Most UHT milk contains a slight amount of sediment, which is not usually sufficient to be a problem (Burton, Reference Burton1988). Several investigators have found that sediment increases with severity of the treatment and is more severe in direct rather than in indirect UHT processed milk (Perkin et al. Reference Perkin, Henschel and Burton1973; Ramsey & Swartzel, Reference Ramsey and Swartzel1984). However, on some occasions a more voluminous sediment appears, for example with goats' milk and with cows' milk which has been supplemented with calcium. Zadow (Reference Zadow1978) found that little sediment was formed in UHT cows' milk if pH was kept above 6·62, below this value sedimentation increased rapidly. In contrast sedimentation was severe in goats' milk when the pH was below 6·9. Similar trends were observed for concentrated skim milk. Milk was stable above pH 6·55 but below this value, severe sedimentation occurred (Zadow & Hardham, Reference Zadow and Hardham1981).
Different stabilisers have been investigated to reduce sediment to acceptable levels and to reduce heat induced thickening and coagulation in concentrated milk. Sweetsur & Muir (Reference Sweetsur and Muir1980) proposed that disodium hydrogen phosphate (DSHP), trisodium phosphate, trisodium citrate (TSC) and sodium bicarbonate should be used if the natural pH falls on the acid side of the heat stability maximum, while sodium dihydrogen phosphate (SDHP) or calcium chloride should be used if the natural pH is alkaline to the maximum, with orthophosphates being generally the most effective stabilisers. Mittal et al. (Reference Mittal, Hourigan and Zadow1990) evaluated sodium hexametaphosphate (SHMP) addition to UHT recombined milk and found that this was effective at retarding gelation. No sediment was found in UHT milk, with or without SHMP.
Goats' milk is much more susceptible to sediment formation on UHT treatment (Zadow et al. Reference Zadow, Hardham, Kocak and Mayes1983). Its ionic calcium level has been reported to be higher compared with cows' milk. Addition of 0·05% CaCl2 further increased ionic calcium from 3·2 to 4·5 mm and further increased sediment. Addition of DSHP reduced ionic calcium and reduced sediment drastically. Montilla & Calvo (Reference Montilla and Calvo1997) observed that a mixture of NaH2PO4, Na2HPO4 and Na3PO4 did not change the pH of the raw goats' milk and increased its heat stability. Use of mixed phosphates was found to be more effective than pH adjustment by means of NaOH.
The alcohol (ethanol) stability test has been used as a simple indicator of cows' milk freshness and suitability for UHT processing (Shew, Reference Shew1981), and cows' milk should be stable in 74% alcohol to be suitable for UHT treatment. Goats' milk has been found to have a much lower ethanol stability than cows' milk (White & Davies, Reference White and Davies1958; Horne & Parker, Reference Horne and Parker1982; Guo et al. Reference Guo, Wang, Li, Qu, Jin and Kindstedt1998). Sediment formation is still reported to be problem during UHT goats' milk production, even with addition of DSHP (Sutton, Reference Sutton2004).
This paper investigates the effects of different stabilisers and the role of pH and ionic calcium on the voluminous sediments arising from goats' milk, using a stable ion electrode system described by Lin et al. (Reference Lin, Lewis and Grandison2006).
Materials and Methods
Milk samples and processing conditions
Raw goats' milk was supplied by Delamere Dairies (Yew Tree Farm, Knutsford, Cheshire, UK) and Willowbrook Farm, Garford, Abingdon, UK. It was subjected to indirect UHT treatment using an APV Junior plate heat exchanger, described and characterised in terms of its temperature/time heating profiles and corresponding B* and C* by Browning et al. (Reference Browning, Lewis and MacDougall2001). Four batches of raw goats' milk were processed using the same conditions of 140°C for 2 s. Upstream homogenization during the heat treatment was at a pressure of 180 bar. Four different stabilisers were added in different concentrations, namely SDHP, SHMP, TSC and SDHP; the latter was only used in the first two trials. The samples were collected in sterile pots (250 ml; Bibby Sterilin Ltd, UK) in a laminar air-flow cabinet. In trial 4, raw goats' milk was processed at 125°C and 114°C, to observe the effects of temperature on sediment formation.
Milk Analyses
Analyses were performed on raw milk and UHT milk samples. Protein, fat, solids-not-fat and lactose contents were measured using a DairyLab II Analyser (Multispec Limited, York, UK) and were the average of two readings.
The pH of the milk samples was measured using a Sentron 3001 pH meter, which was calibrated prior to the analyses with standard buffer solutions of pH 4·0 and 7·0.
Ionic calcium was measured using a Ciba Corning 634 ISE Ca2+/pH Analyser (Lin et al. Reference Lin, Lewis and Grandison2006). The instrument was calibrated in its millivolt ouput mode with solutions of 1·0, 1·5, 2·0, 2·5, and 3·0 mm-Ca2+ daily, prior to use. There was a linear relationship between log (ionic calcium) and mV output, with correlation coefficients greater than 0·99.
Ethanol stability was determined by mixing equal volumes (2 ml) of milk and a range of ethanol solutions, and examining for the presence of clots when poured into a Petri dish. Depending upon the formation of clots, ethanol solutions of increasing or decreasing concentration were used and the highest concentration of ethanol which did not cause coagulation was defined as the ethanol stability. All the above analyses were done at room temperature.
Sediment was measured by a centrifugation method. The milk was well shaken in its container. Approximately 40 ml was accurately weighed and poured into a calibrated tube and centrifuged (Centaur 2 at 4200 rpm for 15 min), corresponding to 2760 g maximum. After removing the supernatant, the sediment volume was then measured (expressed as ml/100 ml). The sediment wet weight was measured and the sediment was then oven-dried at 102°C to constant weight to determine its dry weight (g/100 ml). This method was also used on a selection of commercial cows' milk and goats' milk products.
After removal of the sediment, the supernatant was examined for protein, fat and lactose, to determine losses into the sediment. Ash content was measured using the AOAC method (1995), except that dried sediment was used instead of the dried milk. Results were expressed as percentage of ash in sediment dry weight.
The concentration of total phosphorus (P) in milk was determined according to British Standard Methods (BS 1741-12: 1992). This method was adapted to determine the total P content of the milk sediment, which was dry-ashed.
Total Ca concentration of the sediment from the milk samples was determined by the method of Murthy & Rhea (Reference Murthy and Rhea1966) employing a Pye Unicam SP9 Flame Emission Spectrophotometer (Pye Unicam Ltd., Cambridge, UK).
Results
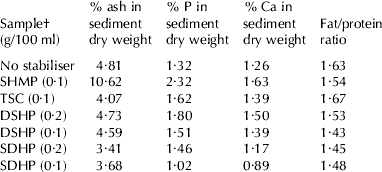
Sediment was estimated within 24 h of UHT production. In raw milk without stabiliser, over 5% sediment (dwb) was produced in three of the four batches, which is equivalent to over 40% of the total milk solids (Table 1). Sediment in the 4th batch was lower, but still substantial at 3·5%. However, on this occasion, the temperature fell within two minutes from 140 to 135°C, suggesting heavy fouling of the heat exchanger. This lower temperature may have accounted for the reduced sediment. There was considerable variation in ionic calcium for the four raw goat's milk samples, ranging from 1·94 to 2·80 mm, whilst ethanol stability ranged between 50 and 64% (Table 1). All these milks were below the threshold value of 74%, recommended for UHT treatment (Shew, Reference Shew1981). Sediment appeared after standing for less than 1 h and the milk had an unacceptable chalky mouthfeel. Sediment after one week and 2 weeks was also measured in the first trial and further changes over this period were minimal, the one exception being milk containing 0·2% SDHP, which increased from 6·05 to 9·48%. Sediment separation by centrifugation permitted measurement of its volume, its wet weight and its dry weight (g/100 g). There was a good agreement between the three methods, although dry weight was felt to be the most useful, as it could be directly related and compared to solids in milk.
Table 1. Ethanol Stability, pH and calcium ion concentration before and after UHT treatment and sediment formation for all four trials with goats' milk and the effects of selected stabilisers

† Stabilisers evaluated were sodium hexametaphosphate (SHMP), trisodium citrate (TSC), disodium hydrogen orthophosphate (DSHP), and sodium dihydrogen orthophosphate (SDHP)
‡ Temperature fell quickly to 135°C
SDHP was not effective at reducing sediment: adding it to raw milk reduced pH and had little effect on ionic calcium. Milk containing SDHP caused noticeable fouling during UHT treatment, within minutes of starting the process.
On the other hand, SHMP, DSHP and TSC were all effective; each reduced ionic calcium and increased ethanol stability. The combined results for these three stabilisers for all four trials are presented in Table 1. Ethanol stability, pH and ionic calcium were evaluated before and after UHT treatment, except for trial 2, where results prior to heat treatment are not available. There was a good general correlation between ethanol stability and ionic calcium prior to heat treatment. The correlation coefficient was significant (R2=0·85; Fig. 1). Thus, ethanol stability is a useful test for indicating changes in ionic calcium brought about by addition of these stabilisers.

Fig. 1. Effect of stabiliser addition on ethanol stability.
Again, UHT treatment was found to reduce ionic calcium in most cases. However, changes in ethanol stability were less consistent: in 6 cases it was higher and in 18 cases it was similar or lower. This did not appear to be related to the stabiliser type. Figure 2 shows the relationship between ethanol stability and sediment formation for these three stabilisers. The trend shown is that increasing ethanol stability reduced sediment formation for the three stabilisers. The overall correlation coefficient is R2=0·34. However, the relationships are offset, and relatively small improvements in ethanol stability are more effective for DSHP (R2=0·72) than for TSC (R2=0·57) and least effective for SHMP (R2=0·22). Thus ethanol stability appears not to be a reliable indicator of sediment formation for SHMP.

Fig. 2. Relationship between sediment formation and ethanol stability for different additions of ◆, sodium hexametaphosphate (SHMP); ■, trisodium citrate (TSC) and ▲, disodium hydrogen orthophosphate (DSHP).
Figure 3 shows how sediment formation was influenced by ionic calcium for all three stabilisers. The overall correlation coefficient is R2=0·43. However, a different picture emerges when the individual stabilisers are examined. For each stabiliser, there is a clear reduction in sediment as ionic calcium is reduced. The best correlation is for TSC (R2=0·80), whereas SHMP is effective but more erratic in its performance. (R2=0·50). When comparing their performance at similar levels of ionic calcium, DSHP is the most effective (Fig. 3) although its R2 value is 0·62.

Fig. 3. Relationship between sediment formation and ionic calcium for different additions of ◆, sodium hexametaphosphate (SHMP); ■, trisodium citrate (TSC) and ▲, disodium hydrogen orthophosphate (DSHP).
Some samples, with low ionic calcium levels, produced more sediment than expected, for example 0·3% TSC and 0·2% SHMP. It is possible that reducing the ionic calcium too much may destabilise casein micelles, rendering them more susceptible to heat induced aggregation. Figure 4 shows the relationship between pH and sediment formation, plotted for the three stabilisers. The overall correlation coefficient is R2=0·17. It is interesting that there is no effect for TSC and for SHMP, but there is a positive correlation for DSHP (R2=0·95), which may help explain why it is more effective than TSC and SHMP, when ionic calcium levels are similar. (see Fig. 3). There was also a poor correlation between ethanol stability and pH (R2=0·08).

Fig. 4. Relationship between sediment formation and pH for different additions of SHMP, TSC and DSHP.
The effect of temperature on sediment was evaluated on goats' milk with no added stabiliser. Sediment formation was above 5% at 140°C in the first three trials and 3·5% in the fourth trial, although it was observed that the temperature quickly fell to 135°C. When processing temperature was reduced to 125°C and 114°C in the fourth trial (holding time=2 s), sediment formation was much lower, at 0·7% in both cases. This shows that sediment formation is quite temperature dependent.
The sediment from the first two trials was analysed for phosphorus and calcium. In the first trial, phosphorus ranged between 1·22 to 2·03%, being highest for SHMP. Calcium ranged between 0·99 to 1·36%. The ratio of P/Ca ranged from 1·05 to 1·50 and did not show any change one week later. In trial 2, the ash content was also analysed and found to range between 3·4 and 10·6% (dwb), being highest for SHMP (Table 2). P ranged between 1·12 and 2·32% and calcium between 0·89 and 1·63%. The low ash content of the sediment suggested that it was predominantly fat and protein. This was confirmed by the reduction in fat and protein found in UHT milk, following removal of the sediment. Based on the assumption that all the fat and protein lost from milk ended up in the sediment, the ratios of protein to fat were estimated to be between 1·43 and 1·67. Overall, there was a very good correlation between loss of protein and fat and the amount of sediment formed. The R2 values for the three trials investigated were 0·98, 0·96 and 0·88. Freezing point depression did not change following UHT treatment. This also suggested that lactose and minerals had changed little in the milk as a result of the heat treatment. The type of stabiliser did not appear to grossly affect the chemical composition of the sediment formed, but they may have produced subtle changes in their mineral contents. Sediment formed with SHMP seemed to produce slightly higher amounts of calcium and phosphate.
Table 2. Percentage ash, phosphorus (P) and calcium (Ca) in sediment dry weight and fat/protein ratios (2nd trial)
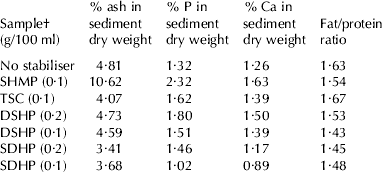
† See Table 1
Sediment (dwb) was measured in raw goats' milk (0·4%), commercial UHT goats' milk (0·29%), pasteruised semi-skim goats' milk (0·19%), semi-skim cows' milk (0·08%) and sterilised semi-skim cows' milk (0·13%) These results suggest that all milk shows a small amount of sediment, which is in agreement with Burton (Reference Burton1988). One question relates to what is an acceptable level of sediment and how much is present before it can be perceived.
Discussion
Sediment in UHT goats' milk is one manifestation of poor heat stability. Its formation appears to involve aggregation of casein micelles, promoted by ionic calcium. In general, addition of TSC, DHSP and SHMP all reduced ionic calcium, increased ethanol stability and reduced sediment. At similar levels of ionic calcium, DSHP was most effective. However, the overall mineral content in the sediment was low. Since homogenization was upstream, the fat globules would be coated with casein micelles and thus be incorporated into the sediment. However, despite containing substantial fat, the sediment was more dense than the serum, and no separate layer of fat was observed. The ratio of phosphorus to calcium found in the deposits (0·96 to 1·50) was higher than that found in milk (approx. 0·78) and in the casein micelle fraction (approx. 0·48). Material responsible for sediment was formed as a result of the heat treatment.
The role of pH is less clear. Walstra & Jenness (Reference Walstra and Jenness1984) illustrated that pH of milk will fall below 6·0 at 140°C. This will also increase ionic calcium and zeta potential; all these factors combined will influence its heat stability. What is probably of importance is the absolute pH value of the milk at 140°C, lower values will also lead to higher levels of ionic calcium and have a destabilising influence. This will be influenced by the buffering capacity of the milk, so milk with a higher buffering capacity will be subject to a smaller drop in pH and thus be less susceptible to sediment formation. This type of mechanism has been attributed to the role of urea in improving heat stability (Metwalli & Boekel, Reference Metwalli and Boekel1996) and it may also be the reason for the effectiveness of DSHP as a stabiliser. Since the fall in pH will be less as temperature is reduced, this may be a contributory factor to the lower level of sediment observed at 125 and 114°C.
The UHT conditions used in this work correspond to an F0 value of 5·3, C* of 0·39 and B* value of 1·25 (Browning et al. Reference Browning, Lewis and MacDougall2001). On occasions, fouling of the heat exchanger was also observed, which was sometimes rapid, indicated by a reduction in the temperature in the holding tube. Thus it is problematic for two reasons, firstly the adverse effects on the sensory characteristics of the milk caused by the presence of the sediment; and secondly the detrimental consequences of fouling, such as shortened processing runs and additional cleaning costs. Burton (Reference Burton1968) suggested that sediment is produced by the same mechanism which is responsible for fouling of heat exchangers, with sediment representing fouling material which has not become attached to heat exchanger surface. The fact that sediment formation was often accompanied by an observed deterioration in the performance of the heat exchanger would support this. Further support is provided by Prakash et al. (Reference Prakash, Datta, Lewis and Deeth2007), who have investigated fouling behaviour during UHT processing of goats' milk, with and without stabilisers.
In commercial processing, monitoring pH, ethanol stability and ionic calcium routinely would be useful for understanding factors affecting sediment formation and also plant performance and its susceptibility to fouling. This would also help understand the effects of natural variations in milk composition. Milk from individual animals and to a lesser extent bulk milks have been found to show considerable variations in ionic calcium levels (Lin, Reference Lin2002), although the exact reasons for this have not been established. Montilla & Calvo (Reference Montilla and Calvo1997) suggested that one factor that affects the heat stability of milk is ionic calcium concentration, although they did not measure it. The heat stability of milk increases by the addition of calcium sequestering agents in the form of mixed phosphate salts. Ionic calcium is higher in goats' milk than cows' milk. Fox & Hoynes (Reference Fox and Hoynes1976) and Horne & Parker (Reference Horne and Parker1982) attributed the low stability of goats' milk to their different chemical composition and their different casein profiles. Tziboula (Reference Tziboula1997) demonstrated that the heat stability of caprine milk is dependent on the casein genotype, with milks having a high content in αs1-casein being of lower heat stability than those having low αs1-casein content. The complexity of the casein fraction is highlighted by the fact that since the 1970s, when it was thought that goats' milk contained no αs1-casein, 13 alleles have been identified in the various amounts of αs1-casein in caprine milk (Ng-Kwai-Hang & Grosclaude, Reference Ng-Kwai-Hang, Grosclaude, Fox and McSweeney2003). Guo et al. (Reference Guo, Wang, Li, Qu, Jin and Kindstedt1998) suggested that the low ethanol stability of goats' milk may be related to the ratio of sodium to potassium. Morgan et al. (Reference Morgan, Jacquet, Micault, Bonnin and Jaubert2000) showed that caprine milk is more heat stable when it contains less soluble Ca and more P. The level of citrate was found to be an important parameter that governs the ionic calcium level of goats' milk and therefore its stability towards heat treatment. Citrate is 40% lower than in bovine milk.
Thus overall, any factors which change the negative charge on the micelle (e.g. Ca2+, H+, Na+, K+ and Mg2+), or induce changes in ionic calcium activity, such as differences in phosphates and citrates, or the casein fraction components, will influence heat induced sediment formation.
Where ionic calcium measurement is not available, strategies which increase ethanol stability would be useful to reduce sediment. There is scope for further reducing it by downstream homogenisation and by using different homogenisation pressures, but probably not by direct UHT processing (Ramsey & Swartzel, Reference Ramsey and Swartzel1984; Perkin et al. Reference Perkin, Henschel and Burton1973).