Introduction
The generic term ‘folate’ represents all forms of folate including synthesized fully-oxidized ‘folic acid’ and the polylglutamates naturally present in foods. It is an important B vitamin involved in essential functions of cell metabolism such as DNA replication, repair, methylation and synthesis of nucleotides (Iyer & Tomar, Reference Iyer and Tomar2009). The daily recommended intake (DRI) as approved in European Union (EU) is 400 μg/d for adults (FAO/WHO 2002; IOM 2004). Since folate deficiency has been associated with the incidence of neural tube defects during the embryo development (Daly et al. Reference Daly, Kirke, Molloy, Weir and Scott1995), higher intake (600 μg/d) is recommended for women before and during pregnancy. Also as folic acid is also important for lactating women so to fulfil the demands of breastfeeding, the Recommended Daily Allowance (RDA) for lactating women in the United States is 500 μg dietary folate equivalents (DFE) per day (Food and Nutrition Board, 1998; Yates et al. Reference Yates, Schlicker and Suitor1998). Folate deficiency has also been implicated in a wide variety of disorders from Alzheimer's to coronary heart diseases, neural tube defects, increased risk of breast and colorectal cancer (LeBlanc et al. Reference LeBlanc, Giori, Smid, Hugenholtz and Sesma2007, Reference LeBlanc, Giori, Smid, Hugenholtz, Sesma and Mendez-Vilas2011; Tomar & Iyer, Reference Tomar, Iyer, Tomar, Singh, Singh, Arora and Singh2011). Owing to the health benefits associated with increased folate intakes many countries now have mandatory folate enrichment programmes in place. Lately, a number of studies have shown that unlike natural folate, high intakes of folic acid, the chemically synthesized form (tolerable upper intake level, 1000 μg/d), can cause adverse health effects such as the masking of the early haematological manifestations of vitamin B12 deficiency, leukaemia, arthritis, colon cancer and ectopic pregnancies (Lucock & Yates, Reference Lucock and Yates2005; Sweeney et al. Reference Sweeney, McPartlin and Scott2007; Wright et al. Reference Wright, Dainty and Finglas2007). Therefore, naturally produced folates seem to be more rationale for fortification purposes and it requires the availability of rapid and sensitive analytical methods to detect and differentiate folate and folic acid level in foods.
Generally the multiplicity of forms and low levels in foods makes quantitative analysis of folate a difficult task (Arcot & Shrestha, Reference Arcot and Shrestha2005; Iyer & Tomar, Reference Iyer and Tomar2009). Numerous reports have been published (Forssen et al. Reference Forssen, Jagerstad, Wigertz and Witthoft2000; LeBlanc et al. Reference LeBlanc, Giori, Smid, Hugenholtz and Sesma2007; Indyk, Reference Indyk2010; Tomar & Iyer, Reference Tomar, Iyer, Tomar, Singh, Singh, Arora and Singh2011) regarding food folate content and the various methods of folate determination including: biological, microbiological, bio-specific procedures (radio binding or immuno assay), electrochemical, spectrophotometric or chromatographic methods such as gel or high-pressure-liquid chromatography (HPLC). Microbiological assay (MA) serves as the traditional folate analysis method and continues to be the only food folate method enjoying official status by American Association of Analytical Chemists (AOAC). It relies on the turbidimetric bacterial growth of Lactobacillus rhamnosus (ATCC 7469) which is used as an assay organism (Tamura et al. Reference Tamura, Mizuno, Johnson and Jacob1997; Shrestha et al. Reference Shrestha, Arcot and Paterson2000). It responds to most native folates, although the response decreases as the number of glutamyl residues linked to the pteroyl group increases. In order to measure all the polyglutamated forms, these must be enzymatically deconjugated prior to analysis. Once hydrolyzed these folates can support the growth of Lb. rhamnosus or be quantified by other methods. Treatment with conjugase alone is not very effective; thereby the use of additional enzymes, proteolytic or amylolytic, has shown to liberate folate from foods (Rader et al. Reference Rader, Weaver and Angyal1998; Johnston et al. Reference Johnston, Lofgren and Tamura2002).
The limitation of Lb. rhamnosus and other assay organisms in differentiating folate derivatives in the folate extract has prompted the use of chromatographic techniques. These techniques involve two distinct steps: separation and purification of deconjugated extract followed by detection and quantification of eluted monoglutamates (Arcot & Shrestha, Reference Arcot and Shrestha2005). This method has been applied to separate and detect the individual forms of folates, especially 5-CH3-H4-folate; H4-folate and synthetic folate mostly involving fluorescence detection (Eitenmiller & Landen, Reference Eitenmiller, Landen, Eitenmiller and Landen1999; Kariluoto et al. Reference Kariluoto, Vahteristo and Piironen2001; Jastrebova et al. Reference Jastrebova, Witthöft, Grahn, Svensson and Jägerstad2003). However, the problems associated with HPLC are the rigorous sample clean-up procedure prior to final injection, the complex sample extraction and purification procedures which cause loss of sensitive derivatives that lead to the lower folate value. Hence, no HPLC method per se has yet been approved for food analysis in general (Finglas et al. Reference Finglas, Wigertz, Vahteristo, Witthoft, Southon and de Froidmont-Gortz1999) and a limited number of studies have been published on folate derivatives in milk and dairy products based on HPLC analyses (Wigertz & Jagerstad, Reference Wigertz and Jagerstad1995; Vahteristo et al. Reference Vahteristo, Ollilainen and Varo1997).
Immunological methods such as Enzyme Linked Immuno Sorbent Assay (ELISA) for folate and folic acid estimation require specific antibodies. ELISA performed on a micro-titration plate format for a specific folate form is fast proving to be well-suited to routine food analysis (Finglas & Morgan, Reference Finglas and Morgan1994; Ruggeri et al. Reference Ruggeri, Aguzzi and Carnovale1999; Arcot et al. Reference Arcot, Shrestha and Gusanov2002; Indyk, Reference Indyk2010). In spite of the speed and convenience of these assays, their application to food analysis is limited due to varying affinity (cross reactivity) for different forms of folate. The mention of these methods has been traced to a limited number of reports on folate concentrations in milk (Wigertz & Jagerstad, Reference Wigertz and Jagerstad1995).
In view of the adverse health effects of high doses of synthetic folate and availability of only a few reports on the determination of folate/folic acid fortification level in food products, this study was aimed at the concurrent determination of folate forms and their levels in milk by microbiological assay with Lb. rhamnosus as assay organism, competitive binding rapid ELISA kit (RIDASCREEN®) and HPLC.
Materials and methods
Culture, Reagents and Instrumentation
Standard culture of Lb. rhamnosus MTCC 1408, (ATCC7469/DSM20021) the assay organism in folate estimation for MA, was obtained from Microbial Type Culture Collection (MTCC), Chandigarh, India. Alpha-amylase (Cat. No. A6211), protease (Cat. No. P6911), human serum (Cat. No. H4522) as a conjugase source and standard folic acid (Cat. No. F7876) were procured from, Sigma, St. Louis, Missouri, USA. Folic acid casei medium (Cat. No. M543, Hi Media Ltd, Mumbai, India was used as the basal medium in the assay. Chromatographic grade Acetonitrile was purchased from Merck (HPLC grade, Lichrosolv®, 60003010001730, Merck Specialties Pvt. Ltd., India) and other reagents were all of analytical grade. The RIDASCREEN® FAST Folsaure (folic acid) kit containing ELISA microtitre plates coated with anti-folic acid antibodies was procured from R-Biopharm AG, Darmstadt, Germany.
Collection and pre-treatment of milk samples
One litre samples of fresh raw pooled cow, buffalo, sheep and goat milk samples from a set of at least 5 different animals of each breed were collected from National Dairy Research Institute's cattle yard and other places near by in and around Karnal city in pre-cleaned and sterilized bottles while Yak milk sample was procured from pooled milk of 5 animals in the same milking season from National Research Centre on Yak, Dirang, Arunachal Pradesh, India. Sample collection was repeated 6 times on different days during one milking season for replication of the results. The bottles containing samples were immediately secured in an ice-box to maintain temperature at 4±0·5 °C during transport. These samples were brought to the laboratory post collection and the sample bottles were immediately refrigerated. The pooled milk samples were allowed to cool to a stable temperature of 4 °C.
Sample preparation
The sample preparation was done as per the method described by Keagy (Reference Keagy, Augustin, Klein, Becker and Venugopal1985), with additional tri-enzyme treatment (Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009; Tomar et al. Reference Tomar, Srivatsa, Iyer and Singh2009). Tri-enzyme treatment was performed by using α-amylase, protease and human plasma conjugase. The order and time of incubation for different enzyme treatments was standardized to 4 h for α-amylase, 6 h for protease and 12–16 h for conjugase treatment at 37 °C. An enzyme blank was also made with distilled water followed by enzyme treatment. This sample extract was used for folate estimation by all the three methods viz. MA, HPLC, ELISA.
Estimation by microbiological assay
Folate contents were estimated by MA with the use of Lb. rhamnosus as assay organism (Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009). For standard curve, pure standard folic acid of 0·1 ng/ml concentration was prepared. In triplicates 0·6, 1·2, 2·5 ml of standard folic acid (0·1 ng/ml) solution and sample extract was taken in test tubes (Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009). To all tubes sufficient distilled water was added to bring the volume in each to 2·5 ml followed by addition of equal volume of 2X basal medium (Folic acid casei medium) was added. A blank was also run having no standard or sample solutions with the remaining steps same as above. Aseptically, Lb. rhamnosus culture inoculum was added to all tubes including standard and blank and incubated for 18–24 h in well stirred water bath maintained at 37 °C. After incubation the contents of the tubes were shaken with a vortex mixer and the percent transmittance was read set at 550 nm by spectrophotometer (Genova, Jenway Ltd., Felsted, Dunmow, Essex CM6 3LB, UK).
Standard curve was prepared by plotting % transmittance values against logarithmic values of folic acid content in respective dilutions (Keagy, Reference Keagy, Augustin, Klein, Becker and Venugopal1985; Tamura et al. Reference Tamura, Mizuno, Johnson and Jacob1997; Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009). This standard curve was further used for the estimation of folic acid content in assay samples by interpolating% transmittance values over this standard curve. The interpolated values of ng per tube were divided by the volume of the sample extract in that tube to obtain the ng folic acid per ml sample extract. The folic acid content of the sample was calculated by Eq. (1)
Further, a known concentration of the folic acid standard was added to the milk samples to raise the level of folic acid by 5 and 10 μg/l respectively and the recovery was calculated from difference in estimated values between milk with and without added folic acid.
Estimation by ELISA
A competitive binding rapid ELISA kit (RIDASCREEN®) containing the ELISA microtitre plates coated with anti-folic acid antibodies was used for the quantitative estimation of folic acid of milk . Each milk sample extract was used in ELISA plate in triplicate (4 wells for each sample extract). The procedure used was as recommended by the manufacturer's instructions handbook.
A volume of 50 μl milk or sample extract (pre-treated) was dispensed into the wells of the ELISA plate. An equal volume of folic acid-peroxidase conjugate supplied with the kit was then added to the wells. The contents were gently mixed by manually shaking the ELISA plate making sure there was no overflow or mixing of the contents of the wells. The plate was covered with aluminium foil and incubated for 15 min at room temperature. The contents of the wells were poured off post incubation and the wells were washed thrice with distilled or deionized water. Then 100 μl substrate (urea peroxide) was added to each well using a multi-channel micropipette. The plate was incubated at room temperature (20°–25 °C) for 10 min in the dark. The chromogenic reaction was stopped by the addition of 100 μl stop reagent (1N H2SO4, contained in the kit) to each well using the multi-channel dispenser. The plate was gently shaken and the absorbance recorded in a Microscan ELISA plate reader (Model No. M55605A), Electronic Corporation of India ltd., Hyderabad, India, at 450 nm within 10 min.
A standard curve for folic acid was prepared by using standards solutions (ready to use) supplied with the kit, in the range of 0, 1, 2, 5, 10, 25 μg/l in duplicates. The percent absorbance at each concentration of folic acid was calculated using the Eq. (2)
where, Abs.=Absorbance
The zero standard is thus made equal to 100% and the absorbance values are quoted in percentages. The standard curve was plotted between the log A/A o (where A=Absorbance of the Standard Solution and A o=Absorbance of the Zero Standard)×100 on the Y-axis against the standard folic acid concentration (μg/l) on the X axis. The correlation coefficient was calculated using computer software MS-Excel 2003 from Microsoft Corp. The folic acid concentration (μg/l) corresponding to the extinction of each sample was read from the calibration curve.
Further, a known concentration of the folic acid standard was added to the milk samples to raise the level of folic acid by 5 and 10 μg/l respectively and the recovery was calculated from difference in estimated values between milk with and without added folic acid.
Estimation by HPLC
The HPLC system (Shimadzu Prominence LC-20AD Quaternary Gradient HPLC System, Shimadzu Corporations, Kyoto, Japan) equipped with a LC20AD pump, DGU20A5 on line degasser, 7725 Rheodyne manual injector with 20 μl loop, SPD-20A UV detector, CTO20A column oven, Phenomenex Luna C18 column (5 μm particle size, 100 Å pore size, 4·6×250 mm) and CBM20A system controller connected to a desktop PC with Chromatography CLASS VP™ software was used. The predominant native folate form in dairy foods, 5-CH3-H4-folate, is detectable by fluorescence rather than UV detector (Arcot & Shrestha, Reference Arcot and Shrestha2005) while folic acid is primarily detected by UV detector. A stock solution of folic acid (concentration=1·005 mg/ml) was prepared in 0·1 m phosphate buffer, pH 6·5, which was further diluted in the range of 1, 10, 100, 500, 1000 μg/ml for determination of retention time (RT) and relationship between folic acid concentration and peak area.
Twenty microlitre standard folic acid or extract from milk samples was loaded after passing through 0·22 μm nylon filter. The column and UV detector cell temperatures were maintained at 40 °C. Column equilibration and gradient elution were achieved with the mobile-phase consisting of acetate buffer (acetic acid 0·166 mol/l; potassium hydroxide 0·01 mol/l; pH 2·8) and acetonitrile filtered through 0·45 μm nylon filter and degassed by sonication before use. The HPLC system was conditioned with the mobile phase until the triplicate injections of standard solution showed identities of retention time ((RT) and peak area. Detection was carried out in the ultra-violet region, at 290 nm at a flow rate of 1 ml/min, using 10% acetonitrile plus 90% buffer aqueous phase in the beginning, changing to 24% acetonitrile plus 76% buffer aqueous phase after 12 min.
Further folic acid was estimated in skim milk with and without added (5 μg/l) folic acid and the recovery of added folic acid was calculated from difference in estimated values between skim milk with and without added folic acid.
Statistical analysis
Each determination was carried out in triplicate. Results were expressed as mean+sd. Calculation of average concentration, standard error (se), standard deviation and coefficient of variation was performed by the computer Systat software (Version 6, Systat Software Inc., Chicago, IL).
Results
Earlier also in our laboratory the applicability of MA had been evaluated for the estimation of total folic acid in milks from different Indian milk species (cow, buffalo, sheep and goat) with an additional tri-enzyme extraction method using Lb. rhamnosus as an assay organism (Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009) and the results showed significant correlation with the published data.
In the present study the standard curve for folic acid by the MA was found to have a R 2 value of 0·99 (Fig. 1a) indicating a perfect linear relationship. The average values of folic acid in cow, buffalo, sheep, goat and yak milk were found to be 44, 60, 56, 10 and 50 μg/l, respectively. Recovery rate of 98·3% with an accuracy (R.E.%=1·0) and repeatability (CV%=2·0–5·6) was observed which validated the efficiency of MA.

Fig. 1. Standard graph of folic acid by different analytical techniques: (a) Microbiological assay, (b) Enzyme Linked Immunosorbant assay and (c) High pressure Liquid Chromatography.
The standard curve for ELISA (Fig. 1b) was found to be linear, as per manufacturer's recommendations (R 2=0·992). The assayed level of folic acid in different milks (Table 1) was found to be in agreement with the values reported earlier using MA or other assay techniques by several workers (Kon & Cowie, Reference Kon and Cowie1961; Wigertz & Jagerstad, Reference Wigertz and Jagerstad1995; Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009). A good accuracy (R.E.%=4·3), repeatability (CV%=3·0–14·5), recovery rate (99·8%) in just 30 min experimentation time indicated a quantitative estimation of folic acid thereby validating the reliability of ELISA.
Table 1. Folate content* of milk of different Indian milch species by Microbiological Assay, High Performance Liquid Chromatography and Enzyme Linked Immno Sorbant Assay
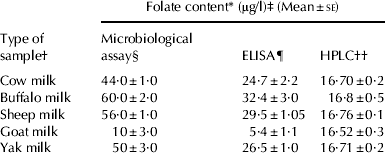
† Pooled milk samples of animals n=5
‡ Average of six samples collected on different days during one milking season
§ Microbiological assay as approved by AOAC (Association of Analytical Chemist) using Lactobacillus rhamnosus MTCC 1408
¶ ELISA(Enzyme Linked Immuno Sorbent Assay) by the RIDASCREEN®FAST Folic Acid kit
†† HPLC (High Pressure Liquid Chromatography) using C18 column with acetonitrile and potassium acetate buffer (pH 2·8) by gradient elution at UV(Ultraviolet) region (290 nm)
* Microbiological assay gives the total folate value of the milk while HPLC and ELISA methods only provide value for folic acid
The HPLC chromatogram depicts the response in volts on Y axis and retention time in minutes on X axis. The Fig. 2 shows the standard folic acid peak at a RT of 15·8±02 with the solvent peak at 5·8±0·1. The standard curve (Fig. 1c) obtained by HPLC showed a linear relationship between peak area and concentration (R 2=0·99). The chromatogram of milk samples yielded several peaks corresponding to various compounds present in tri-enzyme treated sample extracts besides folic acid. Figure 3 shows the significant difference in the peak area in the HPLC profile of the plain skim milk sample and the sample spiked with a known concentration (5 μg/l) of folic acid. Thus, the recovery of 99·4% with an accuracy of 4·3 (R.E.%), and repeatability of 4·0–13·2 (CV%) indicated extraction of folic acid from milk with the above buffer system to be quantitative.

Fig. 2. Magnified high performance liquid chromatogram of Standard Folic acid solution (10 μg/ml): The arrow indicates peak of folic acid at retention time (RT) of 15·75 with a peak area (PA) of 1247624.

Fig. 3. Magnified high performance liquid chromatogram of: (a) Sample extract of skim milk without addition of folic acid (RT=16·06; PA=5263). (b) Sample extract of skim milk with 5 μg/l folic acid added (RT=15·82; PA=6096).
Discussion
A concurrent determination of folate versus folic acid in milk by MA, ELISA and HPLC was done in the present study for detection of the folate form and its level. Food composition tables and review papers based on MA reports total folate values for milk in the range of 5–7 μg/100 g (Forssen et al. Reference Forssen, Jagerstad, Wigertz and Witthoft2000; Arcot & Shrestha, Reference Arcot and Shrestha2005). In our first ever study (Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009) on estimation of total folate of milks of different milk species by MA, buffalo milk was found to contain the highest level of folate compared with that of cow, sheep or goat milk which is in general agreement with values reported earlier in literature (Kon & Cowie, Reference Kon and Cowie1961; Vahteristo et al. Reference Vahteristo, Ollilainen and Varo1997; Clark & Sherbon, Reference Clark and Sherbon2000; Arcot and Shrestha, Reference Arcot and Shrestha2005; Branigan, Reference Branigan2008; Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009).
Although the folate estimation by MA with the additional tri-enzyme extraction (Hyun & Tamura, Reference Hyun and Tamura2005) is somewhat time consuming it has been found to be highly sensitive, versatile, reproducible and provides significant quantitative data on the total folate content of the sample analysed. Moreover it requires low equipment set up costs, and can measure mono- to polyglutamates even at nanogram level. MA with the tri-enzyme method leads to complete hydrolysis of polyglumates to simpler monomeric form (Pffeiffer et al. Reference Pffeiffer, Rogers and Gregory1997; Forssen et al. Reference Forssen, Jagerstad, Wigertz and Witthoft2000; Arcot & Shrestha, Reference Arcot and Shrestha2005) and which then proportionally support the growth of the assay organism Lb. rhamnosus, which shows similar response to all folate isomers and thus gives a total folate value of the product. The results obtained from this study reveals that the values obtained by microbiological assay with the tri-enzyme method correspond not only to folic acid but total folate content of samples. Thus MA led to a reproducible determination of total folate content of milk samples which are in significant correlation with published data (Kon & Cowie, Reference Kon and Cowie1961; Vahteristo et al. Reference Vahteristo, Ollilainen and Varo1997, Clark & Sherbon, Reference Clark and Sherbon2000; Arcot and Shrestha, Reference Arcot and Shrestha2005; Branigan, Reference Branigan2008; Iyer et al. Reference Iyer, Tomar, Singh and Sharma2009).
ELISA is an alternative to MA for rapid, sensitive and quantitative estimation of folic acid (Finglas & Morgan, Reference Finglas and Morgan1994; Ruggeri et al. Reference Ruggeri, Aguzzi and Carnovale1999). ELISA performed on micro-titration plate format is proving particularly well suited to food analysis (Andrew-Kabilafkas, Reference Andrew-Kabilafkas2001; Arcot et al. Reference Arcot, Shrestha and Gusanov2002; Arcot & Shrestha, Reference Arcot and Shrestha2005; Hoegger et al. Reference Hoegger, Morier, Vollet, Heini, Reymond and Rossier2007). Though these procedures are highly specific yet show cross reactivity towards other forms of folate besides the targeted folate derivative. It is clear from the study that kit assay offered potential specificity and sensitivity for the measurement of food folates though less than MA as its specificity apparently was limited to folic acid and H2 folic acid. Besides, as the range of folic acid standards used was very large, e.g. 1, 2, 5, 10 and 25 μg/l which make them less sensitive than the microbiological method in which a much narrower standard range of 0·2–1·0 μg/l was used. As ELISA exhibited a lower response to other folate derivatives, it yielded lower folate content comprising folic acid and dihydrofolate.
Various workers have estimated folate and folic acid in milk using HPLC (Wigertz & Jagerstad, Reference Wigertz and Jagerstad1995; Finglas et al. Reference Finglas, Wigertz, Vahteristo, Witthoft, Southon and de Froidmont-Gortz1999; Verwei et al. Reference Verwei, Arkbage, Havenaar, Van den Berg, Witthöft and Schaafsma2003). HPLC is highly specific, and so any peak activity emerging from the analytical column represents specific folate derivative (Pffeiffer et al. Reference Pffeiffer, Rogers and Gregory1997; Bagley & Selhub, Reference Bagley and Selhub2000; Poo-Prieto et al. Reference Poo-Prieto, Haytowitz, Holden, Rogers, Choumenkovitch, Jacques and Selhub2006).Our study describes the use of the reverse phase HPLC method with UV detector for quantitative estimation of folic acid in milk. Calibration curves show a linear response for folic acid in a concentration range of 0·1–1000 μg/ml at UV detection (Fig. 1c). The average values of folic acid in cow, buffalo, sheep and yak milk were found to be somewhat similar, as this solvent system with UV detector detects only folic acid due to its high sensitivity and specificity without any cross reactivity as in case of ELISA (Arcot & Shrestha, Reference Arcot and Shrestha2005; LeBlanc et al. Reference LeBlanc, Giori, Smid, Hugenholtz and Sesma2007).
The absolute values from HPLC were lower than that of MA, probably because of the limitation of the UV detector to identify other folate derivatives. The limitation of Lb. rhamnosus and other assay organism in differentiating folate derivatives in the folate extract has prompted the use of chromatographic techniques. Results of present study are in agreement with the findings of European inter-laboratory report (Kariluoto et al. Reference Kariluoto, Vahteristo and Piironen2001; Ginting & Arcot, Reference Ginting and Arcot2004; Arcot & Shrestha, Reference Arcot and Shrestha2005) which also found HPLC results much lower (30–40%) than microbiological results (Horne et al. Reference Horne, Briggs and Wagner1981). Therefore this suggests that HPLC with UV detector measures folic acid alone while MA measures total folate and thus the difference represents the amount of folate derivatives except folic acid.
Further, the folic acid assay which is more challenging than many other micronutrients owing to its sensitivity to physical environments and presence of various forms warrants a good knowledge of folate chemistry and appropriate extraction and detection techniques. The extraction techniques may differ with the type, nature, state, origin of foods as well as with the methods of detection. Among the various methods MA, HPLC and ELISA, each has its pros and cons.
In the present study the of folate estimation in milk by HPLC, ELISA and microbiological assay showed a comparative analysis of the estimated average values of folate by all the three methods. In agreement with earlier studies our results also suggest that among all three methods, HPLC being a more sensitive method can quantify the different folate forms with a superior specificity instantaneously in contrast to other conventional detection methods. On the other hand MA is labour-intensive, tedious and time-consuming but is an efficient and reproducible method which gives the total folate content of the sample analysed. Therefore, MA being a premium method of total folate estimation, has the potential to be employed as a dietary folate estimation assay especially in nutraceutical preparations whilst HPLC seems more efficient and feasible for quantitative determination of recommended folic acid level in fortified products.






