The principal role of chymosin or other enzymes in rennets is to destabilize the casein micelle and thus cause the gelation of milk, but a small amount of coagulant is retained in the cheese curds and contributes to the ripening of many cheese varieties (Visser, Reference Visser1977; Visser & de Groot-Mostert, Reference Visser and de Groot-Mostert1977; O'Keeffe et al. Reference O'Keeffe, Fox and Daly1978; Fox & McSweeney, Reference Fox and McSweeney1996, Reference Fox, McSweeney and Law1997; Lane et al. Reference Lane, Fox, Johnston and McSweeney1997, Sousa et al. Reference Sousa, Ardo and McSweeney2001; Upadhyay et al. Reference Upadhyay, McSweeney, Magboul, Fox, Fox, McSweeney, Cogan and Guinee2004). Some peptides produced by rennet action are small enough to influence cheese flavour directly. Other peptides produced by rennet action are further hydrolysed by microbial proteinases and peptidases to small peptides and amino acids. Peptides produced by the coagulant during ripening contribute at least to background flavour, and perhaps, to bitterness if the activity of the coagulant is excessive (see Lemieux & Simard, Reference Lemieux and Simard1991, Reference Lemieux and Simard1992). Catabolism of amino acids by microbial enzymes and perhaps alterations via chemical mechanisms, leads to a range of sapid compounds that are major contributors to characteristic cheese flavours (Visser et al. Reference Visser, Hup, Exterkate and Stadhouders1983; McSweeney et al. Reference McSweeney, Olson, Fox, Healy and Hojrup1993). Coagulant retained in cheese curd is also associated with softening of cheese texture. It was thought that hydrolysis of the Phe23-Phe24 bond of αs1-casein was responsible solely for the marked softening characteristic of the early stages of the ripening of Cheddar cheese (Visser, Reference Visser1977; Visser & de Groot-Mostert, Reference Visser and de Groot-Mostert1977; O'Keeffe et al. Reference O'Keeffe, Fox and Daly1978). However, recent work (O'Mahony et al. Reference O'Mahony, Lucey and McSweeney2005) suggests that the solubilization of calcium phosphate during the early stages of ripening is of more significance.
The level of coagulant retained in cheese curd depends on several factors related to the milk or the procedure of cheese manufacture, e.g., concentration of the caseins in milk, ratio of different caseins, casein micelle size, ionic strength of milk, heat treatment of milk, extent of acidification of milk prior to addition of rennet, pH at whey drainage, cooking temperature, level of moisture and cheese age (Stadhouders & Hup, Reference Stadhouders and Hup1975; Holmes et al. Reference Holmes, Duersch and Ernstrom1977; Green et al. Reference Green, Glover, Scurlock, Marshall and Hatfield1981; Matheson, Reference Matheson1981; Vassal & Grippon, Reference Vassal and Gripon1984; Creamer et al. Reference Creamer, Lawrence and Gilles1985; Garnot et al. Reference Garnot, Molle and Piot1987; Zoon et al. Reference Zoon, Ansems and Faber1994; Rampilli et al. Reference Rampilli, Raja and Gatti1998). The quantity of rennet retained in cheese curd also varies with the type and ratio of enzymes in the rennet, their stability to pH and temperature during cheesemaking and the influence of pH on their ability to bind to the caseins (Holmes et al. Reference Holmes, Duersch and Ernstrom1977; Creamer et al. Reference Creamer, Lawrence and Gilles1985; Garnot et al. Reference Garnot, Molle and Piot1987).
Because of these factors, the amount of residual rennet activity in cheese differs between varieties. The level of residual rennet activity ranges from almost negligible in high-cooked varieties through about 15% of the original amount of added activity in Gouda cheese to about 50% in high-moisture varieties like Camembert cheese (Ohmiya & Sato, Reference Ohmiya and Sato1972; Stadhouders & Hup, Reference Stadhouders and Hup1975; Garnot et al. Reference Garnot, Molle and Piot1987). Proportionate distribution of chymosin between the aqueous phases of whey and cheese curd during cheesemaking suggests that about 5% of added chymosin activity should be retained in the curd (Dunnewind et al. Reference Dunnewind, de Roos and Geurts1996).
Considering the importance of residual rennet during the cheese ripening, much work has been done to develop methods for the determination of residual rennet in cheese curd (see review by Baer & Collin, Reference Baer and Collin1993). Although several studies have been conducted to quantify residual coagulant in individual cheese varieties, there is a lack of comparative data between varieties. Also, most of the previous work involves quantification methods which are tedious and insensitive. The object of this study was to evaluate the partition of chymosin between whey and curd and the total recovery of the coagulant added to cheesemilk in a miniature Cheddar cheese system, by a rapid and sensitive method using a synthetic chromophoric heptapeptide substrate (Pro-Thr-Glu-Phe-[NO2-Phe]-Arg-Leu) specific for aspartic proteinases (Hurley et al. Reference Hurley, O'Driscoll, Kelly and McSweeney1999). The level of residual coagulant activity was also measured and compared in nine varieties of cheeses, including internal bacterially ripened, surface-mould ripened and surface ripened varieties, using the same method.
Materials and Methods
Cheese manufacture and purchase of market samples
Whole milk (3·85% fat; 3·35% protein) was standardized to a casein: fat ratio of 0·70:1·0, pasteurized at 63°C for 30 min and cooled to 30°C for cheesemaking. Miniature (20 g) Cheddar cheeses were manufactured in triplicate according to the method of Shakeel-Ur-Rehman et al. (Reference Shakeel-Ur-Rehman, McSweeney and Fox1998) using a direct-vat-set (DVS, R604, Christian Hansen, Hoersholm, Denmark) starter and fermentation-produced calf chymosin (Chy-Max, 206 IMCU/ml; Christian Hansen) at a level of 1% (v/v) and 43·5 μl/200 ml milk, respectively; the miniature cheeses were not brined to allow calculation of a mass balance. The cheeses were vacuum-packed and stored at 8°C overnight. The masses of milk, whey and cheese were measured accurately. The samples of milk, whey and cheese were analyzed in triplicate for residual coagulant activity.
Three different samples each of nine varieties of cheeses, Parmigiano Reggiano, Cheddar, Emmental, Gouda, Feta, Mozzarella di Bufala Campana, low-moisture part-skim (LMPS) Mozzarella, Camembert and Port du Salut were purchased from three different retailers and analyzed for moisture content by oven drying (International Dairy Federation (IDF), 1982) and residual coagulant activity.
Measurement of residual coagulant activity
Milk, whey and cheese samples were analyzed for residual coagulant activity according to the method of Hurley et al. (Reference Hurley, O'Driscoll, Kelly and McSweeney1999), except that the pH of the trisodium citrate buffer was adjusted to 6·5. Dispersions of milk, whey or cheese in citrate were incubated with the heptapeptide substrate (Pro-Thr-Glu-Phe-[NO2-Phe]-Arg-Leu) in 0·2 m-sodium formate buffer at 37°C and pH 3·2; the incubation time for milk and whey samples was 3 h. However, the incubation time was varied with cheese variety, from 6 h for high moisture varieties, e.g. Camembert, to 24 h for high-cook varieties, e.g. Parmigiano Reggiano, to ensure that the quantity of the substrate did not become limiting during the reaction. At the end of incubation, hydrolysis of the substrate was terminated by heating at 70°C for 10 min and the samples were centrifuged at 16,000 g for 10 min and then filtered through Titan Syringe filters, RC 0·45 μm (Antech, Waterford, Ireland). The filtrate was analyzed using a reverse-phase (RP)-HPLC. The residual coagulant activity was expressed as peak area units (PAU) h−1 or PAU h−1 mg−1 dry matter.
Statistical Analysis
Analysis of variance on data for the residual coagulant activity of the cheeses was performed by one-way ANOVA using the statistical analysis software SPSS Version 14.0 for Windows XP (SPSS Inc., Chicago, IL, USA). Results presented are the mean of triplicate analyses.
Results and Discussion
In the three batches of miniature Cheddar cheeses prepared, the residual enzyme activity recovered in whey and cheese samples was measured and expressed as % of that added to milk (Table 1). The total recovery of the coagulant added to milk was in the range of ~99 to 111%, of which ~89 to 95% was in the whey and ~10 to 18% in cheese, suggesting that the method for measuring the residual rennet used in this study gives satisfactory results for the distribution of coagulant activity between curd and whey. These results are in general agreement with the values of residual coagulant reported in literature for cheese (Ohmiya & Sato, Reference Ohmiya and Sato1972; Stadhouders & Hup, Reference Stadhouders and Hup1975; Holmes et al. Reference Holmes, Duersch and Ernstrom1977; Winwood, Reference Winwood1989; Dunnewind et al. Reference Dunnewind, de Roos and Geurts1996).
Table 1. Residual chymosin activity measured by reversed phase-HPLC as % of that added to milk recovered in whey and miniature Cheddar cheeses
Values are means±standard deviations for n=3
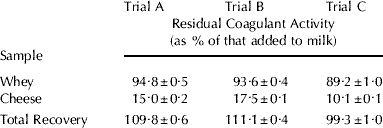
The residual coagulant activity in the market samples of different cheeses varied significantly not only between varieties but also within some varieties of cheese (Table 2). The variations within a cheese variety may be due to slightly different protocols, such as different types of coagulants, different temperatures during manufacture etc., used for the manufacture of different samples of the same variety. The residual coagulant activity in Emmental, Parmigiano Reggiano, LMPS Mozzarella and Mozzarella di Bufala Campana cheeses was lower than the other varieties analyzed (Table 2), presumably because of the high cooking temperature (50–54°C) used during the manufacture of Emmental and Parmigiano Reggiano cheeses and due to the cooking and stretching step in hot water (usually at 70–82°C) during the manufacture of Mozzarella cheese (Robinson & Wilbey, Reference Robinson and Wilbey1998). Stadhouders & Hup (Reference Stadhouders and Hup1975), Singh & Creamer (Reference Singh and Creamer1990), Matheson (Reference Matheson1981), Boudjellab et al. (Reference Boudjellab, Rolet-Repecaud and Collin1994) and Rampilli et al. (Reference Rampilli, Raja and Gatti1998) reported that there is very little or no coagulant activity in high-cooked and pasta-filata varieties of cheeses. There were large differences in the residual coagulant activity between the three samples of Mozzarella di Bufala Campana. A lower stretching temperature might have been used for the manufacture of Sample 1 than other samples.
Table 2. Residual coagulant activity measured by reversed phase-HPLC as peak area units h−1 mg−1 dry matter in different varieties of cheeses
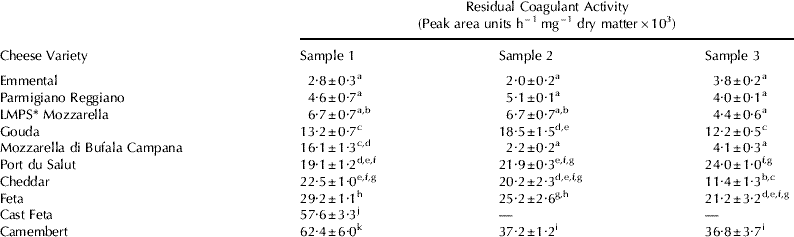
Values are means±standard deviations for n=3
Means with different superscripts are significantly different (Tukey's HSD, P<0·05)
* Low-moisture part-skim mozzarella
The residual coagulant activity in Cheddar, Feta and Port du Salut cheeses was in the range ~20,000 to 30,000 PAU h−1 mg−1 dry matter, except for one sample each of Cheddar and Feta cheese, which had significantly lower and higher activity, respectively, than other samples (Table 2). Singh & Creamer (Reference Singh and Creamer1990) reported that the Cheddar and Feta cheeses contain similar quantities of residual coagulant. The Danish Feta cheese was made from milk concentrated by ultrafiltration (UF) before cheesemaking (Dr. S. Lillevang, Arla Foods, Denmark, personal communication) and had higher residual coagulant activity than that of other Feta-type cheese samples. This can be explained as there is little or no whey drainage during the manufacture of cast Feta-type cheese, and hence all the coagulant added to cheesemilk is retained in the cheese. Hesari et al. (Reference Hesari, Ehsani, Mosavi and McSweeney2007) found higher residual coagulant activity in UF Iranian white cheese compared with traditional white cheese. The residual coagulant activity in Gouda cheese was significantly lower than that of most of the samples of Cheddar and Feta cheeses (Table 2). This may have been due to the loss of coagulant during the washing of cheese curds in the manufacture of Gouda cheese (Robinson & Wilbey, Reference Robinson and Wilbey1998). Similar results were reported by Singh & Creamer (Reference Singh and Creamer1990).
Camembert cheese samples had significantly higher residual coagulant activity than the other varieties (Table 2). During the manufacture of Camembert cheese, the gel is broken and poured into moulds where it develops acidity and whey drains slowly at 18–20°C for ~12 h (Robinson & Wilbey, Reference Robinson and Wilbey1998). Part of the whey thus drains at pH values lower than those typical of drainage during the manufacture of other varieties of cheeses. The low pH at whey drainage and the high moisture content of the cheese may be the reasons for the higher residual coagulant activity in Camembert cheeses. Lowering the pH at whey drainage significantly increases the retention of coagulant in cheese (Garnot et al. Reference Garnot, Molle and Piot1987; Bansal et al. Reference Bansal, Fox and McSweeney2007). Garnot et al. (Reference Garnot, Molle and Piot1987), Singh & Creamer (Reference Singh and Creamer1990) and Boudjellab et al. (Reference Boudjellab, Rolet-Repecaud and Collin1994) reported that Camembert cheese has higher residual coagulant than in other varieties of cheeses. One of the three samples of Camembert cheese had a significantly higher residual coagulant activity than the others, although according to the manufacturers, all the three samples were manufactured traditionally.
Conclusions
The amount of residual coagulant retained in the cheese curd differed between and also, within varieties. Among the varieties analyzed, the high moisture varieties, e.g., Camembert and Feta, had the highest and the high-cook varieties, Emmental and Parmigiano Reggiano, and Mozzarella had the lowest residual coagulant activity. The differences within a variety were presumably due to differences in manufacturing protocols.




