Several in vitro and in vivo studies carried out in humans and laboratory animals have demonstrated that fatty acids (FA) can modulate immune functions (de Pablo & de Cienfuegos, Reference de Pablo and de Cienfuegos2000; Calder et al. Reference Calder, Yaqoob, Thies, Wallace and Miles2002).
We have previously documented that high plasma non-esterified fatty acids (NEFA) or high concentrations of the same FA (palmitic, palmitoleic, stearic, oleic, and linoleic acid) in the culture media are associated with impairment of lymphocyte response to mitogens both in sheep (Lacetera et al. Reference Lacetera, Bernabucci, Ronchi and Nardone2001, Reference Lacetera, Franci, Scalia, Bernabucci, Ronchi and Nardone2002) and cows (Lacetera et al. Reference Lacetera, Scalia, Franci, Bernabucci, Ronchi and Nardone2004, Reference Lacetera, Scalia, Bernabucci, Ronchi, Pirazzi and Nardone2005). On the basis of these findings, the increase of plasma NEFA consequent to lipomobilization is now assumed to represent one of the possible mechanisms to explain the immunodepression taking place in early lactating dairy cows suffering from negative energy balance (Kehrli et al. Reference Kehrli, Neill, Burvenich, Goff, Lippolis, Reinhardt, Nonnecke, Sejrsen, Hvelplund and Nielsen2006). Furthermore, a recent in vitro study indicated that bovine peripheral blood mononuclear cells (PBMC) may be functionally influenced by the presence of polyunsaturated fatty acids (PUFA) in their environment (Thanasak et al. Reference Thanasak, Müller, Dieleman, Hoek, Noordhuizen and Rutten2005).
Little is known about the immunomodulatory properties of exogenous FA when administered to ruminants. However, Lessard and co-workers reported that a series of PBMC functions in transition dairy cows is modulated by composition of FA in the diet, that such modulation may depend on n-6 to n-3 FA ratio, and that effects may vary according to the length of diet administration (Lessard et al. Reference Lessard, Gagnon and Petit2003, Reference Lessard, Gagnon, Godson and Petit2004).
The present study aimed to assess whether changes in fatty acid profile of blood lipids due to intravenous infusion of triacylglycerol (TAG) emulsions derived from different lipid sources affect PBMC responses to mitogens in fasted dairy cows.
Materials and Methods
Reagents
Reagents, TAG emulsion preparation, animals, and treatments for the study were described elsewhere (Mashek et al. Reference Mashek, Bertics and Grummer2005). A brief description of each follows.
Tallow (TA; HRR Enterprises Inc., Chicago, IL), linseed oil (LO; Archer Daniels Midland, Decatur, IL), and menhaden fish oil (FO; Omega Protein, Inc., Hammond, LA) were donated. Lecithin (60% purity) was purchased from ICN Chemicals (Irvine, CA).
Emulsion Preparation
Each of the three lipid sources contained the same amount of ethoxyquin to prevent peroxidation. In separate containers, 200 g lipid source or 765 ml water were heated to approximately 70°C. Lecithin (12 g) was added to the water and the mixture was homogenized in a blender. Glycerol (50 g) was then added to the heated lipid followed by the lecithin and water mixture. A coarse emulsion was prepared by homogenizing the mixture in the blender. Subsequently, the emulsion was passed through an homogenizer. The recipient flask was placed in cold water during the homogenization process to cool the emulsion. After adjusting the pH to 8·3 with 1 m-NaOH, emulsions were autoclaved. The flasks were then cooled in water and the contents were aseptically transferred to sterile bottles and stored at 4°C with the exception of emulsions containing TA. These were never allowed to cool below room temperature and were stored at 37°C to prevent creaming.
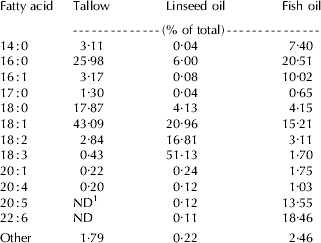
Fatty acid composition of TAG emulsions is shown in Table 1. In the TA emulsion, the most common FA in ruminants, C16:0, C18:0, and C18:1 accounted for 86·9% of total FA, whereas PUFA were not detectable (C20:5 and C22:6) or represented at very low concentration (C18:2, C18:3, C20:1, and C20:4). Compared with TA, in the LO and FO emulsions the proportion of PUFA was much greater and accounted for 68·5 and 39·6% of total FA, respectively.
Table 1. Fatty acid composition of triacylglycerol emulsions
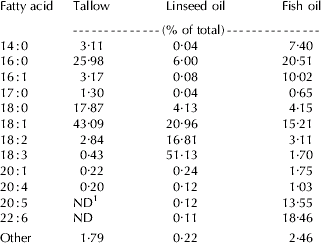
1 ND=Not detected
Animals and Treatments
Six multiparous, non-pregnant, non-lactating Holstein cows were randomly assigned to treatments in a replicated 3×3 Latin Square design. The average age of cows was 4·7 yr, and average BW and BCS were 735 kg and 3·6, respectively. Treatments consisted of fasting and an intermittent intravenous infusion of a 20% TAG emulsion derived from TA, LO, or FO. During each of the three infusion periods, 2 cows were infused with TA, 2 with LO, and 2 with FO emulsions. Therefore, by the end of the experiment each cow had been infused with each of the three emulsions. During the infusion periods, cows were fasted to induce energy deficit and lipid mobilization. TA was chosen as a control treatment because it represents the FA composition normally found in ruminants, and its use ensured isocaloric conditions across treatments. The emulsions were given via drip infusion over a 20 to 30 min period every 4 h at a rate of 0·54 g TAG/kg BW daily for 4 d. Between the three infusion periods, cows were given 24 d during which they were fed a basal diet consisting of alfalfa grass hay and a supplement containing corn grain, soy hulls, and minerals and vitamins to meet or exceed NRC recommendations (NRC, 2001). During infusion periods, cows were given approximately 100 g of the supplement with high concentrations of minerals and vitamins to meet their daily requirements. Cows were housed on a bedded pack between infusions and in tie stalls during the infusion period. Cows were allowed to exercise in an open lot for 2 h/d during the infusion period and were offered water ad libitum. The University of Wisconsin Animal Care and Use Committee approved all animal related procedures.
Sampling and Analysis
Twenty ml blood were sampled every 24 h throughout the 4 d infusion/fast period and placed in Vacutainer tubes containing Na-heparin. Ten ml blood were centrifuged and plasma samples were analysed for NEFA (NEFA-C kit; Wako Fine Chemical Industries USA, Inc., Dallas TX); the remaining 10 ml were utilized to assess DNA synthesis, IgM and interferon-gamma (IFN-γ) secretion in PBMC stimulated with mitogens.
The DNA synthesis was evaluated as previously described (Lacetera et al. Reference Lacetera, Bernabucci, Ronchi and Nardone2001). After isolation, PBMC were resuspended at a concentration of 1×106 cells/ml RPMI-1640 enriched culture medium. Triplicate cultures were assayed, by using 96-well tissue-culture plates. Each well contained 1×105 mononuclear cells in 100 μl enriched culture medium. Control wells contained 100 μl PBMC suspension without mitogens. Additional control wells were used that contained 100 μl enriched culture medium without cells or 100 μl PBMC suspension without the pyrimidine analogue 5-bromo-2′-deoxyuridine (BrdU). An optimal concentration of phytohemagglutinin (PHA, 2·5 μg/ml), pokeweed mitogen (PWM, 1 μg/ml) or concanavalin A (ConA, 2·5 μg/ml; Sigma, Milan, Italy) was added to plates. Plates were incubated in an atmosphere of 95% air and 5% CO2 for 48 h at 39°C. Afterwards, 100 μm-BrdU in 10 μl RPMI-1640 were added to each well, and plates were incubated for an additional 18 h. The DNA synthesis was quantified by an ELISA assay. The assay was performed with a commercial kit (APB, Milan, Italy) that is based on measurement of BrdU incorporated during DNA synthesis in proliferating cells. Values for DNA synthesis were expressed as the optical density (OD) of test wells minus the OD of control wells that did not contain BrdU.
Secretion of IgM was established by growing PWM-stimulated cells (1×106 cells/well) under the same conditions described above. Concentration of PWM was 0·2 μg/ml. Cells were cultured in duplicate for 12 d in 24-well tissue-culture plates. At the end of the incubation supernatants were collected and stored at −20°C until analysed. The IgM released in culture medium were quantified by a single dilution capture ELISA system (Graham et al. Reference Graham, Mawhinney, Adair and Merza1998a, Reference Graham, McShane, Mawhinney, McLaren, Adair and Merzab). Plates were coated with rabbit affinity purified antibody against bovine IgM which were detected by use of a phosphatase-conjugated sheep anti-bovine IgM-μ chain specific polyclonal antibody (Lacetera et al. Reference Lacetera, Scalia, Franci, Bernabucci, Ronchi and Nardone2004). Antibodies were purchased from Bethyl Laboratories (Montgomery, TX). Supernatant samples were tested in duplicate at dilution of 1/24 on plates coated with diluted capture antibody (1/1500). The mean OD of each supernatant at 1/24 was calculated and the corrected OD (COD) obtained by subtracting the mean OD of the control wells. Purified bovine IgM (VMRD, Pullman, WA) at concentration of 20 ng/100 μl (volume added to wells) were used as positive reference sample. Differential IgM secretions were evaluated by calculating the ratio between COD of each sample and COD of positive reference sample.
Secretion of IFN-γ was established by growing ConA-stimulated cells (1×106 cells/well) under the same conditions described above. Cells were cultured in duplicate for 72 h in 24-well tissue-culture plates. At the end of incubation supernatants were collected and stored at −20°C until analysed. The IFN-γ released in culture medium was quantified by a single dilution capture ELISA system, which was carried out by use of a commercial kit (CSL, Victoria, Australia). The IFN-γ concentration (ng/ml) was established by using the same method utilized for IgM quantification. Supernatant samples were tested in duplicate at dilution of 1/8 on plates coated with the capture antibody. The mean OD of each supernatant at 1/8 was calculated and the COD obtained by subtracting the mean OD of the control wells. In this case, bovine recombinant IFN-γ, which was kindly provided by Dr. Stephen Jones (CSL, Victoria, Australia) at concentration of 62·5 pg/100 μl (volume added to wells) was used as positive reference sample. Differential IFN-γ secretions were evaluated by calculating the ratio between COD of each sample and COD of positive reference sample.
Statistical Analysis
Data were analysed using the Mixed procedure of SAS (SAS, 2001). For repeated measurements, the model included a covariate, fixed effects of period, treatment, and time, random effects of cow (square), 2-way interactions of fixed effects, and the residual error. The covariate and interactions were removed if they were not significant (P>0·10) in the model. If factors included in the model were significant (P<0·05), the PDIFF procedure was used to determine differences. Significance was declared at P<0·05.
Results
The infusion of the three TAG emulsions exerted different effects on DNA synthesis of PBMC (Fig. 1). In PBMC isolated from cows infused with TA, a decrease (P<0·01) of DNA synthesis was observed 48 h after initiation of the infusion. Afterwards, values gradually increased, and after 96 h they did not differ from initial values. Basically, in cows infused with LO or FO DNA synthesis of PBMC did not change during the infusion period. However, in cows infused with LO an increase (P<0·01) of DNA synthesis was observed in PBMC stimulated with PHA at the end of the infusion period (96 h). Significant differences were also detected among treatments (LO or FO vs. TA). Compared with TA treatment, 48 h after the beginning of the infusions, DNA synthesis was higher in PBMC isolated from cows infused with LO or FO and stimulated with all 3 mitogens; 72 h after initiation of the infusions DNA synthesis was also higher in LO and FO cows, but only in PBMC stimulated with PHA and PWM; at the end of the infusion period (96 h) superiority of LO and FO cows was detected only for PBMC stimulated with PWM.

Fig. 1. Effects of triacylglycerol emulsions derived from tallow (○- - -○), linseed oil (●- - -●) and fish oil (△- - -△) on DNA synthesis (optical density, OD) in peripheral blood mononuclear cells stimulated with phytohemagglutinin (A), concanavalin A (B), and pokeweed mitogen (C). Significant effects in the model: treatment (P<0·01), treatment×time (P<0·01). Values with different letters indicate significant differences (P<0·01) within treatment. Significant contrasts (*): fish and linseed oil vs. tallow (P<0·01). Data represent least squares means and standard error of the mean.
Infusion of TA did not affect IgM secretion (Fig. 2). Conversely, infusions of LO or FO were responsible for an overall increase of IgM secretion, which became significant (P<0·01) 72 h after the first infusion. Furthermore, 72 h after initiation of the infusions, IgM secreted from PBMC isolated from cows infused with FO and LO were higher (P<0·01) than those recorded in their TA counterparts.

Fig. 2. Effects of triacylglycerol emulsions derived from tallow (○- - -○), linseed oil (●- - -●) and fish oil (△- - -△) on IgM secretion in peripheral blood mononuclear cells stimulated with pokeweed mitogen. Index on the y axis is the ratio between the optical density (OD) of the sample and OD of a positive reference sample. Significant effects in the model: treatment (P<0·01), treatment×time (P<0·01). Values with different letters indicate significant differences (P<0·01) within treatment. Significant contrasts (*): fish and linseed oil vs. tallow (P<0·01). Data represent least squares means and standard error of the mean.
In PBMC isolated from cows infused with TA, a decrease (P<0·01) of IFN-γ synthesis was observed 48 h after initiation of the infusion (Fig. 3). At that time, values of IFN-γ in cows infused with TA were lower (P<0·05) than those recorded in their FO or LO counterparts. Infusion of FO or LO did not modify the ability of PBMC to secrete IFN-γ over the fasting and infusion period.

Fig. 3. Effects of triacylglycerol emulsions derived from tallow (○- - -○), linseed oil (●- - -●) and fish oil (△- - -△) on IFN-γ secretion in peripheral blood mononuclear cells stimulated with concanavalin A. Index on the y axis is the ratio between the optical density (OD) of the sample and OD of a positive reference sample. Significant effects in the model: treatment (P<0·01), treatment×time (P<0·01). Values with different letters indicate significant differences (P<0·01) within treatment. Significant contrasts (*): fish and linseed oil vs. tallow (P<0·01). Data represent least squares means and standard error of the mean.
Discussion
Several authors indicated that conditions of energy deficit and intense lipid mobilization in dairy cows are associated with impairment of leukocyte functions (Szuster-Ciesielska et al. Reference Szuster-Ciesielska, Filar and Kandefer-Szerszen1995; Kaneene et al. Reference Kaneene, Miller, Herdt and Gardiner1997; Hoeben et al. Reference Hoeben, Monfardini, Opsomer, Burvenich, Dosogne, De Kruif and Beckers2000). Furthermore, studies undertaken in periparturient dairy sheep (Lacetera et al. Reference Lacetera, Bernabucci, Ronchi and Nardone2001) or cows (Lacetera et al. Reference Lacetera, Scalia, Bernabucci, Ronchi, Pirazzi and Nardone2005) demonstrated that the intensity of lipid mobilization, as estimated by measuring concentration of plasma NEFA, is positively related with the degree of immune deficiency. Results of the current study reported previously (Mashek et al. Reference Mashek, Bertics and Grummer2005) indicated that during the fasting and infusion periods all cows showed a significant increase of plasma NEFA, and that the only significant difference among treatments was a lower concentration of plasma NEFA in cows infused with LO compared with TA. This would indicate that, in our conditions, differences among treatments in PBMC response to mitogens can be only partially explained by a different intensity of the lipid mobilization or NEFA clearance from blood consequent to fasting.
Further results from the current study also reported elsewhere (Mashek et al. Reference Mashek, Bertics and Grummer2005) indicated that treatments affected FA composition of plasma. Infusion of TA increased the proportion of C16:0 compared with the LO and FO emulsions, whereas infusion of LO increased C18:3 compared with TA or FO emulsions. The C20:5 and C22:6 FA were not detected in plasma NEFA of cows receiving TA or LO, but represented 2·62 and 1·99% of plasma NEFA of cows receiving FO. Furthermore, treatments also affected plasma n-6 to n-3 FA ratio, in that TA infused cows showed a higher n-6 to n-3 FA ratio (4·04) compared with LO (1·26) or FO (1·14) infused cows. These observations suggest that the different effects of treatments on PBMC responses to mitogens stimulation may have been at least partially mediated by the influence of TAG emulsion infusions on plasma FA composition. In particular, previous studies carried out in sheep and cows (Lacetera et al. Reference Lacetera, Franci, Scalia, Bernabucci, Ronchi and Nardone2002, unpublished) pointed out strong inhibitory effects of C16:0 on PBMC functions, whereas Lessard and co-workers (Lessard et al. Reference Lessard, Gagnon, Godson and Petit2004) found that dietary PUFA can attenuate some of the periparturient alterations of lymphocyte functions in dairy cows. In detail, these authors reported that dietary administration of whole flaxseed in periparturient dairy cows reduced blood n-6 to n-3 FA ratio, and was associated with preservation of proliferative response in mitogen-stimulated blood lymphocytes. Studies carried out in other species demonstrated that changes of biophysical and functional properties of cellular membranes (Anel et al. Reference Anel, Richieri and Kleinfeld1993) due to FA administration may explain changes of lymphocyte functions. In our study we did not determine FA compositions of PBMC, so that we can not attribute the effects of treatments to changes of PBMC composition in terms of FA. However, according to a recent study carried out in humans (Skeaff et al. Reference Skeaff, Hodson and McKenzie2006), we can hypothesize that 4 d fasting/infusion period was associated also with changes in FA composition of PBMC membranes. Skeaff et al. (Reference Skeaff, Hodson and McKenzie2006) indicated that few days of dietary administration of FA are sufficient to induce changes both in plasma and blood cells FA composition, and that dietary-induced change in plasma FA is similar to that occurring in blood cells. Authors concluded that their results provide convincing, albeit indirect evidence that the exchange of FA from plasma to blood cells is a major determinant of their membrane FA composition. Furthermore, Zurier et al. (Reference Zurier, Rossetti, Seiler and Laposata1999) demonstrated that incorporation of FA from lymphocytes is a very rapid process, and that a few min incubation of lymphocytes with culture media enriched with FA is sufficient to significantly change FA composition of cells. However, with regard to our study, the hypothesis that effects of treatments were due to changes in FA composition of PBMC would not explain why most of those effects were not evident at the end of fasting/infusion periods. In particular, it would not explain why in TA cows PBMC functions were strongly inhibited during the first 48 h and above all why such inhibition did not last until the end of observation period. Previous studies demonstrated that modulation of cell death via apoptosis or necrosis (de Pablo et al. Reference de Pablo, Susin, Jacotot, Larochette, Costantini, Ravagnan, Zamzani and Kroemer1999) may also represent a mechanism through which FA modulate lymphocyte functions. In our study, as already indicated, most of the differences among treatments were detected between 48 and 72 h, and were basically due to a significant decrease of lymphocyte functions in cows infused with TA. Classical studies (Kerndt et al. Reference Kerndt, Naughton, Driscoll and Loxterkamp1982; Lomax & Baird, Reference Lomax and Baird1983) demonstrated that following 48 h fasting a metabolic adaptation occurs in the body, so that after consuming glucose derived from liver glycogen, cells start to utilize other fuels (basically NEFA and ketones) which become largely available after that time. Results of our study published elsewhere (Mashek et al. Reference Mashek, Bertics and Grummer2005) demonstrated that in our conditions the maximum plasma concentration of NEFA and ketones were reached after 48–72 h from beginning of fasting/infusion. Interestingly, literature data (Hunnicutt et al. Reference Hunnicutt, Hardy, Williford and McDonald1994; Usui et al. Reference Usui, Takata, Imamura, Morioka, Sasaoka, Sawa, Ishihara, Ishiki and Kobayashi1997; Lee et al. Reference Lee, Pinnamaneni, Eo, Cho, Pyo, Kim, Sinclair, Febbraio and Watt2006) indicate that saturated FA (more abundant in plasma of TA cows) are responsible for insulin resistance, which in our conditions would be consistent with higher plasma glucose observed in TA cows (Mashek et al. Reference Mashek, Bertics and Grummer2005). In our conditions such effects of saturated FA might have caused a reduced ability of immune cells from TA cows to utilize glucose when this represented the main energy source (first 48–72 h fasting). In this regard, it must also be noticed that reduced uptake of glucose from immune cells may be responsible for hyporesponsiveness (Frauwirth & Thompson, Reference Frauwirth and Thompson2004) or increased cell death (Alves et al. Reference Alves, Derks, Berk, Spijker, van Lier and Eldering2006; Jacobs & Rathmell, Reference Jacobs and Rathmell2006). Therefore, we can hypothesize that the reduced ability of PBMC from TA cows to respond to lectins during the first 48–72 h fasting might be due to the fact that when isolated from blood, PBMC were in a distressed state due to a reduced glucose uptake. On the other hand, other studies demonstrated that fasting per se is associated with an increase of cell death by apoptosis (Pires et al. Reference Pires, Curi and Otton2006), and that saturated FA increase predisposition of lymphocytes to such event (Carratelli et al. Reference Carratelli, Nuzzo, Vitiello, Galdiero and Galdiero1999). After 48–72 h fasting, increased availability of NEFA and ketones may have restored the ability of immune cells from TA cows to respond to lectins stimulation.
In conclusion, under conditions of energy deficit and lipid mobilization due to fasting, administration of LO or FO would permit to attenuate alteration of lymphocyte responses to mitogens. With regard to practical situations, our findings may be of particular interest for conditions of transient anorexia, which may occur with a certain frequency during transition period (Bertoni et al. Reference Bertoni, Trevisi and Piccioli-Cappelli2004) or disease states (Kulcsar et al. Reference Kulcsár, Jánosi, Lehtolainen, Kátai, Delavaud, Balogh, Chilliard, Pyörälä, Rudas and Huszenicza2005) and that are likely to predispose cows to further health problems. Finally, current results support conclusions from previous studies (Lessard et al. Reference Lessard, Gagnon, Godson and Petit2004) illustrating the potential importance of dietary lipids, and in particular of unsaturated FA, on modulating immune functions during periods that are known to be critical for the immune response.
The research was sponsored by MIUR (PRIN 2005), and Università della Tuscia. The authors recognize also Bioproducts, Inc., Pioneer Hi-Bred International Inc., Degussa Corp., ADM Alliance Nutrition Inc., Kemin Americas, Church and Dwight Co., Inc., Land O'Lakes Inc., Diamond V Mills., and Zinpro Corp., for partial financial support.