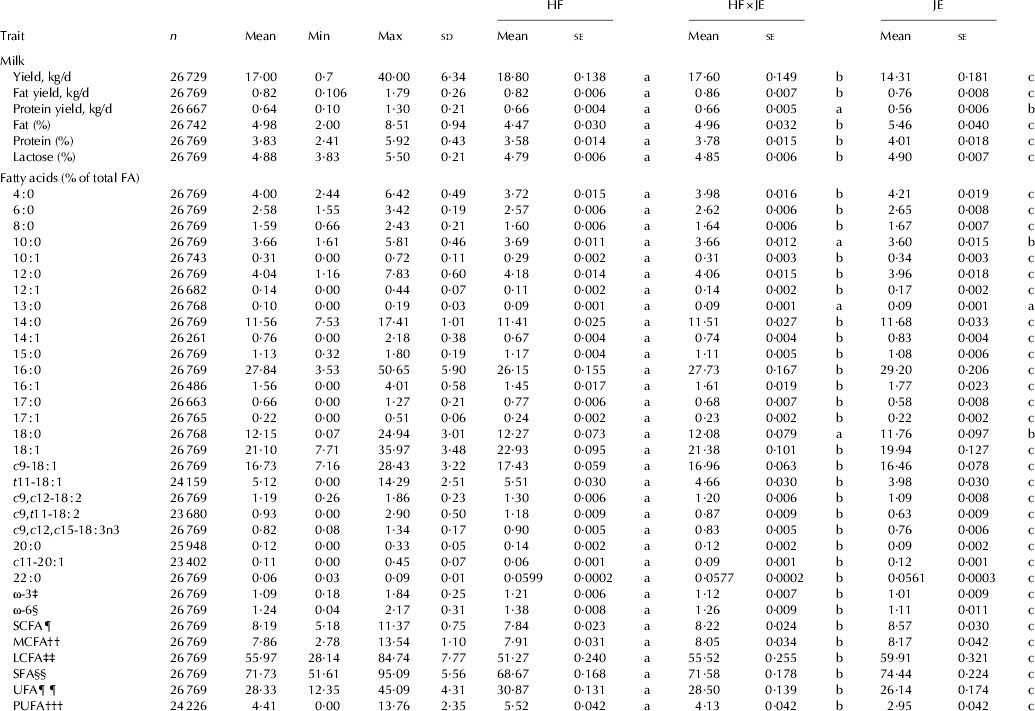
The predicted means for production traits and concentrations of fatty acids in milk fat for each of the breed groups are shown in Table 2. Holstein-Friesian cows produced significantly (P<0·05) higher yields of milk, fat and protein than JE cows, but JE cows produced milk with higher concentration of fat, protein and lactose than HF cows (P<0·05). The HF×JE cows were intermediate between HF and JE for all traits except for fat yield, in which they had produced the highest yield.
Table 2. Predicted means of production traits and concentrations of fatty acids for Holstein-Friesian (HF), Jersey (JE) and first cross HF×JE cows in New Zealand

‡, §, ¶, ††, ‡‡, §§, †††, ¶¶ as defined in Table 1
a,b,cWithin each fatty acid or group of fatty acids, means without common superscripts differ between HF, JE and first cross HF×JE cows (P<0·05)
Table 2 shows the predicted concentrations of FA. In general shorter chain FA (16 : 0 and below) were significantly higher (P<0·05) in JE cows while longer chain, including unsaturated longer chain FA were higher in HF cows.
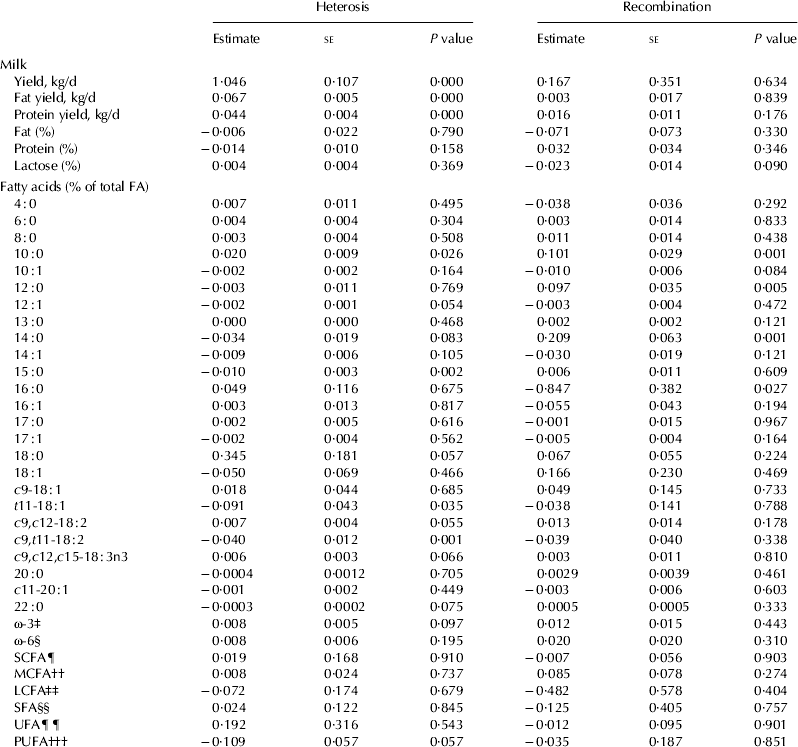
Estimates of the heterosis and recombination effects on production traits and concentration of fatty acids are shown in Table 3. Heterosis effects for yields of milk, fat and protein were positive and significant (P<0·001). Heterosis effects were positive and significant (P<0·05) for the concentrations of 10 : 0 and negative and significant for 15 : 0, t11-18 : 1 and c9,t11-18 : 2. Heterosis effects for concentration of PUFA were negative with P=0·057.
Table 3. Estimates of heterosis and recombination effects for production traits and concentrations of fatty acids in New Zealand dairy cattle

‡, §, ¶, ††, ‡‡, §§, ¶¶, ††† as defined in Table 1.
Recombination effects were positive and significant (P<0·05) for 10 : 0, 12 : 0 and 14 : 0 and negative and significant (P<0·05) for 16 : 0.
Heritabilities
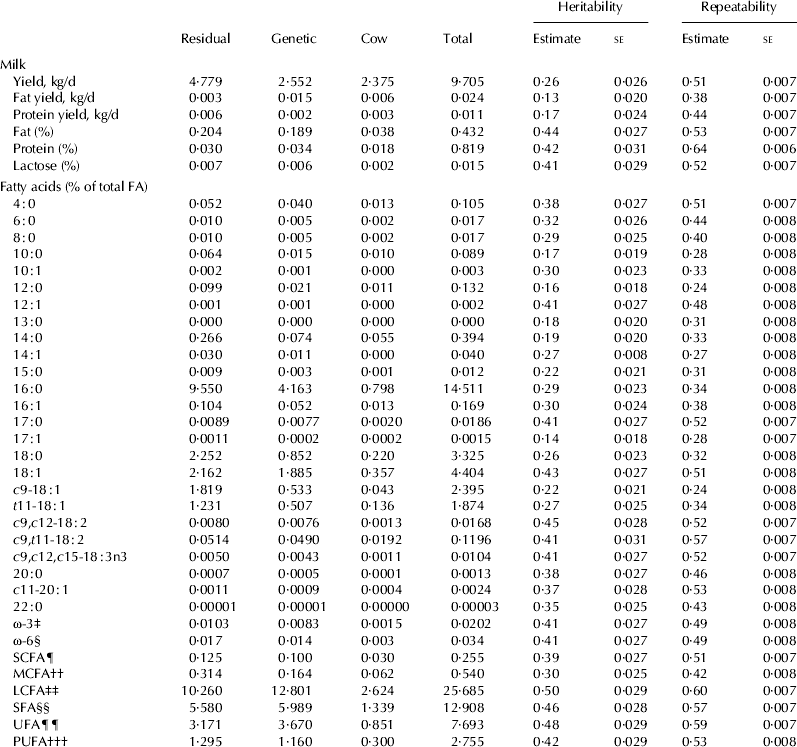
Heritability and repeatability estimates for production traits and concentration of individual fatty acids are shown in Table 4. The heritability estimates for yields of milk, fat and protein were between 0·13 and 0·26 and heritability estimates for concentration of fat, protein and lactose were higher.
Table 4. Estimates of variance, heritability and repeatability for production traits and concentrations of fatty acids in New Zealand dairy cattle

‡, §, ¶, ††, ‡‡, §§, ¶¶, ††† as defined in Table 1.
Heritability estimates for concentration of individual fatty acids were in the range from 0·14 to 45, but heritability estimates for groups of fatty acids, (SCFA, MCFA and LCFA, SFA, UFA and PUFA) were, in general, higher than for individual fatty acids ranging from 0·30 to 0·50). Repeatability estimates for all traits were medium to high (0·24 to 0·60).
Discussion
The concentrations of fat and protein in the data set used to produce the prediction equations were higher than representative New Zealand averages of 4·75 and 3·74%, respectively (LIC & DairyNZ, 2013) because the milk samples used to derive the calibration equations were from a small sample of crossbred cows. Mean values of the concentration of FAs in milk fat are typical of New Zealand dairy cattle (MacGibbon, Reference MacGibbon1996; Auldist et al. Reference Auldist, Johnston, White, Fitzsimons and Boland2004). Of the total FAs, 71·3% were SFA and 28·7% were UFA, which is similar to other populations (Grummer, Reference Grummer1991).
Prediction equations
The R 2V values of the PLS equations to predict fat, protein and lactose were almost 1·0 confirming that our independent algorithm produces almost the same values as the manufacturer's own calibrations.
The ability of calibration equations to estimate the FA concentrations by MIR spectrometry has been investigated previously by several authors (Soyeurt et al. Reference Soyeurt, Dardenne, Dehareng, Lognay, Veselko, Marlier, Bertozzi, Mayeres and Gengler2006a; Rutten et al. Reference Rutten, Bovenhuis, Hettinga, van Valenberg and Van Arendonk2009; Soyeurt et al. Reference Soyeurt, Dehareng, Gengler, McParland, Wall, Coffey and Dardenne2011; Maurice-Van Eijndhoven et al. Reference Maurice-Van Eijndhoven, Soyeurt, Dehareng and Calus2013b). The general conclusion from these studies is that mid-infrared spectroscopy can be used to satisfactorily predict FA sums and ratios (i.e. SFA, MUFA, PUFA, UFA, total trans FA, total trans-C18 : 1 and total cis-C18 : 1 and 16 : 0/cis9-C18 : 1 ratio) but also for individual FA present in medium-to-high concentrations (i.e. 6 : 0, 8 : 0, 10 : 0, 12 : 0, 14 : 0, 16 : 0, 18 : 0, c9-18 : 1, 18 : 1n-7 and c9,t11-18 : 2), but the quality of the prediction decreased when FA are present in low to very-low concentrations. Similar conclusions were obtained by Coppa et al. (Reference Coppa, Ferlay, Leroux, Jestin, Chilliard, Martin and Andueza2010) using near infrared reflectance spectroscopy.
This was confirmed in the present study, the majority of individual FA and groups of FA that are present in high concentrations, were predicted with moderate to high accuracy (based on the R 2 value>0·60 calculated in the validation data set) but those FA that present in low to very-low concentrations (10 : 1, 14 : 1, 16 : 1, 17 : 0, 17 : 1, c9,c12,c15-18 : 3, 20 : 0, c11-20 : 1, 22 : 0 and ω-3) were predicted with low accuracy. However, other FAs with low concentration in milk fat (i.e. 12 : 1 and 13 : 0) were predicted with moderate accuracy (R2=0·63 in the validation data set).
For the purposes of ranking animals based on predicted FA concentration the calibration equations can be considered to have practical utility, except the prediction equations for 16 : 1, 20 : 0 and 22 : 0, because CCC values are lower than 0·60.
Breed, heterosis and recombination effects
A recent Dutch study (Maurice-Van Eijndhoven et al. Reference Maurice-Van Eijndhoven, Bovenhuis, Soyeurt and Calus2013a) demonstrated that prediction equations using mid-infrared spectroscopy can be used to predict the content of most saturated FA in milk for the 5 dairy cattle breeds present in the Netherlands: HF, Meuse-Rhine-Yssel, Dutch Friesian, Groningen White Headed, and JE. The present study demonstrates that FA profile for HF, HF×JE and JE cows in New Zealand can be determined using mid-infrared spectroscopy.
Jersey cows tended to produce milk with more saturated FA than HF cows with an intermediate values for the HF×JE cows. The differences between HF and JE cows agree well with reports on direct measurements in New Zealand dairy cattle (MacGibbon, Reference MacGibbon1996; Auldist et al. Reference Auldist, Johnston, White, Fitzsimons and Boland2004; Palladino et al. Reference Palladino, Buckley, Prendiville, Murphy, Callan and Kenny2010) and other populations (Beaulieu & Palmquist, Reference Beaulieu and Palmquist1995; Soyeurt et al. Reference Soyeurt, Dardenne, Gillon, Croquet, Vanderick, Mayeres, Bertozzi and Gengler2006b). The practical importance of these breed differences is that milk fat from HF cows is softer than milk fat from JE cows (MacGibbon, Reference MacGibbon1996). Also, regardless of breed, there are significant positive correlations between fat hardness and concentrations of 6 : 0, 8 : 0, 10 : 0 12 : 0, 14 : 0, 16 : 0 in milk fat but significant and negative correlations between fat hardness and concentrations of 18 : 1 and c9,c12-18 : 2 in milk fat (MacGibbon, Reference MacGibbon1996).
A point to note in this study is that the concentration of 18 : 0 in JE cows was lower than in HF cows whereas all the aforementioned studies reported that HF cows had lower concentrations of 18 : 0 than JE cows.
Positive effects of heterosis for yields of milk, fat and protein in the present study agree well with results found by Lopez-Villalobos et al. (Reference Lopez-Villalobos, Garrick, Holmes, Blair and Spelman2000) in New Zealand, Penasa et al. (Reference Penasa, Lopez-Villalobos, Evans, Cromie, Dal Zotto and Cassandro2010) in Ireland and Maurice-Van Eijndhoven (Reference Maurice-Van Eijndhoven, Bovenhuis, Soyeurt and Calus2013a) in the Netherlands. However, heterosis effects for concentration of fat, protein and lactose in this study were not significant. Ahlborn-Breier & Hohenboken (Reference Ahlborn-Breier and Hohenboken1991) reported a significant negative heterosis for fat percentage whereas Maurice-Van Eijndhoven et al. (Reference Maurice-Van Eijndhoven, Bovenhuis, Soyeurt and Calus2013a) reported a positive, significant heterosis for fat percentage.
Heterosis effects for concentration of FA in fat were significant for few FA in this study, positive effects for 10 : 0 and negative for 15 : 0, t11-18 : 1, c9,t11-18 : 2 and PUFA, whereas Maurice-Van Eijndhoven et al. (Reference Maurice-Van Eijndhoven, Bovenhuis, Soyeurt and Calus2013a) reported significant positive heterosis effects for several short chain FA concentrations in milk. However, the results from both studies are not comparable because concentrations of FA acids were expressed differently in each of the studies.
Recombination effects for yields of milk, fat and protein and concentrations of fat, protein and lactose were not significant in this study. Maurice-Van Eijndhoven et al. (Reference Maurice-Van Eijndhoven, Bovenhuis, Soyeurt and Calus2013a) reported significant and positive recombination effects for fat and protein percentages and significant and negative for milk and protein yields.
The recombination effects were not significant for most of the FAs considered in this study with few exceptions, positive for 10 : 0, 12 : 0 and 14 : 0 and negative for 16 : 0. Maurice-Van Eijndhoven et al. (Reference Maurice-Van Eijndhoven, Bovenhuis, Soyeurt and Calus2013a) demonstrated significant positive, recombination effects for concentration in milk fat of several FAs with a linear model that did not include fat percentage in milk. Including fat percentage in the model changed the recombination effects to positive values suggesting that when correcting for fat percentage, recombination effects are negative, following the general expectation that recombination effects are negative (Dickerson, Reference Dickerson1969).
Heterosis and additive effects for production, fertility and health traits have been exploited in New Zealand dairy cattle through the use of crossbreeding in combination with selection. Pure HF and JE or crossbred HF×JE bulls of high genetic merit for farm profit are used to produce crossbred replacements. For the production season 2012–13, the proportion of crossbreed HF×JE cows in the national herd was 0·43 followed by HF (0·37), JE (0·12) and other breeds (0·08) (LIC & DairyNZ, 2013). These changes in the breed composition of the national herd can cause changes in the concentration of FA in milk fat and can affect the processing of dairy products. For example, Auldist et al. (Reference Auldist, Johnston, White, Fitzsimons and Boland2004) reported that milk coagulation parameters of cheese processing were correlated with particular fatty acids. Concentrations of 8 : 0, 10 : 0 and 12 : 0 were negatively correlated with rate of curd formation and positively correlated with curd firmness after 1 h of rennet addition. Concentrations of 18 : 1 and 18 : 2 were positively correlated with rate of curd formation. Further studies are required to evaluate the best crossbreeding strategies for farm profitability and desired FA composition.
Heritabilities
Heritabilities for yields of milk, fat and protein and concentrations of fat, protein and lactose agree well with reported in the literature (Lopez-Villalobos, Reference Lopez-Villalobos2012) and recent estimates.
The estimates of heritabilities for FA in this study are in general higher than the estimates reported by Bobe et al. (Reference Bobe, Bormann, Lindberg, Freeman and Beitz2008) in American Holstein cows, Mele et al. (Reference Mele, Dal Zotto, Cassandro, Conte, Serra, Buccioni, Bittante and Secchiari2009) in Italian Holstein cows, Soyeurt et al. (Reference Soyeurt, Gillon, Vanderick, Mayeres, Bertozzi and Gengler2007) in mixed-breed population of the Walloon region of Belgium, and Krag et al. (Reference Krag, Poulsen, Larsen, Larsen, Janss and Buitenhuis2013) in Danish Holstein cows. but agree well with the estimates reported by Stoop et al. (2008) in Dutch Holstein cows. The estimates of heritability provided by Stoop et al. (2008) were obtained from a controlled experiment designed to have approximately 2000 cows with completed pedigree and distributed in 398 commercial herds in the Netherlands. This well designed experiment together with a good milk sampling scheme had to the potential to minimise biases in the estimation of heritabilities of FA. Likewise, the present study aimed to have estimation of genetic and crossbreeding parameters with mimimum biases by using milk samples provided by herds using frequent herd-testing and complete pedigree information.
The estimates of heritability obtained in the present study confirm the existence of genetic variability of FA, which could be used to improve the nutritional and textural properties of milk fat by selective breeding, but as indicated by Stoop et al. (2008) the direction of selection depends on the purpose of the milk product, because changes that are favourable for one product might be unfavourable for others.
This study was funded by ViaLactia Biosciences NZ (Ltd) and the Ministry of Business, Innovation, and Employment. The authors would like to acknowledge the assistance of farm and technical staff for taking all milk samples.