Male dairy calves are commonly transported from the farm of origin for further rearing or slaughter. Frequently, such calves are very young, and previously have been removed from their dams within the first 1–2 d of life. As a consequence, animal welfare concerns have resulted in regulatory restrictions on the transport of these vulnerable livestock. In Australia, for example, regulatory requirements include stipulations that calves transported without their mothers must be at least 5 d old, fed within 6 h of loading, and be transported for no more than 12 h (Animal Health Australia, 2008). Further requirements relate to minimum body weight and total time of feed withdrawal. Regulations within the EU stipulate that calves younger than 14 d old must not be transported for longer than 8 h, among other requirements (European Commission, 2005).
A review by Knowles (Reference Knowles1995) highlighted that the transport of young calves (approximately 1–2 weeks old) is associated with relatively high levels of subsequent morbidity and mortality, and that these adverse effects may be increased by indirect consignment as compared with direct consignment from the farm of origin to the destination. Although the transport of calves to abattoirs for slaughter removes the risk of subsequent morbidity, studies have still shown that mortality can be a problem for these young animals during the transport and lairage process (Cave et al. Reference Cave, Callinan and Woonton2005).
Because calves of this age are unweaned, the transport process effectively involves the withdrawal of both feed and water. There has been some research on the effects of transport up to 18 h and feed withdrawal up to 30 h in young calves (Kent & Ewbank, Reference Kent and Ewbank1986; Todd et al. Reference Todd, Mellor, Stafford, Gregory, Bruce and Ward2000); however, there has not been a comparison of the effects of direct and indirect consignment on calf well-being in this context. It is known from other studies that stress induced by road transport can adversely affect the welfare of livestock, and this can be reflected in altered blood biochemistry (Piccione et al. Reference Piccione, Casella, Giannetto, Bazzano, Giudice and Fazio2013) and haematological changes (Giannetto et al. Reference Giannetto, Fazio, Casella, Marafioti, Giudice and Piccione2011). The objectives of this study were to examine the effects of calf transport (including feed withdrawal of 30 h) on calf well-being, including a comparison of the effects of journey duration and indirect and direct consignment.
Materials and methods
Animals and treatments
The experiment was approved by the institutional Animal Ethics Committee, operating under the Victorian Prevention of Cruelty to Animals Act 1986. This study was conducted on a commercial dairy farm located in central Gippsland, Victoria, Australia (37°60′S, 146°57′W). A total of 60 male Holstein-Friesian dairy calves were allocated to the study. The calves were 5–9 d old (6·9±1·17 d; mean±SD), and weighed 42·8±6·77 kg (mean±SD). The study was conducted in three replicates (5 calves in each of four treatments per replicate) over three consecutive weeks during late winter and early spring (late August to mid September). The minimum temperature recorded during the transport days was −0·1 °C, with other daily minima ranging up to 4·7 °C. Daily maxima ranged from 15·8 to 23·9 °C, and daily rainfall from 0 to 7·4 mm.
Within replicate, and balanced for age and weight, the calves were allocated to four treatments (n=15 per treatment overall): (1) remain in situ without feed for 30 h (Control); (2) no feed for 30 h including transport for 6 h to a new environment (6 h); (3) no feed for 30 h including transport for 12 h to a new environment (12 h); (4) no feed for 30 h including transport for 1 h to a holding pen, remaining for 6 h, then transport for 5 h to a new environment (Indirect). The treatments were designed to simulate the various transport scenarios to which calves may be subjected as part of commercial practice.
Prior to treatment, the calves were managed by farm staff in accordance with standard farm practice, being removed from their dams within 12 h of birth, and housed in small groups (typically 10–15 calves) in an open-fronted, sawdust-bedded barn. Following initial colostrum (milked from the dam and provided to the calf), the calves were offered 2·5 l of fresh, whole milk twice daily for the first 3 d, then 5 l of milk once daily thereafter. The milk was offered from an elevated trough with multiple teats. Water was available at all times. On the day of treatment (day 1), calves were offered their normal once daily milk allocation of 5 l at 6·00. All calves were weighed at 12·00, and then the calves in the 6 h, 12 h and Indirect treatments were loaded and transported on the same vehicle.
The calves were transported in a commercial fixed-body uncovered livestock transport vehicle belonging to a calf transport company. During transport, calves were housed in small groups on unbedded non-slip metal flooring and confined to one of the pens on the truck at a space allowance of 0·3 m2. The fixed width of the internal pen was 1·2 m, and a movable gate was used to maintain the stocking density as the number of calves altered over the 12-h period, as calves were not separated by group during transport. The transport driver drove a set 1-h route over a mixture of main and secondary roads on each of the 3 transport days, returning to the farm at intervals to allow the calves to be checked and blood sampled. Over a typical 6-h period, the vehicle covered 222 km, at a mean speed of 32 km/h, including rest stops.
Calves in the Indirect treatment were unloaded after 1 h of transport (13·00) into an uncovered concrete holding yard with no water trough. Calves in the 6-h treatment were unloaded in the destination environment at 18·00. Calves in the Indirect treatment were re-loaded after 6 h in the holding pen (19·00). Calves in the 12-h and Indirect treatments were then unloaded in the destination environment at 24·00. The destination environment was an open fronted, covered sawdust bedded pen for each treatment group of five calves, and was in a separate facility from the home pen where the Control calves remained. All calves had access to water up to 12·00 and from 24·00. The study concluded after 30 h of feed withdrawal at 12·00 on the second day. At this point all calves were weighed, then fed milk and managed according to commercial practice.
Blood sampling and biochemical measurements
Blood samples were collected via jugular venepuncture at the following time points: 6·00 (day 1, pre-feeding), 9·00, 12·00 (pre-loading), 18·00, 21·00, 24·00, 6·00 (day 2), and 12·00 (day 2, immediately before re-feeding). Blood samples were collected into three different tubes: an 8·5-ml serum separator tube was used for analysis of creatine kinase (CK), lactate, total serum protein (TSP), beta-hydroxybutyrate (BHB) and gamma-glutamyl transferase (GGT, time point 1 only); a 4·0-ml fluoride oxalate tube was used to collect blood for glucose determination; and a 2·0-ml EDTA tube was used to assess packed cell volume (PCV). The serum separator and fluoride oxalate tubes were centrifuged at 2500 g for 5 min. Sera or plasma were then transferred to plain tubes and frozen at −20 °C until analysis. PCV was determined by the microhaematocrit technique within 24 h of collection. Serum concentrations of CK, TSP, lactate, BHB and GGT, and plasma glucose concentrations were determined using a biochemical autoanalyser (RX Daytona, Randox, Co. Antrim, UK). Laboratory reference values for dairy calves 1–14 d old were obtained from Lumsden et al. (Reference Lumsden, Mullen and Rowe1980).
Animal behaviour
Lying behaviour was determined using behaviour loggers (IceTag™, IceRobotics, Midlothian, Scotland, UK). On day 0, calves were fitted with the loggers, attached to the front left leg with a hook and loop strap and a self-adhesive bandage wrapping. The loggers recorded the proportion of each minute spent lying or standing. The data were collected from 6·00 h on day 1 to 12·00 on day 2, and then organised into five 6-h periods: 0–6, 6–12, 12–18, 18–24 and 24–30 h.
Body temperature
On day 1, rectal temperature loggers were fitted to all calves at 5·00. The devices consisted of a small button (Dallas Thermocron iButton® DS1921H/Z, Maxim Integrated Products, Sunnyvale CA, USA) on the end of a short flexible plastic rod, which was placed in the rectum and secured externally by tape to the base of the tail. The design of the probe allowed the animal to defecate normally. The loggers were programmed to record temperature every 2 min.
Statistical analyses
One calf was removed from the study, after the 6-h transport treatment, owing to illness that developed after allocation. Data relating to GGT, BHB, lactate and CK were log10 transformed to normalise residuals. The REML procedure in Genstat (12th Edition, VSN International, Hemel Hempstead, UK) was used for the statistical analysis. Treatment, sampling time (time period for temperature and lying behaviour) and their interaction plus replicate and 0-h value as covariate (for blood biochemistry) were included in the fixed model. Animal, replicate × treatment and, as appropriate, replicate × time and replicate × treatment × time were tested for retention in the random model. The Genstat VSTRUCTURE procedure was used to assess serial correlation and between sampling time heteroskedasticity when analysing post 0-h data as repeated measures. A two-tailed P<0·05 on a wald test was considered to be statistically significant.
Replicate × treatment and replicate × treatment × time were never statistically significant (P>0·05) and were omitted from the random effects. Replicate × time was statistically significant (P<0·05) for all variables except PCV. Both 0 h as covariate and animal effect were always significant (P<0·05). Except for lying behaviour the residuals from the post 0-h analyses, all exhibited between sampling time heteroskedasticity and serial correlation. The latter was fitted as a first order autoregressive correlation structure for all but temperature where correlation applied to only the last three sampling times and an order 2 general correlation matrix was fitted.
Results
Results for calf body weight and serum GGT are presented in Table 1. All treatment groups recorded a decline in bodyweight between 6 h (pre-loading) and 30 h (P<0·001). There were no significant effects of transport treatment (P=0·37). The calves lost 6% of their bodyweight during the measurement period. Serum GGT concentrations, an indicator of transfer of passive immunity via colostrum, did not differ between treatments (P=0·27), although there was a wide range of values (12–1226 IU/l).
Table 1. Calf changes in body weight (BW) with transport treatment and serum gamma-glutamyl transferase (GGT) concentrations
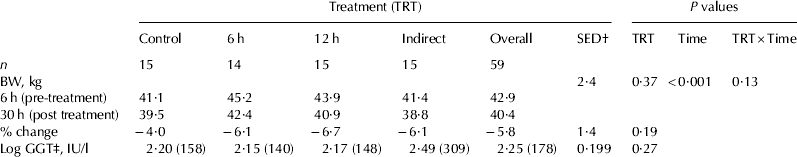
† SED for comparison of treatment means
‡ Backtransformed means are shown in brackets
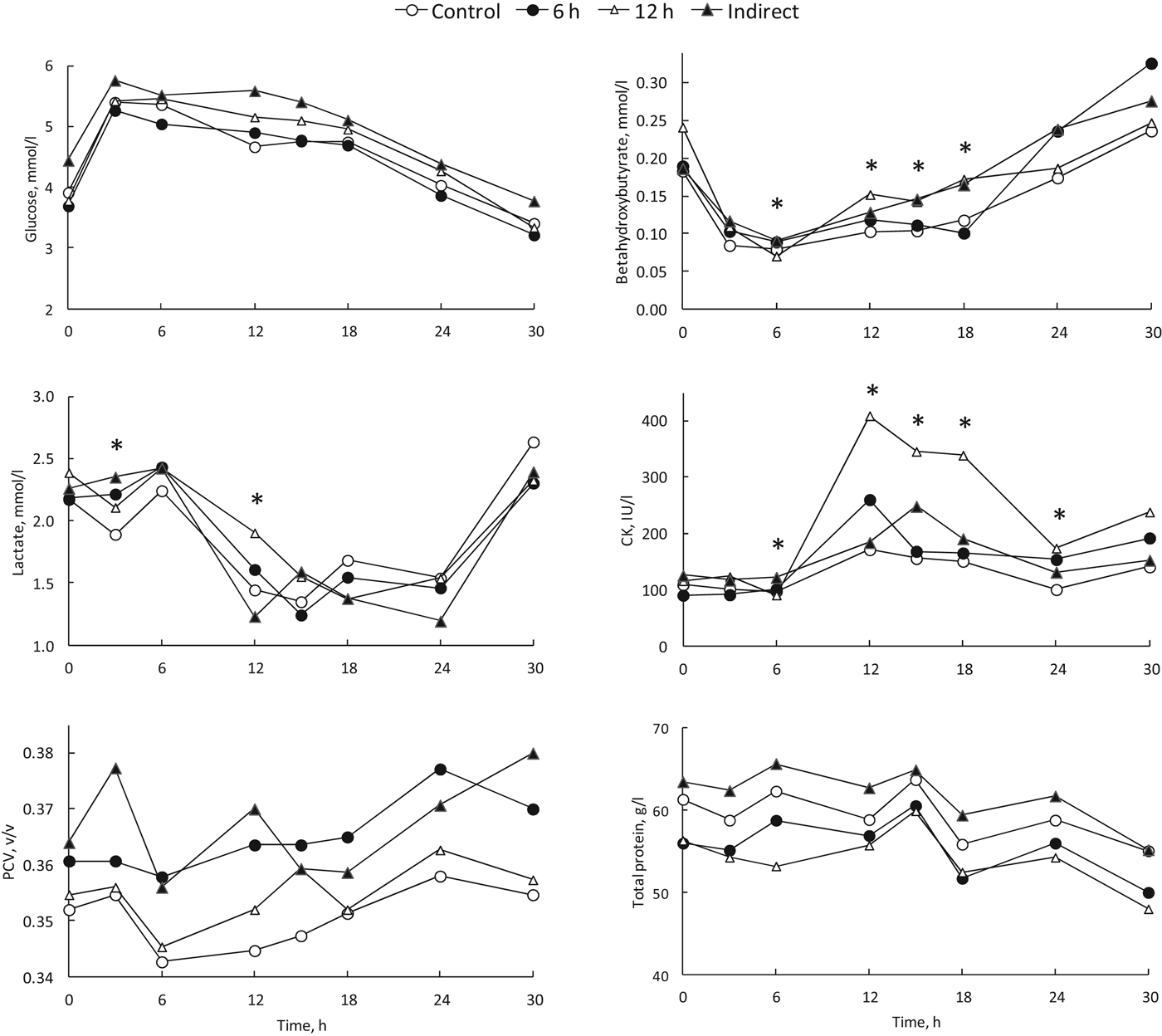
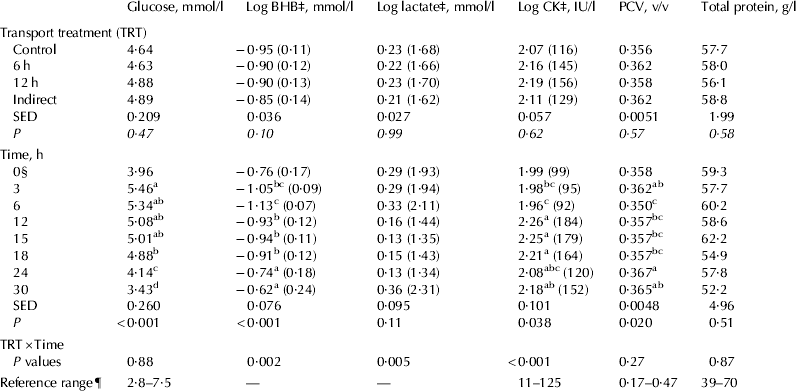
The results for the remaining blood chemistry variables are presented in Fig. 1 and Table 2. Serum glucose concentrations were at a relatively low level before feeding, increased after feeding, remained relatively stable for about 18 h after feeding and then showed a steady decline (Fig. 1). There were no treatment effects (P=0·47) (Table 2). Although mean serum glucose concentrations at 30 h were not below the lower reference value of 2·8 mmol/l, 7 of 59 (12%) calves had values that were below this value at 30 h. BHB concentrations increased over the 30 h of feed withdrawal, but overall values remained low. There were significant treatment × time effects for BHB and lactate concentrations, but these were not consistent with any particular treatments (Fig. 1). Serum CK concentrations showed a significant effect of treatment × time, whereby calves in the 12-h treatment had higher CK concentrations from 12 to 18 h than Control calves. Mean serum CK concentrations were also higher than the reference range, particularly for transported animals. Both total protein and PCV remained within relatively narrow bands and there were no effects of treatment or treatment × time. PCV values increased slightly over the 30 h.

Fig. 1. Effects of transport treatment on concentrations of blood glucose, beta-hydroxybutyrate, lactate, creatine kinase (CK), packed cell volume (PCV) and total protein. Data presented are arithmetic means. *Denotes a significant (P<0·05) effect of treatment at that time point following statistical analysis.
Table 2. Effect of transport treatment, time of sampling and their interaction on calf blood chemistry†

abcMeans within a column without a common superscript are different (P<0·05)
† Data presented are adjusted means. BHB, beta-hydroxybutyrate; CK, creatine kinase
‡ Backtransformed means are shown in brackets
§ Data for 0 h used as a covariate in the repeated measures analysis
¶ From Lumsden et al. (Reference Lumsden, Mullen and Rowe1980)
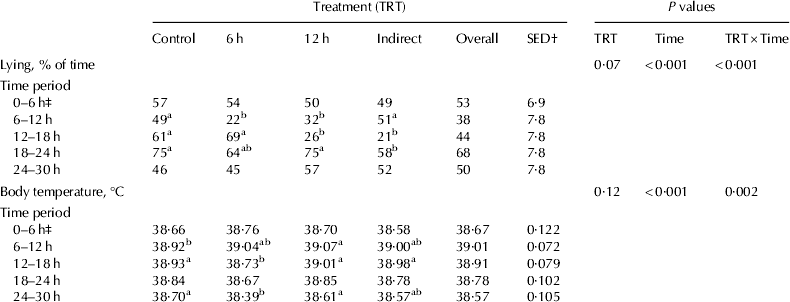
Results for lying behaviour and body temperature are presented in Table 3. The percentage of time spent lying down decreased from approximately 50% in the home pen to 22–32% while on board the travelling vehicle. Calves in the 12-h treatment spent 75% of the time lying down in the destination pen in the 6-h period after their journey; however, this did not differ from Control animals over the same period. Mean body temperature remained within a narrow and normal range. There were some effects of treatment × time for body temperature but these were not consistent across the study.
Table 3. Effect of transport treatment on calf body temperature and lying behaviour

abMeans within a row without a common superscript are different (P<0·05)
† SED for comparison of treatment means
‡ Data for 0–6 h period used as a covariate in the repeated measures analysis
Discussion
The main findings of this study were that transport did not exert a significant additional effect on calf welfare beyond the withdrawal of feed, and that the key effect of 30 h of feed withdrawal in 5–9 d-old calves was declining blood glucose concentrations. It was expected that transported calves would show some deleterious effects in their blood chemistry and metabolism, and that these effects would be exacerbated by the Indirect consignment treatment. However, our findings on the effects of transport are in broad agreement with the results of Todd et al. (Reference Todd, Mellor, Stafford, Gregory, Bruce and Ward2000) who found that either 3 or 12 h of transport had few effects on blood variables different from the effects of feed withdrawal alone, apart from blood glucose. Todd et al. (Reference Todd, Mellor, Stafford, Gregory, Bruce and Ward2000) found that transport of calves at a space allowance of 0·2 m2 per calf initially increased blood glucose compared with control animals, putatively owing to the mobilisation of body reserves induced by standing and bracing during the journey. We did not see such an effect, and this may be due to the ability of calves to lie down on the journey in this study.
The main effects of transport were seen in blood CK concentrations and lying behaviour. CK is an enzyme found in muscle cells, and leaks into the blood in response to increasing muscle cell damage, potentially through significant exertion, and more commonly through direct trauma such as knocks or bruises. Normal values of CK are very low, and thus it does not take much for an individual animal to record increases above this threshold. An increase in CK in itself may not necessarily mean poor welfare, but it can indicate where there has been some degree of physical trauma. Our results indicated an increase in CK due to transport, particularly in the 12-h transport group. The raw data were not normally distributed, with much of the increase coming from a few animals. It would seem that increasing transport per se did not inherently cause an increase in CK, but greater time in transport probably increased the risk of individual animals receiving a bump or a bruise that induced CK elevation. Calves transported up to 18 h by Kent & Ewbank (Reference Kent and Ewbank1986) did not show significant elevations in CK activity, although the vehicle conditions and loading density were not specified. Although lying behaviour in our study was reduced by transport, calves still lay down on the vehicle for 22–32% of the journey on average. This result is similar, although slightly less than that of Kent & Ewbank (Reference Kent and Ewbank1986) who recorded 32–36%. Uetake et al. (Reference Uetake, Tanaka and Sato2011) found that a space allowance of 0·25 m2 compared with 0·35 m2 for calves 11–26 d old reduced the number of animals that lay down. In our study, with younger calves, the 0·3-m2 space allowance was reflective of recommended industry practice and was designed to provide lying opportunity. It may be that further increases in lying could be promoted by factors other than increased space such as bedding, although we did not detect a significant rebound effect in lying behaviour among calves after transport.
The lack of additional impact induced by the Indirect consignment treatment may partly be due to the overall lack of effect of transport itself in comparison with feed withdrawal alone. In our experimental, controlled study, it was not possible to mix calves in with numerous other animals as frequently happens during indirect consignment events in practice. Other research has shown that being held in a lairage environment, which often has many unfamiliar animals and novel aspects, can be stressful for cattle (Giannetto et al. Reference Giannetto, Fazio, Casella, Marafioti, Giudice and Piccione2011). It is also worth noting that these experimental animals were handled and transported with considerable care and diligence, in accordance with experimental animal ethics requirements. It is possible that the realities of commercial practice may at times not incorporate the same levels of care, and it would be for further research to examine calf welfare during commercial supply chains to examine whether the calf responses measured during this study are matched in industry practice.
Although transport did not have a major effect on the calves in this study, it is worth considering how the animals’ metabolic profiles changed during the 30-h period of feed withdrawal. The calves were on a once per day feeding schedule, which is used not infrequently on farms in different countries (Gleeson et al. Reference Gleeson, O'Brien and Fallon2007; Dairy Australia, 2011), although regulations in the EU state that calves should be fed twice daily (European Commission, 2008). Accordingly, the initial blood sample was taken following an effective period of 24 h of feed withdrawal, given that calves were able to rapidly consume their milk allocation. The repeated blood sampling and time series analysis showed that serum glucose increased markedly after feeding, and had returned to approximately pre-feeding values after 24 h. Further time off feed to 30 h resulted in a further reduction in blood glucose. The results show that glucose was in steady decline from about the 18–24-h time period. In examining individual calf glucose concentrations at 30 h, results indicated that 12% of calves were within the reference level, as defined by a lower reference value of 2·8 mmol/l for Holstein dairy calves aged 1–14 d (Lumsden et al. Reference Lumsden, Mullen and Rowe1980). Although the calves did not appear to be dull or depressed, and re-fed themselves normally at the end of the study, our interpretation of these results is that good practice management of the type and age of calves in this study would have a feed withdrawal period of not more than 24 h.
The other indicators of metabolism, particularly BHB, followed a similar response pattern to glucose, but were within narrower ranges. BHB is a by-product of energy metabolism when the body starts to utilise fat reserves. The small rise in BHB is probably due to calves of this age having relatively limited fat reserves compared with other age classes of cattle (Gonzalez-Jimenez & Blaxter, Reference Gonzalez-Jimenez and Blaxter1962; Webster et al. Reference Webster, Gordon and McGregor1978). The 6% loss in body weight was probably due to loss of gastrointestinal fill, because the results for PCV and total serum protein as indicators of hydration in the calves did not change much over time. Typically, values for PCV and serum (or plasma) protein increase with loss of body water, as constituents in the blood become more concentrated. PCV barely changed over the 30 h, and although overall there were some changes with increasing time, this was not biologically significant (Lumsden et al. Reference Lumsden, Mullen and Rowe1980). Total serum protein did not change during the study. Results of Todd et al. (Reference Todd, Mellor, Stafford, Gregory, Bruce and Ward2000) also showed very little change in hydration indicators in response to transport, including feed withdrawal, in dairy calves of a similar age. In a seasonal calving system, very young calves would typically be transported during the cooler months of the year, as in this study, but it is likely that hot weather conditions would increase the risk of dehydration during transport.
Body temperature data showed that the calves generally remained normothermic throughout the study period, and that elevated temperatures or hypothermia were not a problem. Todd et al. (Reference Todd, Mellor, Stafford, Gregory, Bruce and Ward2000) found that 27% of their calves had temperatures above normal (39·5 °C), which is much greater than for calves in this study. As body energy levels decline with increasing duration of feed withdrawal, one might expect an increased risk of hypothermia during transport, and regulations often require that transported calves receive protection from draughts (e.g. Ministry of Agriculture and Forestry, 2011).
In conclusion, best practice transport of 6–12-h duration, including indirect consignment via a holding facility, did not significantly affect calf blood biochemistry and metabolism in terms of glucose, lactate and BHB, in comparison with untransported animals. However, extending the time off feed beyond the daily feeding interval resulted in reduced blood glucose concentrations, suggesting that time off feed needs to be carefully managed in young transported dairy calves. The calves in the study coped with a period of 30 h off feed, but best practice would be represented by a period of not more than 24 h. In addition, further research should examine calf welfare in response to commercial transport conditions, which may vary from those used in research.
The authors thank the participating farm staff and the transport personnel for their help during the study. This study was financially supported by grants from Dairy Australia and the Australian Department of Agriculture, Fisheries and Forestry.