When performing udder health investigations, it is important for veterinarians to know the mastitis pathogens involved in a particular problem. Knowledge of the most prevalent pathogens is essential for practitioners to propose targeted corrective measures that will truly lead to an improvement in a situation. A large proportion of milk samples submitted for bacteriology from cases of clinical/subclinical mastitis, lead to no-growth results: a value of 30% is commonly cited for the ‘no-growth samples’ (Taponen et al. Reference Taponen, Salmiviki, Simojoki, Koskinen and Pyörälä2009) with cited values ranging from 26·5% (Bradley et al. Reference Bradley, Leach, Breen, Green and Green2007) to 49·7% (Makovec & Ruegg, Reference Makovec and Ruegg2003). In addition to the no-growth results, practitioners are often confronted with a high number of samples yielding non-aureus staphylococci (NAS) and Corynebacterium bovis, so-called minor pathogens. In fact, several large-scale studies around the world mention that NAS and C. bovis are the most frequently isolated bacteria from milk samples submitted for detection of pathogens responsible for intramammary infection (IMI) (Wilson et al. Reference Wilson, Gonzalez and Das1997; Makovec & Ruegg, Reference Makovec and Ruegg2003; Pitkälä et al. Reference Pitkälä, Haveri, Pyörälä, Myllys and Honkanen-Buzalski2004). The somatic cell count (SCC) recorded for milk samples that yield these pathogens tends to be low in comparison with samples yielding major pathogens (Wilson et al. Reference Wilson, Gonzalez and Das1997; Pitkälä et al. Reference Pitkälä, Haveri, Pyörälä, Myllys and Honkanen-Buzalski2004). Therefore, practitioners investigating a high bulk tank SCC problem find it difficult to ascribe appropriate significance to a high proportion of NAS and C. bovis isolates. It is difficult to identify the minor pathogen responsibility share, as they may simply be easier to isolate than, for example, Staphylococcus aureus, owing to its intermittent shedding pattern (Sears et al. Reference Sears, Smith, English, Herer and Gonzalez1990) or intracellular location (Brouillette et al. Reference Brouillette, Grondin, Shkreta, Lacasse and Talbot2003).
To overcome the difficulty of major pathogen isolation, freezing milk samples prior to culture has been suggested as a means of improving detection rates (Dinsmore et al. Reference Dinsmore, English, Gonzalez and Sears1992; Sol et al. Reference Sol, Sampimon, Hartman and Barkema2002). However, this method is controversial because some authors have found that freezing led to no effect or to a decrease in detection rates (Schukken et al. Reference Schukken, Grommer, Smit, Vandeeger and Brand1989; Dinsmore et al. Reference Dinsmore, English, Gonzalez and Sears1992), whereas others reported an increased detection of major pathogens (Sol et al. Reference Sol, Sampimon, Hartman and Barkema2002). Other methods have been studied in the past to tentatively increase the detection rate of major mastitis pathogens, including centrifugation of milk samples prior to culturing (Zecconi et al. Reference Zecconi, Piccinini, Zepponi and Ruffo1997) or use of Petrifilm for the increased detection of Staph. aureus (Silva et al. Reference Silva, Caraviello, Rodrigues and Ruegg2005).
Recently a polymerase chain reaction (PCR)-based diagnostic test showed promising results in the detection of intramammary pathogens in milk samples that showed no bacterial growth with conventional culturing (Taponen et al. Reference Taponen, Salmiviki, Simojoki, Koskinen and Pyörälä2009).
The objectives of the present study were a) to determine the effect of freezing on the detection rate of certain bacterial groups in milk samples and b) to determine whether a real-time PCR test could detect mastitis pathogens in culture-negative samples or samples yielding minor pathogens.
Materials and Methods
Study 1
The study was performed on four commercial dairy farms selected on the basis of their geographic location (close to the Faculty of Veterinary Medicine, Technical University of Lisbon) and on the farmers' willingness to participate in the study. Three-month rolling geometric means for the first 3 months of the study were 231, 180, 568 and 407 milk recorded animals per farm, SCC of 255, 246, 334 and 766×103 cells/ml and 305-d milk production of 11 891, 9513, 9589 and 9600 kg, respectively. All four farms were performing the 5-point plan mastitis control programme. Farms were visited every 4 weeks for a total of 20 weeks. On the initial visit, 12 cows were randomly selected from those with individual cow SCC that increased from below to above 200 000 cells/ml in the previous two milk recordings. Quarter milk samples were taken according to National Mastitis Council protocols (NMC, 1999) and kept refrigerated, transported to the laboratory and processed on the sampling day. On subsequent visits, cows from which NAS had been isolated were resampled at quarter-level; other animals were selected according to the above criteria to total 12 cows per visit. None of the samples included in the study came from clinical mastitis cases (i.e. gross changes to milk, udder or to the condition of the cow).
From each milk sample, 0·01 ml of milk was plated onto sheep blood agar (Columbia™, bioMérieux, France) and MacConkey agar (Liofilchem, Italy), incubated at 37°C and observed after 24 h and 48 h of incubation. Colonies of similar morphology were selected for isolation and identification if there was evidence of growth of ⩾500 cfu/ml. Samples yielding more than two morphologically different bacterial isolates were considered to be contaminated. Individual quarter SCC were determined through flow cytometry (CombiFoss™, Foss, Denmark).
To study the effect of freezing on the isolation rates of IMI pathogens, defined groups of microorganisms were classified based on Gram stain characteristics and catalase test results into: Gram-positive, catalase-positive cocci which would include the staphylococci; Gram-positive, catalase-negative cocci, which would include the streptococci; Gram-positive bacilli, which would include Corynebacterium spp.; Gram-negative bacilli, and yeasts, besides culture-negative samples. Immediately after plating, milk samples were frozen at −20°C and replated 24 h later after thawing at room temperature. Subsequent colony identification was made according to the protocol described above. To clarify whether samples evidencing growth of staphylococci post freezing but not in the corresponding samples pre-freezing could yield Staph. aureus, post-freezing isolates were identified to species level by use of a biochemical identification system (ID 32 Staph™, bioMérieux, France). Pre and post-freezing bacteriology was performed on 783 milk samples.
Study 2
In the second study, a commercial real-time PCR test (PathoProof™ Mastitis PCR Assay, Finnzymes Oy, Espoo, Finland) was used to investigate whether further microorganisms could be detected in quarter milk samples with negative culture results or yielding only C. bovis or NAS with conventional bacteriology. Identification of staphylococci was made with a biochemical identification system (ID 32 Staph™, bioMérieux, France) and C. bovis identification was made through the evaluation of growth of Gram-positive bacilli in trypticase soy agar and trypticase soy agar supplemented with 1% Tween 80, according to Watts et al. (Reference Watts, Lowery, Teel and Rossbach2000). Samples with a SCC >500 000 cells/ml that were culture-negative or from which a NAS or C. bovis had been isolated as single isolates, were subjected to real-time PCR analysis (n=162). These included 51 no-growth samples, 79 samples from which C. bovis was isolated as a single pathogen and 32 samples from which NAS were isolated as single pathogens. Milk samples were frozen at −20°C on the sampling day and stored until further analysis by PCR. All PCR protocol details were as described earlier (Koskinen et al. Reference Koskinen, Holopainen, Pyörälä, Bredbacka, Pitkälä, Barkema, Bexiga, Roberson, Sølverød, Piccinini, Kelton, Lehmusto, Niskala and Salmikivi2009). This PCR assay allowed the detection of 11 microorganisms or groups of microorganisms: 1) Staph., 2) Staphylococcus spp., including Staph. aureus and the most relevant NAS (Staph. chromogenes, Staph. epidermidis, Staph. haemolyticus, Staph. hyicus, Staph. lugdunensis, Staph. saprophyticus, Staph. simulans, Staph. warneri and Staph. xylosus), 3) Streptococcus agalactiae, 4) Str. dysgalactiae, 5) Str., 6) Escherichia coli, 7) Enterococcus spp., including Ent. faecalis and Ent. faecium, 8) Klebsiella spp., including K. oxytoca and K. pneumoniae, 9) C. bovis, 10) Arcanobacterium pyogenes and Peptoniphilus indolicus, and 11) Serratia marcescens. Cycle threshold (Ct) values, i.e. the number of PCR cycles at which excitation fluorescence detected by the instrument exceeded a pre-defined threshold, were used to score a reaction as positive (Ct⩽37) or negative (Ct>37).
Statistical analyses
Data were analysed using SPSS version 15.0 (USA). For the first study a McNemar test for paired samples was used to determine whether there were differences in bacteriology results between samples processed with the standard procedure and the same samples after freezing for 24 h. Only samples that led to single isolates were used in the analysis. A Wilcoxon signed-rank test was applied to test for differences between log-transformed individual quarter SCC between samples that yielded the same bacteriology result pre-freezing and post freezing. A Wilcoxon signed-rank test was also used to test for differences in mean genome copies of NAS and of C. bovis between samples that with conventional bacteriology led to their isolation or not. A significant difference was defined as a probability value of P<0·05.
Results
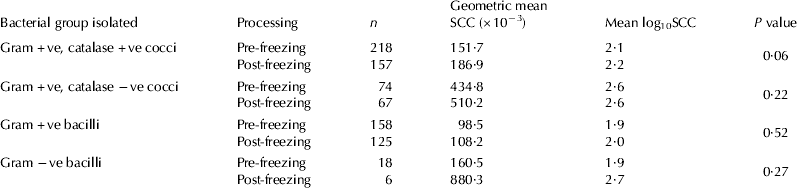
A total of 973 samples were processed pre-freezing and post freezing. Only culture-negative samples or samples yielding single isolates were included in the analyses, which totalled 783 samples. A significant difference in the frequency of isolation of bacteria was observed in several of the microorganism groups between pre- and post-freezing samples (Table 1). Gram-positive catalase-positive cocci, more specifically staphylococci, were detected in 12 samples post freezing while not being detected in the same samples pre-freezing. None of these 12 isolates was identified as Staph. aureus. There was a significant increase in the log-transformed SCC between culture-negative samples pre-freezing and post freezing (Table 2).
Table 1 Bacteriology results for 783 paired samples pre-freezing and post freezing and respective P values according to a McNemar test for paired samples

Table 2 Bacteriology results before and after freezing, number of milk samples submitted for cell count determination pre-freezing, geometric mean individual quarter somatic cell count (SCC), geometric mean log-transformed SCC and P values for the difference between log10SCC of samples yielding the same bacteriology result pre-freezing and post freezing
Results of the PCR testing of the 162 quarter milk samples are displayed in Table 3. Out of the 51 no-growth samples, no pathogens could be detected by the PCR assay in 26 samples. Overall, 1 or 2 pathogens were detected in 47% of the no-growth samples (23/49).
Table 3 Microorganisms detected with a real-time PCR assay on milk samples with an individual quarter somatic cell count (SCC) >500 000 cells/ml that with standard bacteriology were culture-negative or led to the isolation of Corynebacterium bovis as a single isolate or to non-aureus staphylococci (NAS) as a single isolate

† Arcanobacterium pyogenes/Peptoniphilus indolicus
Out of the 79 samples from which C. bovis had been isolated in conventional culturing, 3 or more pathogens were detected in 5 samples. Excluding these samples, the presence of C. bovis was confirmed in 59 samples, in 41 samples as a single pathogen and in 18 samples with another pathogen. Overall, detection of a pathogen other than C. bovis was made in 35% of the samples that with conventional bacteriology yielded C. bovis (26/74).
Out of the 32 samples from which NAS had been isolated in conventional culturing, 3 or more pathogens were detected in 2 samples. Excluding these samples, the presence of NAS was confirmed in 28 samples, in 21 samples as a single pathogen and in 7 samples with another pathogen. On a sample where Staph. intermedius was identified with conventional bacteriology, Staph. aureus was detected with the PCR assay. Overall, detection of bacteria other than NAS was possible in 25% of the NAS samples (7/28).
The number of genome copies obtained for each bacterial species detected is displayed in Table 4. The mean number of copies of NAS and of C. bovis detected in the samples where these had been found with conventional bacteriology was significantly higher (P<0·05) than in the samples where these had not been found.
Table 4 Mean number of genome copies per ml of milk, mean cycle threshold values (Ct) and respective sd for bacterial species identified with a real-time PCR-based kit

† For these 3 pathogens, the values displayed are the ones obtained for single identifications and not true means
Discussion
Study 1
Our results show that freezing milk samples before performing bacteriology with the conventional culture method did not lead to an increased detection of mastitis pathogens.
Many large-scale studies mention that over 50% of milk samples are culture-negative or yield minor mastitis pathogens. Makovec & Ruegg (Reference Makovec and Ruegg2003) reporting on the results of 83 650 samples submitted from cases of clinical and subclinical mastitis as well as samples obtained for mastitis surveillance programmes, found 46·6% of samples with either no growth or isolation of C. bovis or NAS. Wilson et al. (Reference Wilson, Gonzalez and Das1997), reporting on bacteriology results of 105 083 composite milk samples submitted from every animal on most farms of the study, found 66·0% of samples with one of the three aforementioned results and Pitkälä et al. (Reference Pitkälä, Haveri, Pyörälä, Myllys and Honkanen-Buzalski2004), reporting on 12 661 quarter samples representing the totality of the animals on each farm, found 90·5% of samples were culture-negative or yielded C. bovis or NAS. Many of the samples from which C. bovis and NAS are isolated, may correspond to true cases of IMI due to these agents, because it is well established that they cause mastitis (Honkanen-Buzalski & Bramley, Reference Honkanen-Buzalski and Bramley1984; Simojoki et al. Reference Simojoki, Orro, Taponen and Pyörälä2009). However, there is evidence that C. bovis often leads to teat canal colonization rather than to true IMI (Black et al. Reference Black, Marshall and Bourland1972; Honkanen-Buzalski & Bramley, Reference Honkanen-Buzalski and Bramley1984). Therefore, frequent isolation of these minor pathogens might simply attest they are easier to find than major mastitis pathogens truly responsible for the deleterious effects of IMI, but more difficult to detect through conventional techniques. The possibility that minor pathogens outnumber major pathogens in the samples could also be part of the problem, potentially occurring with Staph. aureus owing to its intermittent shedding pattern (Sears et al. Reference Sears, Smith, English, Herer and Gonzalez1990).
It is now well established that Staph. aureus and Str. uberis are capable of internalizing in mammary epithelial cells and neutrophils (Brouillete et al. Reference Brouillette, Grondin, Shkreta, Lacasse and Talbot2003; Tamilselvam et al. Reference Tamilselvam, Almeida, Dunlap and Oliver2006), and it has been hypothesized that freezing milk samples could lead to the lysis of mammary somatic cells and to higher isolation rates of intracellular pathogens (Dinnsmore et al. Reference Dinsmore, English, Gonzalez and Sears1992). In our study, freezing milk samples at −20°C for 24 h did not lead to a higher rate of isolation of major pathogens. In fact, there was a net loss of microorganisms, which was significant for most groups of bacteria.
Out of the 783 pairs of samples analysed, a significant reduction in the proportion of samples yielding the following groups of bacteria was observed after freezing: Gram-positive, catalase-positive cocci (27% decrease in proportion); Gram-negative baccilli (70% decrease) and Gram-positive bacilli (19% decrease). Only for the Gram-positive, catalase-negative group was a significant reduction not recorded (9% decrease). Conversely, the proportion of samples that were culture-negative after freezing showed a significant increase (137% increase). There were 12 samples where no growth of staphylococci was recorded prior to freezing, but where their growth was recorded after freezing. None of these was identified as Staph. aureus by use of a biochemical identification system, which like other identifications systems, has its limitations (Sampimon et al. Reference Sampimon, Zadoks, De Vliegher, Supré, Haesebrouck, Barkema, Sol and Lam2009). These results disagree with earlier reports. Schukken et al. (1989) reported a decrease in Esch. coli isolation and an increase in NAS isolation; however, these authors froze their samples for increased lengths of time (4–16 weeks) and observed that the longer the samples were frozen, the higher was the number of cultures positive for NAS. Dinnsmore et al. (Reference Dinsmore, English, Gonzalez and Sears1992) also reported a decrease in Esch. coli isolation and an increase in the number of NAS after freezing samples overnight at −20°C, even though their results were not statistically significant. The work of Sol et al. (Reference Sol, Sampimon, Hartman and Barkema2002) is not directly comparable with the current study because although the authors froze their samples for 24 h at −20°C, they also incubated them for 24 h at 37°C.
An identification of the isolated microorganisms to species level was not performed in the first study, but rather microorganisms were gathered in groups according to their Gram stain and catalase characteristics. Therefore, Gram-positive catalase-positive cocci would include staphylococci but also other genera with the same characteristics (Micrococcus spp. and Kocuria spp.). Similarly Gram-positive catalase-negative cocci would include not only streptococci, but also Enterococcus spp. and Aerococcus spp. This approach was chosen because the focus of the study was not on individual species but rather on morphologically similar groups of pathogens. It would appear, however, judging by the geometric mean SCC for each group, that the isolated microorganisms were not contaminants but were being deleterious in terms of udder health. The significant difference observed between samples that led to no growth pre-freezing and post freezing, could suggest that many of the samples that post freezing showed no growth actually had an IMI, and therefore the viability of the pathogens responsible for those infections could have been affected by the process of freezing.
The results of our study showed that simply freezing milk samples overnight did not increase the detection of mastitis pathogens in milk samples and therefore this procedure should not be recommended. In fact, the differences observed could lead to questioning of the usefulness of frozen milk samples for the diagnosis of IMI, even though it can be argued that the numbers of viable bacteria in samples with clinical or subclinical mastitis would be different from the numbers present in some of the samples used in the study. Several anecdotal reports mention that glycerol can be incorporated into milk samples to protect against the effects of freezing on milk samples. There have been studies on the use of glycerol to protect against the negative effects of freezing on the viability of bacteria in other types of samples (Ternent et al. Reference Ternent, Innocent, Filshie, Taylor, Steele, McEwen, Reilly, Gunn, Reid and Mellor2004) but to the best of our knowledge the effect of its use on milk samples has not been scientifically tested.
Study 2
We used a real-time PCR test to evaluate whether DNA from mastitis pathogens could be detected in milk samples with a high quarter SCC. After exclusion of samples where PCR detected the presence of DNA from 3 or more pathogens, we analysed the results of a total of 151 samples: 49 samples that were culture-negative with standard bacteriology, 74 samples from which C. bovis was isolated as a single microorganism and 28 samples from which NAS was isolated as a single microorganism with standard bacteriology. The decision to exclude samples with 3 or more pathogens from the analysis was made based on the recommendation of considering a sample as contaminated when 3 or more types of colonies are present at >500 cfu/ml when performing conventional bacteriology (IDF, 1999; NMC, 1999). Applying the same principle when utilizing a PCR test is debatable, especially when quantitative data are available. Still it was decided to apply the same principle as a precautionary measure. Bacterial DNA was detected in 47% of the no-growth samples, a value close to the 43% recently obtained by Taponen et al. (Reference Taponen, Salmiviki, Simojoki, Koskinen and Pyörälä2009) for clinical mastitis samples. For samples from which C. bovis or NAS were isolated as single isolates, 35% and 25% of samples, respectively, were positive for another pathogen. Out of the 151 samples, several major pathogens were found: Esch. coli was detected in 20 samples, Str. uberis in 5 samples, A. pyogenes/P. indolicus in 2 samples, Str. dysgalactiae in 1 sample. Staph. aureus was detected in only 1 sample, which conventional bacteriology had identified as Staph. intermedius. The analytical accuracy of the real-time PCR test has been shown to be 100% in identification of Staph. aureus and NAS, across a large collection of strains (Koskinen et al. Reference Koskinen, Holopainen, Pyörälä, Bredbacka, Pitkälä, Barkema, Bexiga, Roberson, Sølverød, Piccinini, Kelton, Lehmusto, Niskala and Salmikivi2009). Hence, it is likely that this single case observed in the current study was due to a biochemical test misidentification, which is not uncommon (Sampimon et al. Reference Sampimon, Zadoks, De Vliegher, Supré, Haesebrouck, Barkema, Sol and Lam2009).
The number of genome copies quantified in our study was generally lower than the numbers found by Taponen et al. (Reference Taponen, Salmiviki, Simojoki, Koskinen and Pyörälä2009), which may be a reflection of the fact that their study focused on samples from clinical mastitis cases, whereas our focused on subclinical cases. The significantly higher number of copies of NAS and of C. bovis detected in the samples where these pathogens had been found with conventional bacteriology supports the idea that the number of genome copies detected influences the likelihood of isolating mastitis pathogens with conventional bacteriology. As the test is quantitative, it is possible to identify the bacterial species present in higher numbers, which is potentially useful information when determining the most relevant bacterial species in terms of pathogenicity.
Use of this real-time PCR technique can thus help in the detection of mastitis pathogens in milk samples with high SCC yielding minor pathogens or that are culture-negative. This assay can be used on frozen or bronopol-preserved samples, so culture-negative samples or samples leading to the growth of a minor pathogen can be submitted for further diagnosis when bacteriology is not informative enough.
In Portugal, where the samples were collected, as well as in other parts of the world, detection of Mycoplasma bovis is important, as it has been demonstrated to have a large impact on udder health on some farms (L Pinho, personal communication). However, the version of the PCR assay used at the time of the study did not allow DNA from this pathogen to be detected.
Bacteriology results for milk samples with over 50% of samples either culture-negative or leading to the isolation of a minor pathogen are worrying whenever a targeted approach to quarters with mastitis is being used, because significant resources are being used with little information being gained for practitioners or milk-quality advisors. Chronically high-SCC milk samples that are culture-negative or from which only a minor pathogen is isolated may correspond to false negative results, i.e. negative cultures in cases where there is actually an IMI and false positive results, i.e. detection of minor pathogens not truly responsible for mastitis. On the basis of the findings from the two studies reported here, there seems to be limited benefit in freezing milk samples to enhance detection of pathogens as there was a significant reduction in the number of bacteria isolated in several of the groups tested. However, use of this PCR assay may help to reduce the number of false negatives as it detected bacterial DNA of potential mastitis pathogens in samples that were culture-negative or from which only minor pathogens were isolated with conventional bacteriology.
R Bexiga holds grant number SFRH/BD/36759/2007 from the Foundation for Science and Technology. The financial support of the Interdisciplinary Centre of Research in Animal Health (CIISA/FMV) is gratefully acknowledged. The authors also acknowledge the access given to farms by farmers and practitioners.






