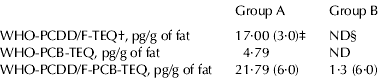Dioxins, lipophilic compounds with a small molecular weight, are bioaccumulative, persistent and highly toxic environmental contaminants (Matsumura, Reference Matsumura2003). In blood plasma, dioxins are transported to the liver, via lipoproteins, for a detoxification attempt mediated by cytochrome P450 (Mandal, Reference Mandal2005) and then gradually distributed to other tissues, mostly according to their lipid content. Cytochrome P450 enzymes contribute to dioxin-induced toxicity, as they catalyse the epoxidation of these compounds, thus generating highly reactive oxygen species (ROS) that may lead to cellular macromolecule damage and tissue injury (Mandal, Reference Mandal2005). Increased lipid peroxidation, enhanced production of the superoxide anion, reduced glutathione (GSH) levels and increased DNA fragmentation were observed in the liver, after activation of dioxin-induced detoxification enzymes (Shertzer et al. Reference Shertzer, Vasiliou, Liu, Tabor and Nebert1995; Zhao & Ramos, Reference Zhao and Ramos1998; Slezak et al. Reference Slezak, Hatch, DeVito, Diliberto, Slade, Crissman, Hassoun and Birnbaum2000). Some of the complex actions of dioxin are caused by stress response reactions of affected cells, which are temporary measures to save cells and the affected organism (Matsumura, Reference Matsumura2003). Stress alters physiological homeostasis and stimulates numerous pathways leading to increased levels of ROS. The damaging action of ROS, if not counteracted or prevented by antioxidant defences, causes cell death, tissue oxidative stress and organ ageing or failure (Parke & Parke, Reference Parke and Parke1995; Halliwell & Gutteridge, Reference Halliwell, Gutteridge, Halliwell and Gutteridge2000). In physiological conditions, the antioxidant defence system, provided by enzymes and antioxidants, scavenges ROS, thus limiting or preventing oxidative damage. Deficiencies of natural protective substances or excess exposure to stimulators of ROS production may result in oxidative stress, which occurs when pro-oxidants exceed the capacity of antioxidants (Halliwell & Gutteridge, Reference Halliwell, Gutteridge, Halliwell and Gutteridge2000). Oxidative stress may impair health and performance of dairy cows, both directly and indirectly (Miller et al. Reference Miller, Brzezinska-Slebodzinska and Madsen1993). In this context it is worth mentioning that the enzymic antioxidants superoxide dismutase (SOD) and glutathione peroxidase (GPx) were classified as preventive antioxidant systems, as participating in prevention of oxidative damage that is superoxide or hydroperoxide-mediated. Ascorbate (Asc), α-tocopherol (Toc) and retinol (Ret) are widely distributed non-enzymic antioxidants, which scavenge most oxygen and nitrogen-reactive species (Halliwell, Reference Halliwell1994) and integrate the endogenous antioxidant activities to prevent oxidative damage (Miller et al. Reference Miller, Brzezinska-Slebodzinska and Madsen1993). Ret and Toc, the major liposoluble antioxidants, are important in host defence and immunological reactivity (Grimble, Reference Grimble1990; Jialal et al. Reference Jialal, Devaraj and Kaul2001). Indeed, Ret contributes to maintaining cell-mediated immunity and plays a role in resistance to infectious disease, particularly mastitis (Grimble, Reference Grimble1990; Miller et al. Reference Miller, Brzezinska-Slebodzinska and Madsen1993). Toc exerts a protective role against lipid oxidation in biological systems and in food (Miller et al. Reference Miller, Rice-Evans, Davies, Gopinathan and Milner1993; Faustman et al. Reference Faustman, Chan, Schaefer and Havens1998) and is essential for growth, reproduction, performance of feedlot cattle, prevention of various diseases and protection of the integrity of tissues (Smith et al. Reference Smith, Harrison, Hancock, Todhunter and Conrad1984; Herdt & Stowe, Reference Herdt and Stowe1991; Miller et al. Reference Miller, Mueller, Thomas, Madsen and Coelho1991; Miller et al.1993; Mallard et al.1998; Azzi & Stocker, Reference Azzi and Stocker2000; Galli & Azzi, Reference Galli and Azzi2010). Asc, in turn, is a major antioxidant in body fluids, protecting cells against deleterious effects of oxidative stress (Frei et al. Reference Frei, England and Ames1989) and restoring the antioxidant properties of oxidized Toc.
The objective of this study was to compare the blood redox status of lactating buffalo cows exposed to high or low environmental dioxin levels. We therefore measured the plasma concentration of Ret, Toc and Asc, as well as of specific markers of oxidative stress, and the activity of SOD and GPx, in order to obtain indices of the antioxidant defence system and of the extent of oxidative damage to lipids and proteins. To our knowledge, there are no indications in the literature about the effects of exposure to dioxin on plasma redox status in river buffaloes.
Materials and Methods
Materials
Bovine serum albumin fraction V (BSA), chemicals of the highest purity, goat anti rabbit IgG-peroxidase (GAR-HRP), rabbit anti-dinitrophenylhydrazine (anti-DNP) IgGs, and standards for HPLC were purchased from Sigma-Aldrich (via Gallarate 154, 20151, Milan, Italy). Rabbit anti-Nitrotyrosine IgG was from Calbiochem (Boulevard Industrial Park, Padge Road, Beeston Nottingham, UK). Nucleosil 100-NH2 columns (5-μm particle size, 250×4·6 mm i.d.) and Nova-PAK C18 columns (4-μm particle size, 125×2 mm i.d.) were obtained from Macherey-Nagel (distributed by Delchimica Scientific, via fratelli Ruffini, 20123, Milan, Italy). Organic solvents were purchased from Romil (distributed by Delchimica Scientific, via fratelli Ruffini, 20123, Milan, Italy). Polystyrene 96-well plates were purchased from Nunc (distributed by VWR International, via Stephenson, 94, I-20157, Milan). Nitrated BSA (nitrotyrosine-BSA) and the kit for titration of lipoperoxide of Cayman Chemical (distributed by Inalco, via Calabiana 18, 20139, Milan, Italy) were used.
Animals
The study was carried out on two commercial dairy farms of Campania region (Southern Italy), localized in two different provinces, and exposed to different levels of environmental dioxins. According to local health sanitary control, the origin of dioxins was mainly the illegal burning of domestic waste. The extent of dioxin exposure was defined by evaluating dioxin level in milk samples. In particular, the concentration of dioxins was measured in the pool of milk samples obtained from animals of group A (n=21; pool A) and in the pool of milk samples obtained from animals of group B (n=29; pool B). Analyses of dioxin level in milk samples (Table 1) were carried out by an Italian accredited laboratory (Laboratorio Cavallo, Salerno, Italy) under the national control of local health units of competence (Istituto Zooprofilattico del Mezzogiorno, Portici, Napoli, Italy), and in collaboration with BDS (BioDetection Systems, Amsterdam, The Netherlands). Fifty lactating buffalo cows, homogeneous for parity (=3), days in milk (DIM=83·4±9·0), body condition score, previous milk production and health condition, were used in this study. In detail, 21 lactating buffalo cows were selected, on the basis of the above mentioned parameters, in the farm exposed to higher environmental dioxin concentration (group A) and 29 lactating buffalo cows were selected in the farm exposed to lower dioxin concentration (group B). The animals were fed ad libitum a diet containing (g/kg dry matter): 155·4 g crude protein, 402·3 g neutral detergent fibre and 0·84 Milk Forage Units (1 MFU=7·11 MJ). The same ration, supplemented with a vitamin-mineral mix, was used on both farms. The Ret or Toc supplement was about 450 mg/kg and 520 mg/kg of feed respectively. Daily food intake was 18–20 kg. Chemical analysis of the diet was carried out according to published procedures (Van Soest et al. Reference Van Soest, Robertson and Lewis1991) and the energy value was calculated by validated methods (INRA, Reference Jarrige1988).
Table 1. Dioxin levels in the pool of milk samples obtained from buffalo cows of group A, and in the pool of milk samples obtained from buffalo cows of group B

† WHO: World Health Organization; TEQ: Toxic Equivalents; PCDD/F: polychlorinated dibenzo-para-dioxins and furans; PCB: polychlorinated biphenyls
‡ Maximum levels legally admitted are reported in parenthesis (pg per g of milk fat)
§ ND: not detected
On each farm blood samples were collected, early in the morning, on the same day and in the same environmental conditions, into heparinized tubes. Plasma was obtained by centrifugation at 500 g at 4°C for 15 min and was processed, by the same operator, for titration of antioxidants, SOD and GPx activity, TAC, N-Tyr, PC and LPO.
Determination of antioxidants and lipid hydroperoxides
Plasma samples for determination of Asc concentration were prepared as previously described (Spagnuolo et al. Reference Spagnuolo, Cigliano, Sarubbi, Polimeno, Ferrara, Bertoni and Abrescia2003) and analysed by HPLC using an anion exchange column (Nucleosil 100-NH2, 5 μm, 250×4·6 mm i.d.; Macherey-Nagel). Chromatography was carried out at a flow rate of 0·8 ml/min, with 50 mm-NaH2PO4:CH3CN (35:65, v:v) as mobile phase. A UV-spectrophotometer was used to detect the elution of Asc, which was identified by retention time and absorbance spectrum. Absorbance at 254 nm, and calibration curves (r 2⩾0·9997) obtained by injecting different amounts (n=12) of standard were used for quantitative analysis.
Samples for determination of Ret and Toc were prepared according to a published procedure (Spagnuolo et al. Reference Spagnuolo, Cigliano, Sarubbi, Polimeno, Ferrara, Bertoni and Abrescia2003) and analysed by HPLC. A reverse phase C18 column was chosen for chromatography (Nova-PAK C18, 4 μm, 125×2 mm i.d.; Macherey-Nagel). The analysis was carried out with isopropanol:methanol:water (46·25: 46·25: 7·5, v: v: v) as mobile phase, at 0·2 ml/min, using a programmable fluorescence spectrometer for detection. In particular, Ret was detected by setting λEX=325 nm and λEM=465 nm from 0 to 5 min, while Toc was detected by λEX=295 nm and λEM=335 nm from 5 to 8 min. Calibration curves (r 2⩾0·9998), obtained by injecting different amounts (n=12) of standard, were used for quantitative analysis.
LPO concentration was measured by a colorimetric quantitative assay, using the lipid hydroperoxide assay kit of Cayman Chemical according to the manufacturer's instructions.
Determination of total antioxidant capacity
The plasma total antioxidant capacity was measured by the trolox equivalent antioxidant capacity assay (TEAC) according to a published procedure (Miller et al. Reference Miller, Brzezinska-Slebodzinska and Madsen1993). Plasma samples were reacted with the radical 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonate) [ABTS], and the antioxidant capacity was measured as the decrease of the absorbance at 734 nm, and expressed as μm concentration of trolox equivalents (Miller et al. Reference Miller, Brzezinska-Slebodzinska and Madsen1993; Spagnuolo et al. Reference Spagnuolo, Cigliano, Balestrieri, Porta and Abrescia2001).
Determination of nitrotyrosine (N-Tyr) in plasma samples
Nitrated protein levels in plasma samples were measured by ELISA according to Spagnuolo et al. (Reference Spagnuolo, Cigliano, Balestrieri, Porta and Abrescia2001). Plasma samples were diluted (1:100, 1:500, 1:1·000, 1:3·000, 1:6·000, and 1:10·000) with coating buffer (7 mm-Na2CO3, 17 mm-NaHCO3, 1·5 mm-NaN3, pH 9·6) and incubated in the wells of a microtitre plate overnight at 4°C. Standard curves were obtained with serial dilutions of nitrated BSA. N-Tyr was detected by incubation with rabbit anti-N-Tyr antibody (1: 500 dilution in 130 mm-NaCl, 20 mm-Tris–HCl, 0·05% Tween 20, pH 7·3, supplemented with 0·25% BSA; 1 h, 37°C) followed by goat anti rabbit IgG-horseradish peroxidase linked (GAR-HRP) diluted 1:2·000 as the primary antibody. Colour development was monitored at 492 nm, as previously described (Spagnuolo et al. Reference Spagnuolo, Cigliano, Sarubbi, Polimeno, Ferrara, Bertoni and Abrescia2003). Data were reported as nmol of N-Tyr per mg of protein.
Determination of protein-bound carbonyls
PC in plasma samples were measured by ELISA according to Buss et al. (Reference Buss, Chan, Sluis, Domigan and Winterbourn1997). Protein derivatization was carried out with a dinitrophenylhydrazine (DNP) solution (10 mm in 6 m-guanidine hydrochloride, 0·5 m-potassium phosphate buffer, pH 2·5) to give a final protein concentration of 3 mg/ml. Samples were incubated at room temperature for 45 min, vortexing every 10–15 min. Each sample was then diluted (1:6·000–1:50·000) with 10 mm-sodium phosphate buffer, pH 7·0, containing 140 mm-NaCl, and incubated overnight at 4°C in the wells of a microtitre plate. PC were detected by incubation (1 h, 37°C) with rabbit anti-DNP antibody (Sigma-Aldrich, St Louis MO, USA) diluted 1:2·000 with PBS supplemented with 0·2% gelatine and 0·05% Tween 20, followed by GAR-HRP antibody (diluted 1:3·000 as the primary antibody). Colour development was monitored at 492 nm as previously described (Spagnuolo et al. Reference Spagnuolo, Cigliano, Sarubbi, Polimeno, Ferrara, Bertoni and Abrescia2003). A six-point standard curve of oxidized BSA was included with each plate. A blank for DNP reagent in PBS without protein was subtracted from all other absorbances. Data were reported as nmol of carbonyls per mg of protein.
Evaluation of plasma activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD)
GPx activity was measured indirectly by a coupled reaction with glutathione reductase (GR), using the glutathione peroxidase assay kit of Cayman Chemical, according to the manufacturer's instructions. In this assay, oxidized glutathione, produced from reduction of hydroperoxide by GPx, is recycled to its reduced state by GR and NADPH. GPx activity is expressed as nmol of NADPH oxidized per min per ml of sample.
SOD activity was measured with the superoxide dismutase assay kit of Cayman Chemical according to the manufacturer's instructions. This kit utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. SOD activity is expressed as U/ml. One unit of SOD is defined as the amount of the enzyme needed to exhibit 50% dismutation of the superoxide radical.
Statistical Analysis
Samples for determining the SOD and GPx activities, protein-bound carbonyls, nitrotyrosine, or lipoperoxide concentration were processed at least in triplicate. Titration of Toc, Ret and Asc was carried out on duplicates. Values are expressed as mean±sem. The program “Graph Pad Prism 3″ (Graph Pad Software, San Diego CA, USA) was used to obtain trend curves, perform t test, and calculate significance.
Results
Analysis of antioxidants and total antioxidant capacity
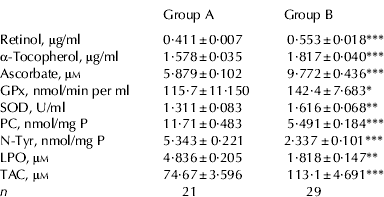
Blood samples, obtained from river buffalo cows of group A and B were collected and separately used to obtain the plasma for titration of Ret, Toc and Asc, and for determining GPx and SOD activity, the antioxidants here selected as markers of the antioxidant defence system. Samples were also analysed for total antioxidant capacity (TAC) with the TEAC assay. Concentrations of Ret, Toc and Asc were higher in group B than in group A (P=0·0001; Table 2). According with this result, TAC was higher in group B than in group A (P⩽0·0001). Plasma titres of Ret and Toc were positively correlated both in group A (r=0·645, P=0·002) and in group B (r=0·809, P=0·0001) as expected, since both are dietary liposoluble antioxidants. Plasma concentration of Asc was positively correlated with TAC, both in group A (r=0·8044; P⩽0·0001) and in group B (r=0·7377, P⩽0·0001). This finding suggests that a major part of plasma antioxidant capacity, as measured by trolox equivalents capacity, might be accounted for by Asc. As shown in Table 2, both GPx activity and SOD activity were higher in group B than in group A (P=0·04 and P=0·006, respectively).
Table 2. Markers of the antioxidant defence system and markers of oxidative stress in plasma of buffalo cows exposed to dioxins (Group A) and cows not exposed (Group B)†
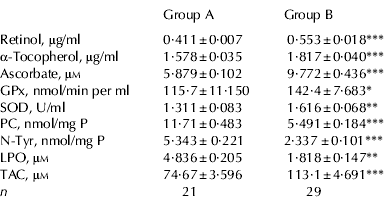
† Concentration of antioxidants, PC, N-Tyr, LPO, and TEAC in plasma samples of buffalo cows exposed to dioxins (group A; n=21) and not exposed to dioxins (group B; n=29).
* P<0·05;
** P<0·01;
*** P<0·001. PC, protein-bound carbonyls (nmol per mg of protein); N-Tyr, nitro-tyrosine (nmol per mg of protein); LPO, lipid hydroperoxides (μm); TAC, total antioxidant capacity (μm concentration of trolox equivalents); GPx, glutathione peroxidase activity (expressed as nmol of NADPH oxidized per min per ml); SOD, superoxide dismutase (U/ml)
Analysis of PC, N-tyr and LPO
Plasma concentrations of PC, N-Tyr and LPO were measured in order to obtain data on the extent of oxidative modifications to proteins and lipids. The amount of modified amino acids in plasma samples was determined by ELISA. As shown in Table 2, significantly higher plasma levels of PC and N-Tyr were found in group A than in B (P=0·0001). Plasma levels of PC were negatively correlated with plasma levels of Asc, both in group A (Fig. 1, panel A; r=−0·70, P=0·0004) and in group B (Fig. 1, panel B; r=−0·951, P=0·0001) thus suggesting a major role for circulating Asc in preventing protein oxidative modifications induced by carbonyls in blood of buffalo cows. Further, plasma levels of PC were negatively correlated with TAC, both in group A (r=−0·6771; P=0·0007) and in group B (r=−0·5659; P=0·001); (data not shown). Asc was reported to be a scavenger of peroxynitrite (Halliwell, Reference Halliwell1997). Accordingly, we found that plasma level of N-Tyr was negatively correlated with that of Asc in group B (r=−0·803; P=0·0003; data not shown) but not in group A. Also, we found a negative correlation between SOD activity and plasma concentration of N-Tyr in group B (r=−0·952; P=0·001) but not in group A. Finally, plasma level of lipoperoxide was higher in group A than in group B (P=0·007), suggesting that exposure to dioxins is associated with a high extent of oxidative damage also to the lipid compartment in blood.

Fig. 1. Correlation between the concentration of ascorbate (Asc) and protein-bound carbonyls (PC) in the plasma of lactating buffalo cows. Asc and PC were measured in plasma samples from buffalo cows exposed to dioxins (panel A, n=21) and from cows not exposed to dioxins (panel B, n=29). The statistical program Grap Pad Prism 3 performed the regression analysis and the calculation of P (r=−0·700, P=0·0004, panel A; r=−0·951, P=0·0001, panel B).
Discussion
Dioxin detoxification in the liver is associated with increased production of ROS (Matsumura, Reference Matsumura2003). During an intensive activity of detoxification, the imbalance between ROS production and the antioxidant defence capacity may lead to oxidative stress with consequent increased consumption of antioxidants and accumulation of toxic compounds in blood and tissues. In our study, Asc, Ret and Toc were used as markers of the antioxidant defence system, able to scavenge ROS and prevent oxidative damage, in the hydrophilic and lipophilic compartment. Further, we evaluated the extent of oxidative protein damage and lipid peroxidation by measuring plasma concentrations of N-Tyr, PC and hydroperoxides (LPO). N-Tyr and PC are good markers of ROS-mediated protein oxidation (Berlett & Stadtman, Reference Berlett and Stadtman1997) as the concentration of N-Tyr residues represents the footprint of oxidative damage induced by peroxynitrite (Halliwell, Reference Halliwell1997) and PC, in turn, may be introduced into proteins by direct oxidative attack of proteins (Kristal & Yu, Reference Kristal and Yu1992), or by reactions with aldehydes produced during lipid peroxidation (Uchida & Stadtman, Reference Uchida and Stadtman1993). Finally, the extent of lipid peroxidation, induced by the interaction of free radicals with polyunsaturated fatty acids, is broadly assessed by measuring LPO plasma concentration.
We report here that plasma concentrations of Ret, Toc and Asc, as well as TAC and SOD and GPx activities, were lower in blood of cows exposed to dioxins, suggesting that exposure to dioxins might negatively affect plasma concentration of liposoluble antioxidants and Asc, and might impair the blood antioxidant defence system. We also found that TAC was positively correlated with plasma concentration of Asc, suggesting that a part of the TAC might be accounted for by this water-soluble antioxidant.
In accordance with this result, we found that the lower antioxidant capacity of buffalo cows exposed to dioxins is associated with a significantly higher extent of oxidative modifications of protein and lipid in blood of cows exposed to dioxins. Further, the negative correlation between plasma levels of Asc and PC, in both groups A and B, seems to suggest a major role of circulating Asc in preventing protein oxidative modifications induced by carbonyls in blood of buffalo cows. It is well known that SOD activity, in physiological conditions, keeps the concentration of superoxide anion very low (Halliwell & Gutteridge, Reference Halliwell, Gutteridge, Halliwell and Gutteridge2000). However, when SOD activity is low or absent (i.e. oxidative stress or mutation) superoxide reacts with nitric oxide, thus producing the peroxynitrite (Beckman & Koppenol, Reference Beckman and Koppenol1996). Asc was reported to be a scavenger of peroxynitrite (Halliwell, Reference Halliwell1997). We found that both SOD activity and Asc concentration were negatively correlated with N-Tyr concentration in group B, but not in group A. This result suggests that the perturbation of blood redox homeostasis, induced by exposure to dioxins, might interfere with the protective role of SOD and Asc against peroxynitrite production and damage to protein. Taken together, our results suggest that increased protein oxidative damage might occur as a result of the lower protective capacity as measured by plasma Asc concentration and TAC. In accordance with the elevated levels of PC and N-tyr, and the lower antioxidant capacity, we found significantly increased plasma concentrations of LPO in cows exposed to dioxins.
In conclusion, higher levels of products of oxidative damage (PC, N-tyr and LPO) and decreased levels of antioxidants were detected in buffalo cows exposed to dioxins. This indicates that a higher extent of oxidative stress is present in cows exposed to dioxins. So, we hypothesize that the exposure to dioxins might promote plasma protein and lipid oxidation. The above-reported differences in the blood redox condition between the analysed groups might indicate that exposure to dioxins impairs the antioxidant defence system, and that metabolic processes associated with dioxin detoxification might induce and/or enhance oxidative protein and lipid damage. This adverse influence on blood redox status might have negative implications for animal health and reproduction, and might compromise animal welfare.
The study was supported by the Italian National Research Council under DG.RSTL.083.001.