The effect of dietary physically effective fibre (peNDF) on ruminal pH in dairy cows has been extensively studied. In a meta-analysis, Zebeli et al. (Reference Zebeli, Dijkstra, Tafaj, Steingass, Ametaj and Drochner2008) summarized data from 45 published studies and demonstrated that ruminal pH was increased by increasing dietary peNDF (Mertens, Reference Mertens1997) up to 31% (DM basis), beyond which ruminal pH reached a plateau (daily mean ruminal pH 6·27). Nonetheless, peNDF in that study was able to explain only 50% of the variation in ruminal pH. Additional dietary factors such as ruminal degradable starch from grain and DMI were also shown to affect ruminal pH despite the presence of apparently adequate levels of peNDF. For example, Zebeli et al. (Reference Zebeli, Dijkstra, Tafaj, Steingass, Ametaj and Drochner2008) demonstrated that at a fixed level of peNDF (31% of DM), increasing the dietary ruminal degradable starch from 14 to 22% and DMI from 20 to 25 kg/d increased the duration that ruminal pH was below 5·8.
The effect of ruminal pH on milk fat (MF) is inconsistent in the literature. Some studies reported a reduction in MF concentration with low ruminal pH (Gentile et al. Reference Gentile, Cinotti, Ferri, Famigli-Bergamini, Harigan and Monaghan1986; Stone, Reference Stone1999), whilst others showed no effect of pH on MF concentration (Rustomo et al. Reference Rustomo, AlZahal, Cant, Fan, Duffield, Odongo and McBride2006a, Reference Rustomo, AlZahal, Odongo, Duffield and McBrideb). Additionally, Allen (Reference Allen1997) summarized the association between ruminal pH and MF concentration from 23 studies and concluded that ruminal pH explained 39% of the variation in MF percentage.
Davis & Brown (Reference Davis, Brown and Phillipson1970) defined two conditions for milk fat depression (MFD) to occur. The first was altering microbial processes (i.e., by low fibre diets) and the second was the presence of polyunsaturated fatty acids (PUFA) in the diet. Bauman & Griinari (Reference Bauman and Griinari2001, Reference Bauman and Griinari2003) proposed the biohydrogenation theory which states that: intermediates resulting from altered ruminal biohydrogenation (BH) under specific dietary conditions act on the mammary gland, thus inhibiting de novo synthesis of FA. The role of trans-10, cis-12 conjugated linoleic acid (CLA) (Baumgard et al. Reference Baumgard, Corl, Dwyer, Sæbø and Bauman2000) and cis-10, trans-12 CLA (Sæbø et al. Reference Sæbø, Sæbø, Griinari and Shingfield2005) as potent inhibitors of FA synthesis in the mammary gland have been confirmed. Additional BH intermediates such as trans-9, cis-11 CLA (Perfield et al. Reference Perfield, Lock, Griinari, Sæbø, Delmonte, Dwyer and Bauman2007) and trans-10 18:1 (Shingfield et al. Reference Shingfield, Sæbø, Sæbø, Toivonen and Griinari2009) were identified as antilipogenic, nonetheless, more studies are needed to confirm their role in MFD.
Previously, AlZahal et al. (Reference AlZahal, Or-Rashid, Greenwood, Douglas and McBride2009) demonstrated that diets with moderate forage level, rich in rapidly fermentable starch, and low in PUFA content induced ruminal pH depression, did not cause MFD. Further, the concentrations of BH intermediates known to inhibit lipogenesis in dairy cows (trans-10, cis-12; trans-9, cis-11 CLA) were not different among treatments and were lower than those levels known to cause MFD. In the current study, we hypothesized that PUFA-induced MFD is greater when cows are fed moderate- compared with high-forage diets. The objective of this study was to investigate the effect of ruminal infusion of soybean oil (SBO) with either a moderate- or high-forage-to-concentrate diet on fat concentration, yield and composition in milk from dairy cows.
Materials and Methods
Animals, experimental design and feeding
As described in the previous study (AlZahal et al. Reference AlZahal, Or-Rashid, Greenwood, Douglas and McBride2009), six rumen-fistulated multiparous lactating Holstein cows housed in a tie-stall facility at Elora Dairy Research Centre, University of Guelph, Guelph, Ontario and cared for and handled in accordance with the Canadian Council on Animal Care regulations were used in the study.
The cows were randomly assigned to one of two dietary treatments, a high forage:concentrate (HFC; 74:26; % of DM) or a moderate forage:concentrate (MFC; 56:44; % of DM) total mixed ration. Ingredients and chemical analyses and FA profiles of the experimental TMR are presented in Tables 1 and 2, respectively. The HFC diet was designed to provide a large amount of fibre to maintain high ruminal pH. On the other hand, the MFC diet provided an adequate amount of fibre to maintain DMI and milk production and yet included a large amount of starch (24% of DM) derived from wheat and barley, which are rapidly and extensively fermented in the rumen. The estimated total dietary starch fermentation rate was 34·6%/h for the MFC diet compared with 25·6%/h for the HFC diet (Table 1). The chemical analyses of MFC and HFC TMR agreed closely with formulation targets. The study consisted of 4 weeks of adaptation during which each cow received one of the two TMR without ruminal SBO infusion (AlZahal et al. Reference AlZahal, Or-Rashid, Greenwood, Douglas and McBride2009), followed by 3 weeks (current experiment) during which, cows continued receiving the same diet but with ruminal infusion of SBO [Morrison Bros Ltd., Wingham, Ontario, Canada (g/100 g FA; 17, 16:0; 12, 18:0; 34, 18:1; 28,18:2 n-6; 3,18:3 n-3; and 7, others)].
Table 1. Ingredient composition and chemical analyses of high forage:concentrate (HFC) and moderate forage:concentrate (MFC) total mixed rations (TMR)

† Contained (% of DM): 48%-soybean meal, 24·8; high-protein corn gluten meal, 19·8; canola meal, 10·1; roasted soybean (whole), 10·4; fish meal (herring), 5·1; beet pulp, 1·3; calcium carbonate (limestone), 3·9; dicalcium phosphate, 4·9; soybean hulls (ground), 5·9; sodium bicarbonate, 4·4; salt, 2·8; molasses (in pelleter), 1·8; urea, 2·8; magnesium oxide, 1·0; Organic Ruminant Micro Premix (Floradale Feed Mill Limited, Floradale, ON, Canada), 0·7; sulphur flour (99·5%), 0·4; Rovimix Biotin (H-2, DSM Nutritional Products, Inc., Parsippany, NJ), 0·02
‡,¶ Estimated using CPM-Dairy v 3.0.8 (Miner Institute, Chazy, NY) using the chemical analysis of feed ingredients
§ Nonfibre carbohydrates=100−(NDF+CP+ether extract+ash)
†† Estimated fermentation rate of dietary starch calculated from the CPM-Dairy default values of starch content of dietary ingredients and the ingredients' fermentation rates
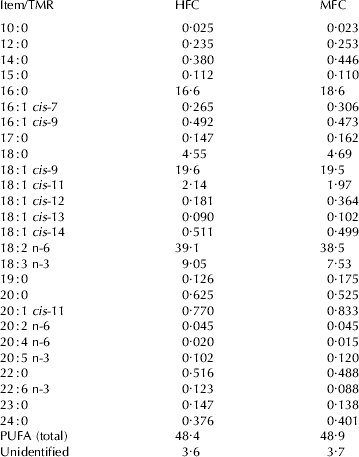
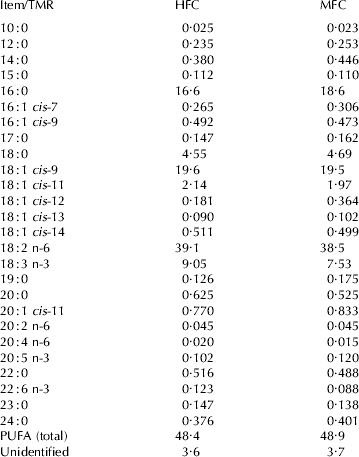
Table 2. Fatty acid profile (g/100 g fatty acids) of high forage:concentrate (HFC) and moderate forage:concentrate (MFC) total mixed rations (TMR)
The TMR (HFC and MFC) were fed twice daily at 07·00 and 13·00 h. The amount of feed was adjusted based on average DMI of the previous week to allow a maximum of 5 kg/d of refusals (as-fed basis). Soybean oil was dosed into the rumen during the experimental period through the cows' fistulae at 13·00 h using a plastic funnel connected to a 1-metre tube. The SBO was pulse-dosed over 10 minutes and distributed evenly into the different compartments of the rumen. The rumen contents were mixed within the rumen through the cannula for 2 min. The amount of added SBO equalled 2% of the individual cow's average DMI of the previous week.
Experimental measures and samples analyses
Ruminal pH was measured and recorded continuously every min for 3 d per week using a pH recording system as described by AlZahal et al. (Reference AlZahal, Rustomo, Odongo, Duffield and McBride2007). pH electrodes were calibrated weekly using standard buffer solutions of pH 4·00 and 7·00 (Fisher Scientific, Fairlawn, NJ). Feed intake and milk yield were monitored daily throughout the experimental period. Total mixed ration samples from each dietary treatment and ort samples from each individual animal were collected 3 times per week and frozen at −20°C until analysis. The orts samples were pooled per cow per week proportionally to the amount of the orts. The TMR samples were pooled per week per treatment. Pooled TMR and orts samples were dried for 48 h in a forced-air oven to determine the DM content for that week. At the end of the experiment, dried TMR samples were ground through a 1-mm screen (Wiley Mill, Arthur A. Thomas Co., Philadelphia, PA) and pooled by treatment across all weeks. Samples were analyzed at Agri-Food Laboratory, Guelph, Ontario, Canada as described previously by AlZahal et al. (Reference AlZahal, Rustomo, Odongo, Duffield and McBride2007).
Cows were milked twice daily at 05·00 and 15·00 h and milk samples were collected in duplicate 3 times per week during morning and afternoon milking throughout the experiment. Milk samples for FA analysis were frozen immediately at −20°C until analysis. Milk samples for component analysis were preserved with 2-bromo-2-nitropropane-1-2-diol and stored at 4°C. Every week, milk samples for components analysis were pooled by cow by day based on a constant proportion of 60:40 (am:pm, respectively) and then were pooled by week using equal proportions and submitted to Laboratory Services Division (Guelph, Ontario, Canada) for analysis using a near-infrared analyzer (Foss System 4000, Foss Electric, HillerØd, Denmark).
Lipids for FA analysis were extracted from pooled milk and feed samples as explained by Or-Rashid et al. (Reference Or-Rashid, Odongo, Wright and McBride2009).
Particle size distribution
The particle size of the experimental TMR was assessed weekly (on the second day of pH recording of each week) in duplicate using the Penn State Forage Particle Size Separator with three sieves and a solid bottom pan (model C24682N, Nasco, Fort Atkinson, WI), as described by Kononoff et al. (Reference Kononoff, Heinrichs and Buckmaster2003) . The materials remaining on each sieve and pan was then removed, weighed and oven-dried at 100°C to determine the distribution of feed DM retained on each sieve and in the pan. The peNDF (of particles >1·18 mm) was determined by multiplying NDF content by the proportion of DM of particles retained on the top, middle, and bottom screens of the separator (Mertens, Reference Mertens1997).
Statistical analysis
Statistical analysis was conducted on weekly averages of DMI, milk yield, milk components and ruminal pH characteristics and FA data. Proc Mixed of SAS (SAS Institute, 2004) was used using the following model: Yijk=μ+Di+Wj+(D×W)ij+eij where Yij=the dependent variable, μ=overall mean, Di=effect of diet (i=1, 2), Wj=effect of week (j=1, 2, 3 ), (D×W)ij=effect of diet x week (ij=1,.., 6), and eij=random residual error.
The effects of week and diet were considered as fixed effects. Week of experiment was used as a repeated measurement with cow within dietary treatment as the subject. Orthogonal polynomial contrast was used to describe the linear and quadratic terms of week effect and week by diet interaction. For each analyzed variable, cow was subjected to five covariance structures: compound symmetry, heterogeneous compound symmetry, autoregressive order 1, heterogeneous autoregressive order 1 and unconstructured covariance structure. The covariance structure that gave the smallest Bayesian information criterion was used (Littell et al. Reference Littell, Milliken, Stroup and Wolfinger1996). The fold-change in a given variable by week or treatment, in case of lack of an interaction, was calculated from the main effects (not shown). In case of a significant interaction, the proportions were calculated for each factor within the levels of the other factor.
Results and Discussion
Ruminal pH
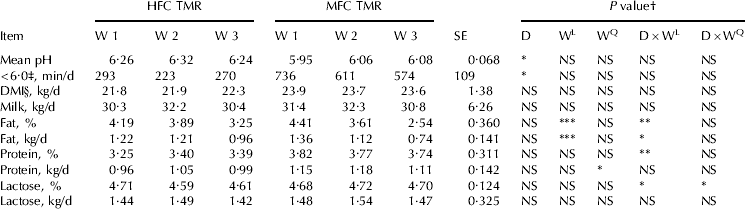
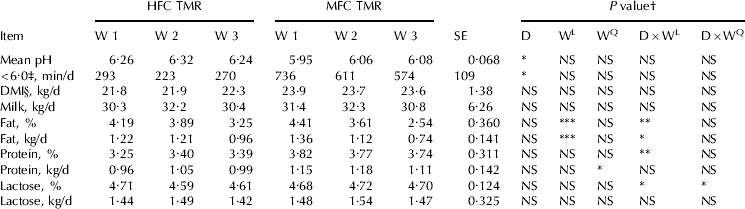
Diet had a significant effect on mean ruminal pH and duration of ruminal pH below 6·0 during adaptation (AlZahal et al. Reference AlZahal, Or-Rashid, Greenwood, Douglas and McBride2009) and during SBO infusion (current experiment, P<0·05, Table 3). Ruminal pH below 6·0 is considered suboptimal for cellulolytic bacteria growth (Russell & Wilson, Reference Russell and Wilson1996) and the pH remained below 6·0 for greater than 9·5 h/d in the MFC diet.
Table 3. Effect of diet (D), week (W), and their interaction (D×W) on ruminal pH characteristics, DMI, and milk yield and components, HFC=high forage:concentrate, MFC=moderate forage:concentrate
† L, Q=linear and quadratic effects, respectively
‡ Duration ruminal fluid pH was below 6·0
§ Including ruminally infused soybean oil (2% of DMI)
*,**, *** Significant at P<0·05, P<0·01 and P<0·001, respectively; NS, non significant P⩾0·05
Although the MFC diet contained sufficient amount of peNDF (29·3% of DM), the provision of highly fermentable carbohydrate (starch from ground wheat and barley) led to a significant depression in ruminal pH. This was in agreement with Zebeli et al. (Reference Zebeli, Dijkstra, Tafaj, Steingass, Ametaj and Drochner2008) who showed that increasing the concentration of ruminally degradable starch can depress ruminal pH despite the provision of a diet containing 31% peNDF (DM basis). This emphasizes the need to take into account total diet starch fermentation rate during formulating lactating cow rations.
Dry matter intake, milk yield and components
The main effect of diet, week, and their interaction had no effect on DMI and milk yield (P⩾0·05, Table 3). Additionally, diet had no effect on protein percentage and yield (Table 3). However, there was a significant (P<0·05) quadratic week effect on milk protein yield (kg/d). The increase in milk protein yield during week two likely reflected the tendency (P=0·06) for milk yield to increase during that week.
In a previous study, AlZahal et al. (Reference AlZahal, Or-Rashid, Greenwood, Douglas and McBride2009) utilized dietary treatments that were low in lipid [2·0 to 2·4% of DM, linoleic acid (LA) intake was approximately 170 g/d/cow for a cow consuming approximately 20 kg/d] to investigate the effect of dietary forage level (74 to 56% of DM, corn-silage-haylage based forage) on MF and demonstrated that forage level had no effect on MF, when low PUFA diets were fed. In the current experiment, which is a continuation of the previous experiment, the same base diets were utilized and SBO was pulse-dosed intra-ruminally daily for 3 continuous weeks. Results showed that both MF concentration and MF yield (kg/d) dropped linearly over-time, with cows receiving the MFC diet having a greater drop in MF concentration and MF yield than cows receiving the HFC diet (P<0·05, significant linear week×diet interaction; Table 3). By the third week of SBO infusion, milk fat concentration dropped by 22 and 42% for the HFC and MFC diet, respectively (Table 3). Similarly, milk fat yield (kg/d) dropped by 21 and 45% for HFC and MFC diet, respectively (Table 3).
The effect of dietary plant oil supplementation on milk fat secretion is well established (Bauman & Griinari, Reference Bauman and Griinari2003). However, there are many factors that mediate the effect of plant oil supplementation on MF content, yield, and FA profile and thus contribute to differences among studies. These factors are the composition of the basal diet used in the study, plant oil type, and duration of plant oil supplementation (Loor et al. Reference Loor, Ferlay, Ollier, Ueda, Doreau and Chilliard2005; Roy et al. Reference Roy, Ferlay, Shingfield and Chilliard2006; Shingfield et al., Reference Shingfield, Ghvenjarvi, Toivonen, Vanhatalo, Huhtanen and Griinari2008).
Roy et al. (Reference Roy, Ferlay, Shingfield and Chilliard2006) observed a reduction in MF content by 39 and 52% with 48:52 and 27:73 forage to concentrate corn-silage-based diets, respectively, following 18 d of sunflower oil supplementation (5% of DM) as the source of LA. Additionally, Roy et al. (Reference Roy, Ferlay, Shingfield and Chilliard2006) showed no change in milk fat content when a similar amount of linseed oil was supplemented to a 64:36 grass-hay-based diet. Furthermore, Shingfield et al. (Reference Shingfield, Ghvenjarvi, Toivonen, Vanhatalo, Huhtanen and Griinari2008) demonstrated that an incremental increase in supplemental linseed oil (from 0 to 750 g/d) to a grass-silage-based diet (60:40, forage:concentrate) had no effect on MF.
Milk fatty acids
Feeding high amounts of PUFA to ruminants inhibit ruminal BH and generate a wide range of BH intermediates that are transferred to the milk, including trans-18:1 FA and CLA isomers, some of which inhibit milk fat synthesis in the mammary gland (Bauman & Griinari, Reference Bauman and Griinari2001, Reference Bauman and Griinari2003). Griinari & Bauman (Reference Griinari, Bauman, Yurawecz, Mossoba, Kramer, Pariza and Nelson1999) suggested a minor BH pathway in which LA is isomerised to form trans-10, cis-12 CLA. This isomer in turn is reduced to trans-10 18:1 and subsequently to C18:0. Recent studies confirmed that trans-10, cis-12 CLA can be synthesized from LA when incubated with Probionibacterium acnes and mixed ruminal bacteria (Wallace et al. Reference Wallace, McKain, Shingfield and Devillard2007) and in-vivo (Shingfield et al. Reference Roy, Ferlay, Shingfield and Chilliard2008). Other CLA such as cis-10, trans-12 CLA (Sæbø et al. Reference Sæbø, Sæbø, Griinari and Shingfield2005) and trans-9, cis-11 CLA (Perfield et al. Reference Perfield, Lock, Griinari, Sæbø, Delmonte, Dwyer and Bauman2007), both intermediates of LA (Wallace et al., Reference Wallace, McKain, Shingfield and Devillard2007), were suggested as antilipogenic. Furthermore, trans-10, trans-12 CLA (Sæbø et al. Reference Sæbø, Sæbø, Griinari and Shingfield2005) and trans-9, trans-11 CLA (Perfield et al. Reference Perfield, Lock, Griinari, Sæbø, Delmonte, Dwyer and Bauman2007) showed no effect on lipogenesis but caused a decrease in Δ9-desaturation indices. The trans 18:1 intermediates investigated include trans-9 (Rindsig & Schultz, Reference Rindsig and Schultz1974), trans-11, and trans-12 (Griinari et al. Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000). These studies showed no direct effect on milk fat synthesis. Studies showed that trans-10 18:1 had a significant association with MFD in dairy cows (Bauman & Griinari, Reference Bauman and Griinari2001). A study by Lock et al. (Reference Lock, Tyburczy, Dwyer, Harvatine, Destaillats, Mouloungui, Candy and Bauman2007) demonstrated that trans-10 18:1 had no direct role in MF synthesis in the mammary gland. Lock et al. (Reference Lock, Tyburczy, Dwyer, Harvatine, Destaillats, Mouloungui, Candy and Bauman2007) infused post-ruminally 42·6 g/d of a pure preparation (95%) of trans-10 18:1, which increased the concentration of milk trans-10 18:1 from 0·47 to 1·11 (g/100 g FA). Provided that during MFD the levels of milk trans-10 18:1 can exceed in some cases 10 g/100 g FA, the study by Lock et al. (Reference Lock, Tyburczy, Dwyer, Harvatine, Destaillats, Mouloungui, Candy and Bauman2007) has been criticized by not infusing a sufficient amount of trans-10 18:1 to induce MFD (Kadegowda et al. Reference Kadegowda, Piperova and Erdman2008). Most recently, Shingfield et al. (Reference Shingfield, Sæbø, Sæbø, Toivonen and Griinari2009) post-ruminally infused a mixture of 18:1 FAME that supplied 92 g/d trans-10 18:1 and provided convincing evidence that trans-10 18:1 may contribute to MFD. However, more studies are needed to confirm the role of trans-10 18:1 on milk fat synthesis using pure isomer at different doses.
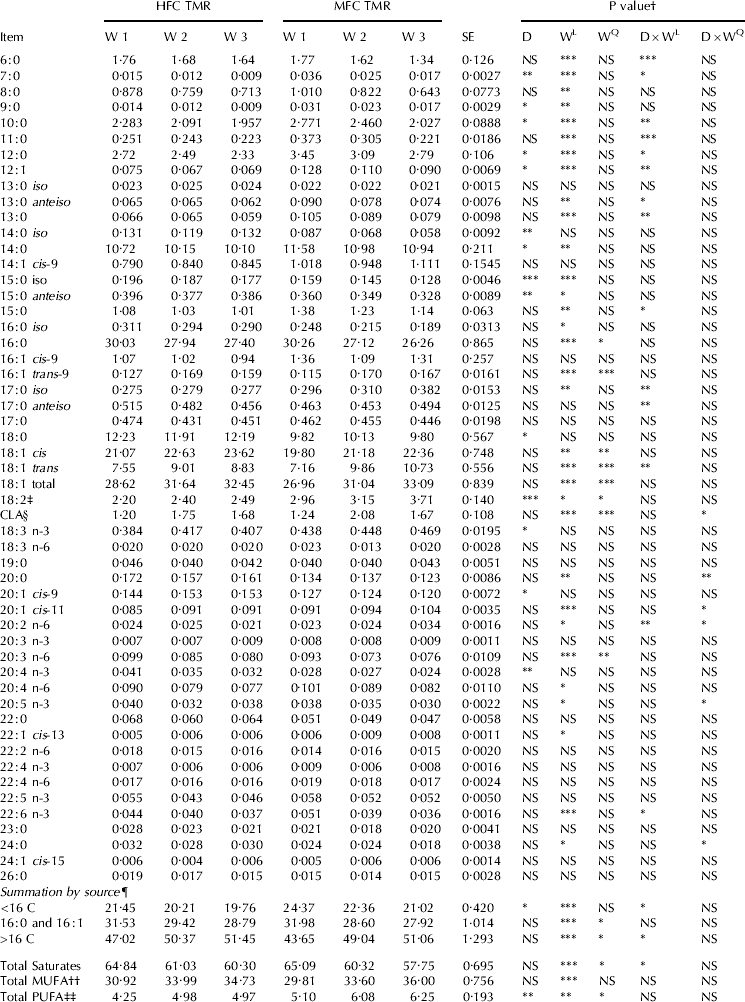
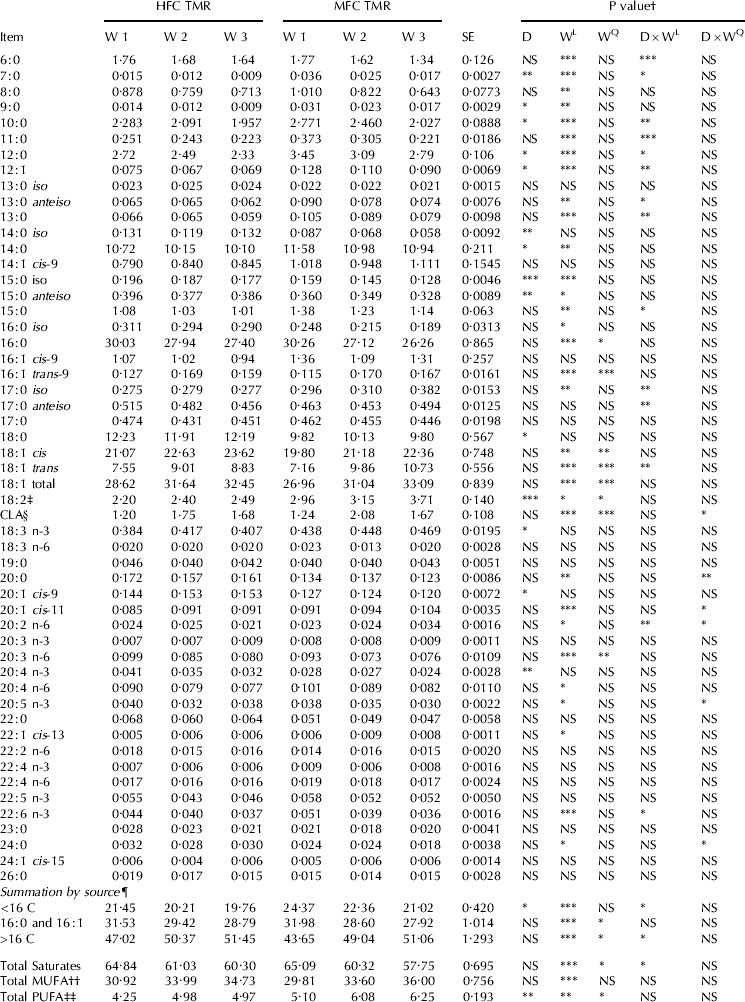
In the current study, MFD was associated with a significant shift in milk FA profile during the SBO infusion weeks (week effect). This shift included a proportional decrease in MF concentration (g/100 g FA) of de novo synthesized FA (FAC 6 to <C16, P<0·05) and a proportional increase in MF concentration (g/100 g FA) of long chain FA (FA >C16, P<0·05) and most BH intermediates (Tables 4, 5 & 6). These changes were more pronounced for cows receiving the MFC diet (week×diet interaction).
Table 4. Milk fatty acid composition (g/100 g total FA), HFC=high forage:concentrate, MFC=moderate forage:concentrate
† D, W, L, Q=diet, week, linear and quadratic effects, respectively
‡ Sum of 18:2 FA excluding isomers of conjugated linoleic acid
§ Total conjugated linoleic acid
¶ FA <16 C originated from de novo synthesis, FA >16C were preformed FA taken up by the mammary gland, and 16:0 and 16:1 FA came from both de novo and preformed sources
†† Monounsaturated FA
‡‡ Polyunsaturated FA
*,**, *** Significant at P<0·05, P<0·01 and P<0·001, respectively; NS, non significant P⩾0·05
Table 5. Milk concentration (g/100 g total FA) of 18:1 FA, HFC=high forage:concentrate, MFC=moderate forage:concentrate

† D, W, L, Q=diet, week, linear and quadratic effects, respectively
*,**, *** Significant at P<0·05, P<0·01 and P<0·001, respectively; NS, non significant P⩾0·05
Table 6. Milk concentration (g/100 g total FA) of 18:2 FA, HFC=high forage:concentrate, MFC=moderate forage:concentrate

† D, W, L, Q=Diet, week, linear and quadratic effects, respectively
‡ Unresolved peak of trans-9, trans-11 and trans-10, trans-12 conjugated linoleic acid
*, **, *** Significant at P<0·05, P<0·01 and P<0·001, respectively; NS, non significant P⩾0·05
By the third week, MF concentration (g/100 g FA) of FA<C16 was reduced by 7·9 and 13·7% for the HFC and MFC treatments, respectively. The concentrations of C6:0, 7:0, 10:0, 11:0, 12:0, 12:1, iso-13:0, ai-13:0 and 15:0 FA were, however, reduced to a greater extent with the MFC treatment (week and week×diet, P<0·05). On the other hand, the concentrations (g/100 g of FA) of total trans-18:1 FA, total CLA and long chain FA were increased to a greater extent with the MFC diet (week and week×diet effect, P<0·05), namely iso-17:0; trans-5 18:1; trans-10 18:1; trans-12 18:1; cis-11 18:1; trans-9, cis-11 CLA; trans-9, trans-11+trans-10, trans-12 CLA (unresolved peak); cis-11 20:1 and 20:2 n-6. Milk concentration of cis-9, trans-11 CLA was increased over week but this increase was more pronounced for the HFC treatment than the MFC diet (42 vs. 28%). Additionally, milk concentration of trans-10, cis-12 CLA was increased over time (P<0·05) but without interaction with diet.
Milk concentration of trans-10, cis-12 CLA was increased when a high-concentrate diet was supplemented with an oil rich in LA (Loor et al. Reference Loor, Ferlay, Ollier, Ueda, Doreau and Chilliard2005; Roy et al. Reference Roy, Ferlay, Shingfield and Chilliard2006). Whilst, milk fat concentration of trans-9, cis-11 CLA was increased when a high-concentrate diet was supplemented with fish oil (Shingfield et al. Reference Shingfield, Reynolds, Lupoli, Toivonen, Yurawecz, Delmonte, Griinari, Grandison and Beever2005) and sunflower oil (Roy et al. Reference Roy, Ferlay, Shingfield and Chilliard2006).
Plant oil supplementation has been shown to increase the amount of trans-11 18:1 leaving the rumen and thus milk concentration of endogenously-synthesized cis-9, trans-11 CLA (Bauman et al. Reference Bauman, Corl, Peterson, Sebedio and Christie2003). However, the persistency of the response of MF trans-18:1; cis-9, trans-11 CLA; and total CLA to oil supplementation was influenced by the composition of basal diet, oil supplement source, and duration of oil supplementation (Roy et al. Reference Roy, Ferlay, Shingfield and Chilliard2006). The authors demonstrated that the concentration of cis-9, trans-11 CLA in MF was consistent over time with high-forage diets supplemented with linseed oil, whereas, the increase in MF concentration of cis-9, trans-11 CLA was rapid and transient and declined over-time with lucerne and maize silage based diets supplemented with sunflower oil. This decline in cis-9, trans-11 CLA was associated with an increase in MF concentration (g/100 g FA) of trans-10 18:1 likely mediated by a time-dependent shift in ruminal biohydrogenation.
In the current study, there was a quadratic increase (P<0·05) in milk concentration (g/100 g FA) of trans-11 18:1 and the concentration (g/100 g FA) of cis-9, trans-11 CLA. During week two of SBO infusion, the concentration of trans-11 18:1 was 3·7 and 4·18 (g/100 g of FA) for the HFC and MFC treatments, respectively. The decline in trans-11 18:1 from week two to week three was associated with an increase in milk concentration (g/100 g FA) of trans-10 18:1 for the MFC treatment (from 1·5 to 2·8 g/100 g FA), whereas there was no change in trans-10 18:1 concentration from week two to week three for the HFC treatment (1·06 and 1·13 g/100 g FA; week two and week three 3; respectively). These results, suggest that there was a shift in the pathway of ruminal BH towards a higher trans-10 to trans-11 18:1 ratio when dietary fibre was replaced with grain in agreement with Bauman & Griinari (Reference Bauman and Griinari2003), and Roy et al. (Reference Roy, Ferlay, Shingfield and Chilliard2006). The shift in the BH pathway can be explained by the fact that LA to cis-9, trans-11 CLA formation in the rumen is mediated by Butyrivibrio fibrisolvens (Kepler & Tove, Reference Kepler and Tove1967), which are cellulolytic bacteria that can be inhibited directly by low ruminal pH (Russell & Dombrowski Reference Russell and Dombrowski1980). Whereas, the formation of LA to form trans-10, cis-12 CLA and other CLA is mainly mediated by lactic acid bacteria (i.e., Propionibacterium and Lactobacillus), which despite their lower occurrence in the rumen environment, are more abundant with concentrate feeding, hence, the increase in trans-10, cis-12 CLA with concentrate diets (Jenkins et al. Reference Jenkins, Wallace, Moate and Mosley2008). Additionally, mechanisms responsible for CLA synthesis in the rumen differ based on bacteria involved in CLA formation and CLA formed (Wallace et al. Reference Wallace, McKain, Shingfield and Devillard2007).
Cows receiving the MFC treatment had, on average, 43% greater MF LA concentration than the HFC treatment (main effect of diet, P<0·05). Additionally, MF concentration of LA was increased (20%) over time (week effect, P<0·05). The difference in LA concentration among treatments might be in response to an inhibition in ruminal lipid lipolysis by low ruminal pH (Van Nevel & Demeyer, Reference VanNevel and Demeyer1996).
Results showed that cows receiving SBO infusion and the MFC diet had a greater degree of milk fat depression than cows receiving SBO infusion and the HFC diet and greater milk concentration of trans-FA and CLA, namely, trans-10 18:1 and trans-9, cis-11 CLA. The results of this study emphasized the two conditions necessary for milk fat depression, namely, the presence of PUFA and low fibre/high concentrate level in the diet.
The authors would like to thank Laura Wright and the staff of the Elora Dairy Research Centre (University of Guelph, ON, Canada) for their technical assistance. We would like to acknowledge the continued support received from the Ontario Ministry of Agriculture Food and Rural Affairs and the Natural Sciences and Engineering Research Council of Canada (BW McBride).