Coagulation of milk on addition of rennet and rheological properties of the gels thus formed are essential factors determining the physical properties, including texture, of cheese. In recent years, there has been a wide interest in application of high-pressure homogenisation (HPH) in processing of milk and manufacture of some dairy products. HPH, which uses dynamic pressures in the range of 100–350 MPa, allows production of food emulsions with smaller particles and narrower particle size distribution than conventional homogenisation (Hayes & Kelly, Reference Hayes and Kelly2003a; Floury et al. Reference Floury, Legrand and Desrumaux2004). Furthermore, several studies have suggested that HPH could inactivate microorganisms and enzymes in milk, thus prolonging its shelf-life (Hayes & Kelly, Reference Hayes and Kelly2003b; Hayes et al. Reference Hayes, Fox and Kelly2005; Pereda et al. Reference Pereda, Ferragut, Quevedo, Guamis and Trujillo2007).
HPH, like conventional homogenisation, disrupts milk fat globules and also modifies their surface layer, mainly by adsorption of casein micelles and their fragments. Therefore, homogenised milk fat globules, in contrast to native fat globules, are expected to act like casein particles and participate in aggregation processes of casein micelles, e.g., rennet- or acid- induced coagulation. The homogenised fat particles can also greatly influence formation of milk gels and their rheological properties, as they interact with the casein matrix (Walstra & Mulder, Reference Walstra, Mulder, Walstra and Mulder1974). Several studies on the effect of the HPH on the coagulation of whole milk for potential application in cheese and yoghurt manufacture have been reported (Serra et al. Reference Serra, Trujillo, Quevedo, Guamis and Ferragut2007; Zamora et al. Reference Zamora, Ferragut, Jaramillo, Guamis and Trujillo2007). It has been shown that the time needed to form a gel for HPH-treated whole milk sample was shorter than that of unhomogenised milk and decreased with homogenisation pressure (Hayes & Kelly, Reference Hayes and Kelly2003a; Serra et al. Reference Serra, Trujillo, Quevedo, Guamis and Ferragut2007, Zamora et al. Reference Zamora, Ferragut, Jaramillo, Guamis and Trujillo2007). The rheological properties of homogenised milk gels mainly depend on the volume fraction of the filler (fat) particles, their rheological properties and the type of interaction with the protein matrix (van Vliet & Dentener-Kikkert, Reference van Vliet and Dentener-Kikkert1982; van Vliet, Reference van Vliet1988). HPH has been shown to affect not only milk fat globules but also casein micelles, and it has been suggested that HPH slightly modifies the surface structure of micelles and reduces the coagulation time of skim milk, possibly due to solubilisation of αS- and κ-caseins (Sandra & Dalgleish, Reference Sandra and Dalgleish2005).
However, limited data on the effects of HPH on the rheological properties of milk gels are currently available. The rheological properties of rennet-induced milk gels, especially at large deformations, and their fracture properties, are particularly relevant to cheese-making. The main purpose of the studies reported herein was to determine the effects of the HPH on the gel formation process of renneted skim milk and standardised milk, as well as their rheological characteristics at large deformations.
Materials and Methods
Preparation of milk samples
Raw whole milk was obtained from a local dairy farm and separated on the same day into skim milk (0·15% fat, w/w) and cream (40–42% fat, w/w) using a mechanical separator. Standardised milk samples were prepared by blending whole milk and skim milk at 55°C to give a casein: fat ratio of 0·70. This corresponded to an average fat and protein content of 38 and 34 g/kg milk, respectively. The standardised milk and skim milk samples were batch pasteurised at 63·5°C for 30 min in a water-bath. Skim milk samples with an average fat content of 0·08% fat (w/w) were prepared by centrifugation of pasteurised skim milk at 5000 g at 4°C for 60 min, after which the residual fat globules were removed by filtration through glass wool. Milk samples were warmed to 55°C and homogenised at a primary stage pressure of 100, 200 or 300 MPa and a secondary stage pressure of 5 MPa using an ‘nm-Gen 7400 H’ homogeniser (Stansted Fluid Power Ltd., Essex, UK). The flow rate of milk in the homogeniser was 300 ml/min and the sample was circulated in the homogeniser for one pass. Milk samples were cooled immediately after HPH treatment to 4°C in iced water and were analysed on the day of preparation.
Compositional analysis
Compositional analysis of raw whole and standardised milk was performed using a Milkoscan FT 120 (Foss Electric, Hilerod, Denmark). Protein content and fat content in skim milk samples were determined using the Kjeldahl (IDF, 1986) and Rose-Gottlieb (IDF, 1996) methods, respectively.
Measurement of casein micelle size and turbidity
Casein micelle size distribution in skim milk samples was determined by photon correlation spectroscopy (Malvern Zetamaster, Malvern Instruments Ltd., Worcestershire, UK). Skim milk samples were diluted in lactose-free synthetic milk permeate (SMUF) (Jennes & Koops, Reference Jennes and Koops1962) to a ratio of 1:5000 (v/v) and filtered through Whatman No. 40 filter paper (Whatman International Ltd., Maidstone, UK). Measurements of casein micelle size were carried out at ambient temperature. For turbidity measurements, skim milk samples were diluted in SMUF to 1:10 (v/v) and the turbidity (absorbance) was measured at 600 nm at ambient temperature using a Carry 300Bio UV-visible spectrophotometer (Varian, Inc. Scientific Instruments, California, USA).
Measurement of fat globule size
The fat globule size distribution in standardised milk samples was determined by light-scattering using a Mastersizer model S (Malvern Instruments, Malvern, UK), equipped with a 3000 F (reverse Fourier) lens and a He-Ne laser (λ of 633 nm). Prior to measurement, milk samples were dispersed in deionised water by stirring in a sample-dispersion unit. The parameters of fat globule size distribution were calculated using the polydisperse optical model, assuming a refractive index of fat globules of 1·46, absorbance of 0·00 and the refractive index of the aqueous medium (water) as 1·33. The volume-to-surface average diameter (d 3,2), the volume-weighted average diameter, d 4,3, and the relative width of the size distribution (c 2) were determined.
Rheological measurements
Milk samples were warmed to 20°C and the pH was adjusted to 6·60 by adding 10% lactic acid (v/v) and stirring for 20 min; after adjusting the pH, milk samples were placed in a water bath at 32°C and maintained at this temperature for 20 min. The dynamic rheological behaviour of rennet-induced milk gels was studied in a concentric cylinder cell (CC 27) of a Physica MCR 301 rheometer (Anton Paar, Hertford Herts, UK). Milk gels were formed at 32°C in the cup of the rheometer by adding 200 μl of diluted (1:10, v/v) rennet (Maxiren 180 (DSM, Food Specialities, Delft, the Netherlands), diluted 1:10 (v/v) in Milli Q water (Milipore Corporation, Bedford, MA, USA)) per 20 ml milk sample; gel formation was followed by measuring the storage modulus (G′) and loss modulus (G″) over 120 min at a frequency of 1 Hz and a strain of 0·01. The gel formation rate (dG′/dt) was determined as a maximum value of dG′/dt from the plots of G′ against time after rennet addition, and t gel was defined as the moment when G′′=G′ (tan δ=45°C).
The behaviour of the rennet-induced milk gels at large deformations (fracture) was studied using concentric cylinder geometry (CC 27) of the rheometer. Rennet-induced milk gels were formed in the cylinder of the rheometer at 32°C for 60 min, after which a constant shear rate of 0·01 s−1 was applied to gels until the fracture (yield) was reached. The apparent fracture stress (σfr) and apparent strain at fracture (γfr) were defined as the shear stress and strain at the yielding point.
Electrophoretic analysis
Proteins in control (unhomogenised) and homogenised skim milk samples were separated by SDS-PAGE on 12·5% acrylamide gels as described by Laemmli (Reference Laemmli1970), with (reducing conditions) or without (non-reducing conditions) β-mercaptoethanol (5%). Skim milk samples (fat content 0·08%, w/w, 30 ml) were also fractionated by ultracentrifugation at 100,000 g for 60 min at 20°C with a LE-80K Optima preparative ultracentrifuge using a 50.2 Ti rotor (Beckman, CA, USA) and supernatants were analysed by reducing and non-reducing SDS-PAGE. Gels were stained with a colloidal Coomassie blue staining procedure (Chevalier et al. Reference Chevalier, Rofidal, Vanova, Bergoin and Rossignol2004) and gel images were acquired with a GS-800 imaging densitometer (Bio-Rad Laboratories, Hercules, California) and analysed with PDQuest software v.7.3.1 (Bio-Rad).
Analytical two-dimensional gel analysis of ultracentrifugal supernatants was carried out with 3·0 μl milk (~100 μg protein) separated with 7-cm strips. Milk samples were mixed with a solubilisation buffer (9 m-urea, 4% CHAPS, 0·05% Triton X100 and 65 mm-DTT). Subsequently, 7-cm strips, with a linear pH gradient from 3 to 10, (Bio-Rad) were re-hydrated in the above solution and isoelectric focusing was carried out using the Protean IEF Cell (Bio-Rad, Hercules, CA, USA) system up to 20,000 kV/h for 7-cm strips. Before the second dimension, the strips were reduced (50 mm-Tris HCl pH 8·8, 6 m-urea, 30% glycerol, 2% SDS and 130 mm-DTT) and alkylated in the same buffer containing 130 mm-iodoacetamide instead of DTT. The strips were then embedded using 0·6% w/v low-melt agarose on the top of a 12·5% acrylamide gel. SDS-PAGE was carried out using the Criterion® Dodeca Cell electrophoresis unit (Bio-Rad) and gels were stained and digitized as described above.
Statistical analysis
All treatments were repeated 2–3 times for each set of experimental conditions. Experimental data were evaluated statistically using one-way ANOVA and linear regression analysis (MINITAB Release 15, Statistical software, Minitab Inc., PA, USA). Statistical differences between quantitiave data obtained from electrophoretograms were calculated using a Student's t-test and a difference between 2 groups was considered to be statistically significant if the P-value was less than 0·05.
Results and Discussion
Effect of HPH on physical properties of casein micelles
Average casein micelle size in skim milk samples decreased significantly compared to that in unhomogenised (control) skim milk after homogenisation at 200 MPa (P<0·05) and 300 MPa (P<0·005) (Table 1). However, HPH treatment of skim milk at lower pressures (100 MPa) did not affect casein micelle size. The turbidity of HPH-treated skim milk samples was also significantly lower (P<0·05) than that of the control sample for all pressures studied (100–300 MPa) and decreased considerably with increasing homogenising pressure (Table 1). The turbidity of skim milk samples appeared to broadly correlate with the average casein micelle size. Hayes & Kelly (Reference Hayes and Kelly2003a) also reported that HPH treatment of skim milk at 200 MPa decreased casein micelle size and Sandra & Dalgleish (Reference Sandra and Dalgleish2005) demonstrated that apparent casein micelle size in reconstituted skim milk powder decreased with increasing homogenising pressure from 41 to 186 MPa and number of passes through a homogenizer (up to 6).
Table 1 Effect of HPH treatment on average casein micelle size and turbidity of skim milk
Values are given as means (n=3)±standard deviation

‡ Control (unhomogenised) skim milk sample
Effect of HPH on protein distribution between serum and micellar phases
Denaturation of whey proteins due to HPH treatment was studied by comparing protein profiles of control and HPH-treated skim milk under reducing or non-reducing conditions (Fig. 1a, b); in addition, distribution of casein and whey proteins in the micellar and serum phases of skim milk were investigated by ultracentrifugation followed by SDS-PAGE and two-dimensional (Fig. 2) electrophoresis. The similarity of electrophoretograms of control and HPH-treated skim milk under reducing and non-reducing SDS-PAGE indicated that no disulphide bonding, i.e., due to denaturation of whey proteins, was induced by HPH treatment (Fig. 1a and b, lanes 1–4).

Fig. 1. SDS-PAGE electrophoretograms under (a) reducing or (b) non-reducing conditions of skim milk (lanes 1–4) and ultracentrifugal supernatants of skim milk (lanes 5–8) treated by HPH at 0 (lanes 1, 5), 100 (lanes 2, 6), 200 (lanes 3, 7) or 300 (lanes 4, 8) MPa.

Fig. 2. Two-dimensional electrophoretograms of ultracentrifugal supernatants of skim milk treated by HPH at 0 (a) or 200 (b) MPa.
The protein profiles of ultracentrifugal supernatants of control and HPH-treated skim milk on SDS-PAGE (Fig. 1a, b, lanes 5–8) were broadly similar, and also similar under reducing and non-reducing conditions, suggesting little dissociation of caseins on HPH treatment, or formation of disulphide bonds; however, the supernatants of milk HPH-treated at 200 MPa showed a slight apparent increase in content of αs1- casein. Further analysis by two-dimensional electrophoresis (Fig. 2) confirmed that the content of αs1-casein in the ultracentrifugal supernatant (serum) of milk HPH-treated at 200 MPa (Fig. 2b) was higher than that in the serum phase of control sample (Fig. 2a). However, there was no apparent difference observed between the amounts of αs2-, β- and κ-caseins in the serum phase of HPH-treated and unhomogenised skim milk, as may be expected if the casein micelles were disintegrated. The mechanism for the release of αs1-casein from the micellar casein fraction to the serum phase remains unclear, and this dissociation did not appear to be correlated with homogenisation pressure (being maximum after treatment at 200 MPa). Sandra & Dalgleish (Reference Sandra and Dalgleish2005) also reported a slight increase in the amounts of non-sedimentable κ- and αs1-caseins in HPH-treated reconstituted skim milk powder, and proposed that only the surface of casein micelles were affected, possibly due to mechanical forces or disruption of hydrophobic bonds. However, no significant dissociation of casein micelles occurred during HPH treatment in the present study, and it appears that this is not related to the decrease in micelle size measured (Table 1).
It may be possible that the fragments of casein micelles or caseins dissociated from micelles as a result of HPH were transferred to the small number of residual fat globules in skim milk, as the amount of αs2-, β- and κ-caseins in the supernatant did not increase; it was previously reported that even a fat content of 0·1%, as may be found in skim milk, presents a relatively large fat surface area, i.e., of about 200 m2/l, as a result of homogenisation (Walstra & Mulder, Reference Walstra, Mulder, Walstra and Mulder1974).
Effect of HPH on rheological properties of rennet-induced skim milk gels
While HPH at 100 MPa did not affect the t gel of skim milk, t gel was significantly shorter for renneted skim samples homogenised at 200 and 300 MPa than for control sample (Table 2). The t gel of renneted skim milk decreased significantly (R 2=0·75, P<0·01) with reducing casein micelle size (Fig. 3). This is in agreement with the report of Sandra & Dalgleish (Reference Sandra and Dalgleish2008) of reduced gelation times for skim milk homogenised at 179 MPa compared with untreated milk. These authors also proposed that HPH had no significant effect on the primary phase of rennet coagulation and that only aggregation of casein particles was influenced; the decrease in t gel of skim milk was mainly attributed to the loss of κ-casein during HPH and thus reduced electrostatic repulsion and steric hindrance of casein particles. However, in the present study, electrophoretic analysis did not indicate any correlation between the t gel and the dissociation of κ-casein in HPH-treated skim milk samples. The decrease in the t gel of the renneted skim milk with a reduction of casein particle size may possibly be explained by perikinetic aggregation theory. Walstra (Reference Walstra and Walstra2003) reported that the rate of perikinetic aggregation is particle-size-dependent and that the initial aggregation rate increases greatly with decreasing particle diameter for a constant volume fraction of particles; therefore, it is expected that the time needed to form a gel becomes shorter for smaller casein micelles.

Fig. 3. The storage modulus at 2700 s after rennet addition (○) and gel formation time (•) of rennet-induced skim milk gels at pH 6·60 as a function of average casein micelle size.
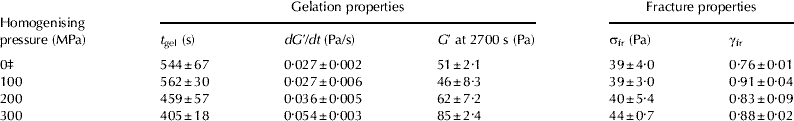
Table 2 Effect of HPH treatment on gel formation time (t gel), gel formation rate (dG′/dt), storage modulus (G′ at 2700 s after rennet addition), apparent fracture stress (σfr) and apparent strain at fracture (γfr) of rennet-induced skim milk gels at pH 6·60
Values are given as means (n=3)±standard deviation
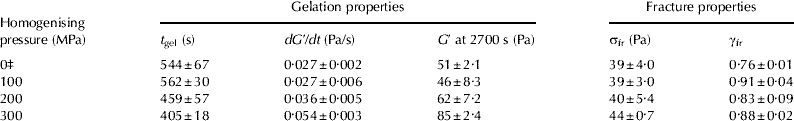
‡ Control (unhomogenised) skim milk sample
The dG′/dt and the G′ at 2700 s after rennet addition (′cutting time′ of curd during cheese making) increased with increasing homogenising pressure (Table 2). There was a significant correlation between the average casein micelle size and the G′ value at 2700 s (R 2=0·92, P<0·005; Fig. 3). It appears that rennet-induced skim milk gels formed from the smaller casein micelles were stiffer, i.e., had a higher G′ value 2700 s after rennet addition, than milk gels made from larger casein micelles. Horne (Reference Horne1996) and Horne & Banks (Reference Horne, Banks, Fox, McSweeney, Cogan and Guinee2004) theoretically demonstrated and experimentally confirmed that the G ∞ value of milk gels is inversely proportional to the cube of casein micelle size at a given concentration, assuming an homogeneous distribution of the particles and equal strength of the bonds. Our experimental results showed that G′ values increased with decreasing casein micelle size, although the dependence between the G′ and the size of casein micelles was weaker than that predicted by the model of Horne (Reference Horne1996). These differences could be possibly due to the polydispersity of the casein micelles in skim milk samples.
There was no significant difference in the σfr between rennet-induced skim milk gels formed from HPH-treated and control milk (Table 2). The γfr values of rennet-induced milk gels were similar for gels formed from HPH-treated skim milk and the gels made from the control skim milk sample. It appears that HPH had no effect on the strength and deformability of the rennet-induced skim milk gels. These findings are in agreement with the report of Walstra & Mulder (Reference Walstra, Mulder, Walstra and Mulder1974) that homogenisation at lower pressures (15–40 MPa) did not change the firmness of skim milk gels.
These results suggest that HPH treatment (100–300 MPa) influenced gel formation time and the small-deformation rheological properties of rennet-induced skim milk gels, possibly due to the changes in casein particle size. However, there was little effect on the rheological behaviour of rennet-induced skim milk gels at large deformations.
Effect of HPH on rheological properties of rennet-induced standardised milk gels
The d 3,2 and d 4,3 values of fat particles in standardised milk decreased significantly (P<0·005) with increasing homogenising pressure (Table 3). However, there were no significant differences observed in d 3,2 values between milk samples homogenised at 200 MPa or 300 MPa. It appears that 200 MPa was a threshold value of homogenising pressure, above which the process was no longer efficient as no further changes in the fat globule size was observed. These findings are in general agreement with data reported by Hayes & Kelly (Reference Hayes and Kelly2003a) and Zamora et al. (Reference Zamora, Ferragut, Jaramillo, Guamis and Trujillo2007).
Table 3 Effect of HPH treatment on volume-surface average diameter (d 3,2) and volume-weighted average diameter (d 4,3) of standardised milk with a fat content of 3·60% (w/w)
Values are given as means (n=3)±standard deviation

‡ Control (unhomogenised) skim milk sample
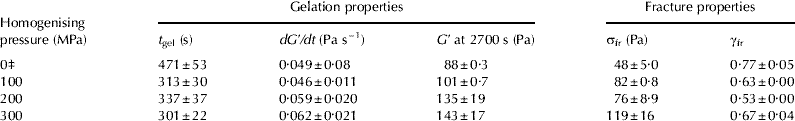
The t gel of HPH-treated standardised milk was significantly shorter (P<0·05) than the t gel of control milk for all homogenising pressures studied, and the dG′/dt increased slightly with increasing homogenising pressure (Table 4). However, there was no difference in the t gel of renneted standardised milk for different HPH treatments. There was also no unequivocal relation between the t gel and the fat particle diameter, d 3,2 and d 4,3. Shorter rennet coagulation times for HPH-treated whole milk were previously reported (Hayes & Kelly, Reference Hayes and Kelly2003a; Serra et al. Reference Serra, Trujillo, Quevedo, Guamis and Ferragut2007; Zamora et al. Reference Zamora, Ferragut, Jaramillo, Guamis and Trujillo2007); the decrease in the t gel of homogenised milk could be mainly attributed to an increase in the effective concentration of casein participating in aggregation process (Walstra & Mulder, Reference Walstra, Mulder, Walstra and Mulder1974; Walstra et al. Reference Walstra, Wouters, Geurts, Walstra, Wouters and Geurts2006) as homogenised fat particles can take part in the gel formation process. Therefore, homogenisation of milk greatly reduces gel formation time, and aggregation of casein micelles proceeds faster. It has previously been demonstrated that gel formation process for particle gels is faster for higher volume fractions of particles (Walstra, Reference Walstra, Mulder, Walstra and Mulder1974, Walstra et al. Reference Walstra, Wouters, Geurts, Walstra, Wouters and Geurts2006).
Table 4 Effect of HPH treatment on gel formation time (t gel), gel formation rate (dG′/dt), storage modulus (G′ at 2700 s after rennet addition, apparent fracture stress (σfr) and apparent strain at fracture (γfr) of rennet-induced standardised milk gels at pH6·60)†
† Values are given as means (n=3)±standard deviation
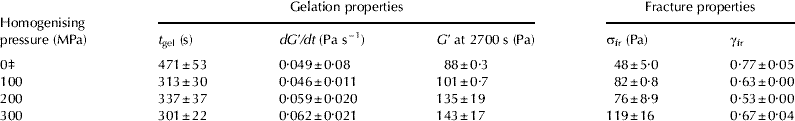
‡ Control (unhomogenised) skim milk sample
Gels made from HPH-treated standardised milk had a higher G′ value 2700 s after rennet addition than gels formed from control sample (Table 4) and there was a strong linear correlation (R 2=0·96, P<0·05) between the G′ at 2700 s and homogenising pressure (100–300 MPa). Higher G′ and dG′/dt for HPH-treated whole milk was also reported by Hayes & Kelly (Reference Hayes and Kelly2003a). The G′ at 2700 s after rennet addition of milk gels increased with reduction of d 3,2 and d 4,3 of milk fat particles. This finding is in agreement with data of Michalski et al. (Reference Michalski, Cariou, Michel and Garnier2002), who also showed that the G′ of rennet-induced milk gels was higher for the larger surface area (smaller diameter) of homogenised fat globules at a constant volume fraction of milk fat. The increase in the G′ (stiffness) of standardised milk gels may be largely attributed to a higher concentration of particles forming a gel, as homogenised fat globules (active filler) interact with casein network during gel formation. van Vliet & Dentener-Kikkert (Reference van Vliet and Dentener-Kikkert1982) and van Vliet (Reference van Vliet1988) demonstrated that the G′ value of the filled acid milk gels increased with increasing volume fraction of recombined milk fat globules or homogenised milk fat particles. Furthermore, van Vliet (Reference van Vliet1988) also showed that the G′ value of gels filled with interacting fat particles greatly depends on the properties of the matrix material and the filler particles, as well as their interactions. The stiffness of filler particles is inversely proportional to their diameter and increases when the interfacial tension is higher (van Vliet, Reference van Vliet1988), which could partially explain an increase in the G′ value for standardised milk gels with decreasing d 3,2 and d 4,3 of homogenised fat globules. The increase in the storage modulus of emulsion-filled protein gels containing smaller oil droplets was previously reported by Rosa et al. (Reference Rosa, Sala, van Vliet and van de Velde2006).
The σfr of the rennet-induced milk gels produced from HPH-treated milk (100–300 MPa) was significantly higher (P<0·005) than the σfr of the milk gels made from control sample (Table 4). The σfr of gels formed from HPH-treated standardised milk increased slightly with increasing homogenising pressure (R 2=0·84, P<0·1). Milk gels produced from HPH-treated milk fractured at lower γfr than gels made from control sample, indicating a more brittle texture, but there was little difference in the γfr of rennet-induced milk gels between the three HPH treatments. There was no clear effect of the homogenised fat particle diameter d 3,2 on the σfr and γfr of milk gels (3·60% fat). It thus appears that HPH treatment had a relatively large effect on the σfr of standardised milk gels, whereas the γfr was less affected. The increase in the σfr of the rennet-induced standardised milk gels made from HPH-treated milk could be due to a higher number of protein-protein bonds per cross-section and possibly increased tortuosity and stiffness of strands. A slight decrease in the γfr of the standardised milk gels formed from HPH-treated milk could possibly be due to the filler particles acting as defects in the structure, causing local stress and strain concentrations, and therefore a decrease in the apparent fracture strain (Luyten, Reference Luyten, van Vliet and Walstra1991).
The authors wish to thank Professor Elke K. Arendt of the Department of Food and Nutritional Sciences at University College Cork for assistance with rheological measurements, and Mr David Waldron for technical assistance. The authors acknowledge the Commission of the European Communities for the financial support given to this research (CRAFT project UHPH-512626).