Camel milk is considered to be an important milk for human nutrition, particularly for people living in arid and semi-arid regions (Al-Haj & Al-Kanhal, Reference Al haj and Al Kanhal2010). For example, the level of vitamin C is three to five times higher than that in cow milk (Al-Haj & Al-Kanhal, Reference Al haj and Al Kanhal2010). In addition, camel milk contains higher levels of essential fatty acids and antimicrobial agents than most other milks and has potential therapeutic properties such as anti-diabetic activity (Shori, Reference Shori2015). It is also considered as a promising new protein source for children allergic to cow milk since camel milk does not contain β-lactoglobulin (El-Agamy et al. Reference El-Agamy, Nawar, Shamsia, Awad and Haenlein2009), although other whey proteins are more abundant than in bovine milk (Kappeler, Reference Kappeler1998).
Camel milk is known to have limit ability for enzymatic coagulation. Indeed, it exhibit a rennet-induced coagulation time two to three-fold longer compared with bovine milk (Farah & Bachmann, Reference Farah and Bachmann1987). This behaviour has been attributed to the specific structure of the casein micelles (El Zubeir & Jabreel, Reference El Zubeir and Jabreel2008). The low content of κ-casein and the large size of camel milk casein micelles compared with those of cow milk prolongs the rennet-induced coagulation of camel milk (Kappeler et al. Reference Kappeler, Farah and Puhan1998).
Heat treatment is an essential step of milk production adopted by the dairy industry either to improve desirable characteristics of the products, or to ensure its safety and shelf-life by reducing the microbial load (Donato & Guyomarc'h, Reference Donato and Guyomarc'h2009). However, this treatment results in irreversible modifications for both the physical and chemical properties of milk (Vasbinder et al. Reference Vasbinder, Rollema and de Kruif2003; Blecker et al. Reference Blecker, Habib-Jiwan and Karoui2012).
In the literature, several studies are available regarding the impact of milk heating on the rheological properties of rennet-induced cow milk gels (Pomprasirt et al. Reference Pomprasirt, Singh and Lucey1998; Renault et al. Reference Renault, Gastaldi, Cuq and Tarodo de la Fuente2000; Blecker et al. Reference Blecker, Habib-Jiwan and Karoui2012), but very limited research is available on camel milk (Hattem et al. Reference Hattem, Manal, Hanna and Elham2011; Felfoul et al. Reference Felfoul, Jardin, Gaucheron, Attia and Ayadi2017). Felfoul et al. (Reference Felfoul, Jardin, Gaucheron, Attia and Ayadi2017) reported that heating camel milk at 80 °C for 60 min induced a complete disappearance of α-lactalbumin and peptidoglycan recognition protein and a decrease of 42% of serum albumin concentration.
Regarding cow milk, it is well known that milk heat treatment at temperatures higher than 55 °C induces the denaturation of whey proteins causing an increase and a decrease of the gelation time and the curd firmness, respectively. This trend has been ascribed to the inhibition of the enzyme action on κ-casein resulting from the interaction of this casein with denatured whey proteins (particularly β-lactoglobulin) allowing the formation of whey protein/κ-casein complexes (Blecker et al. Reference Blecker, Habib-Jiwan and Karoui2012).
Calcium and phosphate are essential elements of the mineral fraction of milk and their levels play a key role in the coagulation process. Gustavsson et al. (Reference Gustavsson, Glantz, Buitenhuis, Lindmark-Månsso, Stålhammar, Andren and Paulsson2014) found that high ionic calcium level in milk improved the gelation properties. This finding was recently confirmed by Ramasubramanian et al. (Reference Ramasubramanian, D'Arcy, Deeth and Eustina2014), who reported that adding calcium to preheated cow milk at 90 °C for 10 min decreased the gelation time and increased the curd-firming rate. The authors ascribed these changes to the reduction of the pH of milk following the addition of calcium which induces a decrease of electrostatic repulsion of micelles allowing the formation of calcium bridges between caseins particles and the increase of calcium colloidal phosphate level. In contrast, the incorporation of phosphate in milk induced a delay in the onset gelation of the rennet-induced coagulation resulting from the decrease of the ionic calcium amount (Guillaume et al. Reference Guillaume, Gastaldi, Cuq and Marchesseau2004).
Although several studies have been published on the effect of milk pre-heating and its enrichment with calcium and phosphate on the physical properties of rennet-induced cow milk gels, to the best of our knowledge the scientific literature does not contains information about the effect of added phosphate on preheated camel milk gels. Thus, the aim of this study was to monitor rheological properties of the rennet-induced coagulation of preheated camel or preheated cow milk gels at 50 and 70 °C enriched with calcium and phosphate at a level of 10 and 20 mM.
Materials and methods
Milk samples
Fresh camel milk, consisting of a volume of 2 l distributed into plastic bottles of 30 ml capacity, was obtained from an experimental station located in the centre of Tunisia (ElJem region, Mahdia, Tunisia) by directly milking into a sterile milking bottle and the milk was transported using an icebox. Camels of Maghrebine genotype aged 6 years were maintained on pasture feeding (yearly pasture of thorny plants and coquelicot) and supplemented with concentrate. They were inspected by a qualified shepherd on a daily basis, and routine animal care and vaccination procedures were conducted as prescribed by best practice protocols.
Fresh cow milk of Holstein Friesian genotype, consisting of a volume of 2 l distributed into plastic bottles of 30 mL capacity, was obtained from a regional farm of France (Nord Pas de Calais, 59000 Lille, France). Cows were maintained on pasture feeding and supplemented with a corn-based concentrate.
Upon arrival at the laboratory, milk samples were kept at −18 °C until analysis. All the analyses were made in duplicate.
Physicochemical analyses of camel and cow milk
The pH, protein, fat and dry matter contents of camel and cow milk were determined as described by Karoui & Dufour (Reference Karoui and Dufour2003).
Size distribution of milk casein micelles
Skimmed milk was obtained by centrifugation at 3740 g for 15 min (Froilabo-SW14R, 69330 Meyzieu, France). Number-based particle size distribution of skimmed milk was determined using a SALD-2300 laser diffraction particle size analyser in wet type mode (Shimadzu Scientific Instruments, 77448 Marne La Vallée, France). Milk samples were directly injected into the dispersion cell (containing deionised water as dispersant) until a light intensity distribution more than 10% was reached and the particle size distributions were measured. The refractive index of 1·55 was used according to the findings of Griffin & Griffin (Reference Griffin and Griffin1985) who have used refractive index value of 1·57. For the dispersant, a blank was measured with deionised water at which the light intensity distribution is less than 10%.
Sample preparation for the gelation
Twenty milk samples in total for each milk species were thawed during 12 h at 4 °C and then kept at room temperature (~18 °C) for 15 min and gently mixed. Milk samples were placed in a water-bath preset at 50 or 70 °C for 10 min. The samples were then cooled for 5 min and placed immediately in another water-bath equilibrated previously at 36 °C during 5 min.
Then, calcium chloride anhydrous (CaCl2, Merck, 64271 Darmstadt, Germany) and di-sodium hydrogen phosphate dihydrate (Na2HPO42H2O, Merck, 64271 Darmstadt, Germany) powder at 10 and 20 mM were separately added to milk samples with stirring for 2 min. A volume of 6·25 µl of the rennet FAR-M®Sticks (12500 IMCU/stick) (Chr. Hansen A/S BoegeAllé, 2970 Hoersholm, Denmark) prepared directly before the test from 1 g of powder FAR-M® Sticks diluted in 20 g of distilled water was added to 25 ml of milk and measurements were immediately performed. Control milk (i.e. preheated milk samples at 50 and 70 °C without added calcium and phosphate) was also studied.
Rheological and pH measurements throughout rennet induced coagulation
The experiments were performed using a controlled-strain rheometer (Physica MCR 301, Anton Paar Company, 73760 Ostfildern, Germany) with low amplitude oscillation shear analysis and a temperature set at 36 °C by applying a Peltier plate. A volume of 20 ml milk was placed in two concentric cylinders, with an inner diameter of 26·66 mm, length of 40·02 mm, and a gap of 1·13 mm. A liquid paraffin layer was placed onto the surface of milk to prevent evaporation during coagulation, and then measurement was started. The oscillation shear analyses were performed during 45 min in the linear viscoelastic region by applying a constant frequency of 1 Hz and a strain of 0·05%. The gels were then subjected to a frequency sweep from 1 to 10 Hz (at 36 °C; 0·05% strain), followed by an amplitude sweep from 0·05 to 150% with a frequency of 1 Hz frequency. Taking into account the elastic modulus (G′), the following parameters were determined: (i) the gelation time defined as the time when G′ = 1 Pa as reported by Klandar et al. (Reference Klandar, Lagaude and Chevalier-Lucia2007) and Ramasubramanian et al. (Reference Ramasubramanian, D'Arcy, Deeth and Eustina2014); (ii) the gelation rate (defined as dG′/dt) corresponding to the slope obtained by linear regression of G′ vs time from onset of gelation over a period of 4 min; and (iii) the asymptotic value of G′ after 45 min of gelation (G′asym).
The pH was determined every 15 min during 45 min of the rennet-induced coagulation.
The different measurements were performed using frozen-thawed milk samples, following a recent study conducted on caprine milk showing that milk frozen for up to 2 months at −27 °C did not present significant effect on the coagulation properties (Kljajevic et al. Reference Kljajevic, Jovanovic, Miloradovic, Macej, Vucic and Zdravkovic2016).
Statistical analysis
In order to detect difference between milk samples, rheological and physico-chemical parameters were compared using a one-way ANOVA (P < 0·05). ANOVA was applied using XLSTAT 2013 (Addinsoft SARL USA, New York, NY, USA) software.
Results and discussion
Physico-chemical properties of camel and cow milk
Camel milk contains significantly lower amounts (P < 0·05) of dry matter, protein and fat (110 ± 10·00, 28·58 ± 0·21 and 34·50 ± 0·51 g/l, respectively) than those of cow milk that reached 126·13 ± 8·48, 34·70 ± 0·55, and 37·50 ± 0·50 g/l, respectively. By contrast, the pH of camel milk was higher (6·51) than that of cow milk (6·42). The size of raw camel milk casein micelles was significantly (P < 0·05) larger (i.e. 471 ± 2·50 nm) than that of raw cow milk (i.e. 138 ± 1·50 nm), in agreement with the findings of Bornaz et al. (Reference Bornaz, Sahli, Attalah and Attia2009) who observed a diameter of casein micelles of camel milk in the 280–550 nm range, larger than that of cow milk (i.e. 90–210 nm).
Throughout the whole gelation time, the pH values of camel milk gels preheated at 70 °C remained constant (6·51). Quite similar results were observed for cow milk preheated at 70 °C, presenting initial pH value of 6·42 that reached 6·50 after 45 min of coagulation, since no significant difference between the pH values was observed (P > 0·05: Fig. 1a, b). These results are in agreement with those of Ramasubramanian et al. (Reference Ramasubramanian, D'Arcy, Deeth and Eustina2014) who indicated that milk preheating had no significant effect on the pH values of the obtained gels. Similar results were observed for camel and cow milk gels preheated at 50 °C (data not shown).

Fig. 1. Evolution of pH as a function of time for (a) camel and (b) cow milk gels preheated at 70 °C for 10 min (data are means of duplicates with sd).
A similar trend was observed following the addition of calcium and phosphate to preheated milk gels at 70 °C for both types of milk. Adding calcium to milk induced a significant decrease (P < 0·05) of pH values compared with control milk gel; for milk fortified with 20 mM calcium, the pH reached after 45 min of rennet-induced coagulation, 5·95 ± 0·01 and 5·92 ± 0·02 for camel and cow milk gels, respectively (Fig. 1a, b), in agreement with others (Farah & Bachmann, Reference Farah and Bachmann1987; Ramasubramanian et al. Reference Ramasubramanian, D'Arcy, Deeth and Eustina2014). Indeed, excess amount of calcium ions added to milk displace H+ ions from hydrogen phosphate, leading to the increase of the concentration of H+ ions in milk gels and thus reducing pH as explained by the following equation (Lewis, Reference Lewis2011):
In contrast, adding phosphate to preheated milk gels at 70 °C induced a significant increase of pH values (P < 0·05) compared with control milk gels, regardless of milk species (Fig. 1a, b). With the enrichment of milk with 20 mM phosphate, this increase reached after 45 min of rennet-induced coagulation, 6·98 ± 0·02 and 6·95 ± 0·01 for camel and cow milk gels, respectively, in agreement with Tsioulpas et al. (Reference Tsioulpas, Koliandris, Grandison and Lewis2010) who attributed this trend to the increase of negatively charged casein because of the reduction of milk ionic calcium. Similar results were observed for camel and cow milk gels preheated at 50 °C (data not shown).
Rheological properties of calcium/phosphate induced milk gels
The gelation properties of camel and cow milk gels including the gelation time, the gelation rate and the G′asym are illustrated in Table 1. Pre-heated cow milk enriched or not with calcium and phosphate allowed the formation of gels. In addition, the pre-heat treatment at 50 and 70 °C of cow milk seemed not to have a significant effect on the gelation properties (Fig. 2b, d and Table 1). A different trend was observed for camel milk since a negative effect on the gelation properties was observed for preheated control camel milk and those enriched with phosphate (10 and 20 mM) at 50 °C (Fig. 2a, c and Table 1). In addition, no gelation was observed for camel milk preheated at 70 °C.

Fig. 2. Storage modulus (G′) of added calcium and phosphate milk samples as a function of time of: (a) camel, (b) cow milk gels preheated at 50 °C for 10 min, and (c) camel, (d) cow milk gels preheated at 70 °C for 10 min (data are means of duplicates).
Table 1. Gelation kinetic parameters of camel and cow milk samples preheated at 50 and 70 °C for 10 min for control milk gels and those enriched with calcium and phosphate

Values are means of 2 replicates with sd, dG′/dt; gelation rate (Pa/min), G′asym; asymptotic value of storage modulus (Pa) after 45 min of gelation, (−); no gelation.
Different small letters (a, b, c, d, e, f, g, h) represent statistical differences between the gelation properties of camel and cow milk (P < 0·05).
Different capital letters (A, B, C, D, E, F, G) and (H, I, J, K, L, M) represent statistical differences within the same type of milk as a function of added mineral for camel and cow milk respectively (P < 0·05).
Although several studies have determined the impact of the added minerals on cow milk gel properties, only limited papers were available in the literature on the camel milk gels. The absence of the gelation for preheated camel milk at 70 °C compared to cow milk preheated at the same temperature could be attributed to the difference in the composition of the two milk samples, particularity, the absence and β-lactoglobulin and deficiency of κ-casein. The level of κ-casein represents 3·47% of the total casein in camel milk vs 13% in cow milk as reported by others (Farah & Atkins, Reference Farah and Atkins1992; Al-Haj & Al-Kanhal, Reference Al haj and Al Kanhal2010). It could be concluded that camel milk is more sensitive to heat treatment than cow milk, in agreement with the findings of Hattem et al. (Reference Hattem, Manal, Hanna and Elham2011) who observed that the gelation time of raw and preheated camel milk gels at 80 °C for 30 min was of 17 and 26 min, respectively.
Our findings are in agreement with those of Montilla et al. (Reference Montilla, Balcones, Olano and Calvo1995) who pointed out that the addition of 10 mM calcium to preheated cow milk gels at 70 °C for 3 min induced a decrease in the rennet clotting time. These findings were recently confirmed by Ramasubramanian et al. (Reference Ramasubramanian, D'Arcy, Deeth and Eustina2014) who observed a decrease and an increase of the clotting time and the gelation rate with added 20 mM calcium to cow milk gels preheated at 90 °C for 10 min compared with preheated milk gels without added calcium, and Hattem et al. (Reference Hattem, Manal, Hanna and Elham2011) who reported that the gelation time of preheated camel milk −63 °C for 30 min- gels was 20 min, while the same preheated camel milk supplemented with 1·8 mM calcium was 12 min.
According to Table 1 and Fig. 2a–d added phosphate at 10 and 20 mM affected significantly (P < 0·05) the gelation properties of camel and cow milk gels. Compared with control milk gels, a significant delay (P < 0·05) of the gelation time and a significant decrease of both the gelation rate and gel firmness were observed with added phosphate to preheated camel and cow milk gels at 50 and 70 °C. The results obtained on cow milk gels were in agreement with the finding of Guillaume et al. (Reference Guillaume, Gastaldi, Cuq and Marchesseau2004) who reported that the addition of phosphate to milk delayed the gelation time. This trend could be explained by the decrease of the ionic calcium level resulting from the formation of a phosphocalcic complex between calcium and added phosphate (Udabage et al. Reference Udabage, McKinnon and Augustin2001; Guillaume et al. Reference Guillaume, Gastaldi, Cuq and Marchesseau2004).
Evolution of storage and loss modulus of calcium/phosphate induced milk gels as a function of frequency
The evolution of G′ as a function of frequency and strain amplitude sweep is depicted in Figs 3 and 4. In general, an increase in the G′ (Fig. 3a–d) and G″ (data not shown) values of calcium and phosphate induced milk gels was observed in the 1–10 Hz frequency range, regardless of milk species and the intensity of heating. These results agreed with the findings of Pomprasirt et al. (Reference Pomprasirt, Singh and Lucey1998) who observed that G′ values of renneted gels made with recombined high total solids milk preheated at 70 °C for 3 min increased with increase of frequency from 0·001 to 1 Hz.

Fig. 3. Storage modulus (G′) of added calcium and phosphate milk samples as a function of frequency of: (a) camel, (b) cow milk gels preheated at 50 °C for 10 min, and (c) camel, (d) cow milk gels preheated at 70 °C for 10 min (data are means of duplicates).

Fig. 4. Shear stress of added calcium and phosphate milk samples as a function of strain of: (a) camel, (b) cow milk gels preheated at 50 °C for 10 min, and (c) camel, (d) cow milk gels preheated at 70 °C for 10 min (data are means of duplicates).
The differences between G′ and G″ were less than 1 log, particularly for camel milk samples, reflecting the formation of weak gels (Lapasin & Pricl, Reference Lapasin, Pricl, Lapasin and Pricl1995). Indeed, an increase of G′ values from 5·4 to 12·15 Pa for control camel milk gels preheated at 50 °C and from 6 to 12·02 Pa for those with added calcium at 20 mM was observed when the frequency passed from 1 to 10 Hz (Fig. 3a). A slight increase (i.e. from 7 to 7·52 Pa with increase of frequency from 1 to 10 Hz) was obtained for camel milk gels preheated at 50 °C with added 10 mM calcium indicting the formation of more stabilised gels. This result reflects that G′ values were independent of frequency and depended on the number of calcium bridges formed in the protein network (Fig. 3a). Similar trends were observed for G′ (Fig. 3c) and G″ values (data not shown) of preheated camel milk gels at 70 °C.
Over the entire used frequency range (from 1 to 10 Hz), the G′ slope obtained using a linear regression of calcium-induced camel milk gels preheated at 50 and 70 °C exhibited lower values in comparison with control milk gels and those with added phosphate (Fig. 3a, c). For instance, slopes obtained from camel milk gels preheated at 70 °C were of ~0·32 (R 2 = 0·85) and ~0·50 (R 2 = 0·91) for respectively milk gels supplemented with 10 and 20 mM calcium. While slopes of ~0·91 (R 2 = 0·90), 0·87 (R 2 = 0·93) and 1·29 (R 2 = 0·95) were observed respectively for control milk gels and those added with 10 and 20 mM phosphate (Fig. 3c). These results reflected that the added calcium to milk induced the formation of strengthen gels than those obtained with added phosphate, indicating the formation of bridges between calcium and casein molecules.
The obtained slopes from preheated camel milk gels are higher than those of preheated cow milk gels at 50 and 70 °C (Fig. 3b, d). For example, control cow milk gels preheated at 70 °C and those enriched with calcium and phosphate presented slopes of ~0·19 (R 2 = 0·99), 0·15 (R 2 = 0·99) and 0·17 (R 2 = 0·99), respectively. This difference between the both types of milk gels reflect the formation of higher cross-links between casein particles, leading to obtain more stabilised cow milk gels compared to camel milk gels. Indeed, the high quantity of protein in cow milk compared to camel milk (34·70 ± 0·55 vs 28·58 ± 0·21 g/l, respectively) increased the number of bonds and contact area in the gel network and thus the formation of more stabilised gel (Sandra et al. Reference Sandra, Ho, Alexander and Corredig2012).
The slope values of gels made from preheated milk at 50 °C were lower than those of preheated milk at 70 °C, indicating the formation of more stabilised gels for preheated milk at 50 °C. The results obtained from cow milk gels were in line with the findings of previous investigations (Lucey et al. Reference Lucey, Munro and Singh1998, Reference Lucey, Munro and Singh1999) reporting that log G′ preheated milk gels at 75 and 80 °C for 30 min vs log frequency gave linear curves with a slope of ~0·15. Sandra et al. (Reference Sandra, Ho, Alexander and Corredig2012) observed a lower slope value obtained with G′ as a function of frequency following the addition of 1 mM calcium due to the formation of higher extent of cross-linking resulting from the formation of calcium bridges in the milk gel protein.
During the strain sweep tests, the stress of milk gels increased the breaking point of the gels (Fig. 4a–d). Irrespective of the considered heat-treatment, the breaking stress of the cow and camel milk gels increased with added calcium. A decrease in the breaking stress values of camel and cow milk gels was recorded following the addition of phosphate mainly for camel milk gels preheated at 70 °C. For example, the maximum breaking stress of preheated control camel milk gels at 50 °C and those with 20 mM added calcium and phosphate exhibited values of 1·60, 2·54 and 1·34 Pa, respectively. The breaking strain with 20 mM added calcium and phosphate showed similar values (i.e. 48%), while it was of 33% for control milk gels (Fig. 4a). The maximum breaking stress of control cow milk gels preheated at 50 °C and those added with 20 mM calcium and phosphate was respectively of 51·10, 69·50 and 28·15 Pa. A similar breaking strain value of 70% was obtained for control milk gels and those added with calcium at 20 mM, while values of 102% were observed for added phosphate at 20 mM (Fig. 4b). Similar results were observed for preheated camel and cow milk gels at 70 °C (Fig. 4c, d), in agreement with the findings of Ramasubramanian et al. (Reference Ramasubramanian, D'Arcy, Deeth and Eustina2014) who found that the breaking stress of gels made from preheated milk at 90 °C during 10 min increased with calcium added at 20 mM, while the breaking strain was in the 70–80% range. The increase of breaking stress could be explained by the increase of calcium level in the casein micelles, inducing the formation of calcium bridges between caseins particles making the gels more resistant to stress (Lucey et al. Reference Lucey, Teo, Munro and Singh1997). In contrast, the decrease of gel breaking stress after the addition of phosphate could be ascribed to the reduction of colloidal calcium phosphate cross-links and the dispersion of casein particles (Ozcan et al. Reference Ozcan, Lucey and Horne2008).
Comparison of the final storage modulus of camel and cow milk gels as a function of heat treatment and the level of added minerals
The G′asym values of control preheated camel and cow milk gels at 50 and 70 °C and those supplemented with phosphate and calcium are depicted in Fig. 5. The G′asym values were in the following order: cow milk gels > camel milk gels. Indeed, irrespective of the applied temperature and the added amount of phosphate and calcium, cow milk gels presented significant higher values (P < 0·05) than camel milk gels. Moreover, the G′asym values of phosphate and calcium induced camel milk gels obtained from milk preheated at 50 °C were significantly higher (P < 0·05) than those made from preheated milk at 70 °C (Fig. 2a, c and Table 1); however, no significant differences were observed between the G′asym values of preheated cow milk gels at 50 and 70 °C. Additionally, for a considered milk species, the G′asym values of added calcium gels were significantly higher (P < 0·05) than those of phosphate, regardless the intensity of heat treatment applied to milk. Compared with control milk gels, the fortification of preheated camel and cow milk at 50 and 70 °C with calcium induced a significant: (i) increase of G′asym and gelation rate values; (ii) decrease of gelation time. The G′asym of control camel milk pre-heated at 50 and 70 °C was of 5·28 and 0·97 Pa, respectively; it passed to 7·33 and 3·65 Pa for those enriched with 10 mM calcium, respectively. The G′asym gel passed from 120 and 107 Pa for control cow milk gels preheated at 50 and 70 °C, respectively to 188 and 182 Pa for those with added 20 mM calcium, respectively (Table 1). These results confirmed the findings of Ramasubramanian et al. (Reference Ramasubramanian, D'Arcy, Deeth and Eustina2014) who observed G′asym values of 248·6 Pa for gels made with preheated cow milk at 90 °C for 10 min and enriched with 20 mM calcium. This trend could be ascribed to the denaturation of β-lactoglobulin and α-lactalbumin in cow milk and α-lactalbumin in camel milk following the heating of milk leading to form whey protein network that strengthen the calcium-induced milk gel by increasing the cross-linking of the micelles during gelation (Riou et al. Reference Riou, Havea, McCarthy, Watkinson and Singh2011). The difference between camel and cow milk gels could be ascribed to the difference in the: (i) protein composition of both type of milk could be due to the absence of β-lactoglobulin in camel milk resulting in a poor stability of camel milk following thermal treatment (Farah & Atkins, Reference Farah and Atkins1992); (ii) difference in the level of dry matter since Ramet (Reference Ramet2001) and El Zubeir & Jabreel (Reference El Zubeir and Jabreel2008) reported that the lower amounts of dry matter and protein in camel milk, the weaker the formed gel; this could be explained by the creation of less bonds per unit space allowing the formation of softer gel. Additionally, the low fat level of camel milk compared to cow milk induced the formation of weak gels as described by Lucey et al. (Reference Lucey, Munro and Singh1998) and El Zubeir & Jabreel (Reference El Zubeir and Jabreel2008) who pointed out that during coagulation fat globules interact with protein matrix improving the firmness of milk gels.
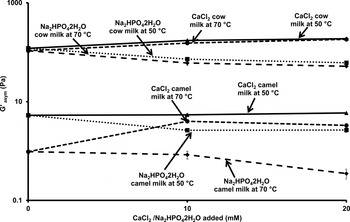
Fig. 5. Effect of the milk preheating at 50 and 70 °C for 10 min and added calcium and phosphate on final storage modulus (G′asym) of camel and cow milk gels (data are means of duplicates).
Furthermore, the size of the casein micelles affected the mechanical properties since smaller casein micelles increased the gels firmness and shortened the gelation times (Ramet, Reference Ramet2001; Glantz et al. Reference Glantz, Devold, Vegarud, Lindmark-Månsson, Stålhammar and Paulsson2010), and greater availability hydrolytic cleavage sites (Niki et al. Reference Niki, Kim, Kimura, Takahashi, Kohyama and Nishinari1994). In the present study, the size of camel milk casein micelles was significantly (P < 0·05) larger (i.e. 471 ± 2·50 nm) than that of cow milk (i.e. 138 ± 1·50 nm), in agreement with the findings of Bornaz et al. (Reference Bornaz, Sahli, Attalah and Attia2009) who observed a diameter of casein micelles of camel milk in the 280–550 nm range, larger than that of cow milk (i.e. 90–210 nm).
In summary, the present study showed differences between the gelation properties of camel and cow milk gels preheated at 50 and 70 °C. A significant difference was observed between the gelation properties of preheated camel milk gels at 50 °C and those obtained from camel milk gels preheated at 70 °C. In contrast, no difference was noticed between the gelation characteristics of preheated cow milk gels at 50 and 70 °C. The addition of calcium at 10 and 20 mM improved the gelation properties of camel and cow milk gels compared to control milk gels, regardless of the intensity of heat treatment. Whereas, added phosphate at 10 and 20 mM to preheated milk gels at 50 and 70 °C induced the formation of softer camel and cow milk gels, and even no gelation for preheated camel milk at 70 °C.
This work has been carried out in the framework of Alibiotech project which is financed by European Union, French State and the French Region of Hauts-de-France. We acknowledge Mr. Ismail MERDASSI for providing us camel milk samples from his experimental station located in the centre of Tunisia (ElJem, Mahdia governorate). CHR HANSEN A/S (Hoersholm, Denmark) is acknowledged for sending us the FAR-M®sticks.








