Whey proteins such as β-lactoglobulin (β-Lg) and α-lactalbumin (α-La) have found use in functional foods and beverages, infant formulas and sport diets as well as being a very good source of bioactive peptides (Chatterton et al., Reference Chatterton, Smithers, Roupas and Brodkorb2006; Smithers, Reference Smithers2008; Cheison et al., Reference Cheison, Schmitt, Leeb, Letzel and Kulozik2010; Lisak Jakopović et al., Reference Lisak Jakopović, Barukčić and Božanić2016; Miletić et al., Reference Maletić, Aleksić, Vejnović, Nikšić, Kulić, Đukić and Ćirković2016). Researchers performed numerous experiments in order to fractionate either α-La or β-Lg from whey. Specifically, Bramaud et al. (Reference Bramaud, Aimar and Daufin1997) selectively precipitated and then fractionated α-La by heating and Ca2+-chelation using citrate at pH 4.2 and obtained a process which left β-Lg and other proteins in solution. Toro-Sierra et al. (Reference Toro-Sierra, Tolkach and Kulozik2013) reported an improvement of that method, combined with membrane fractionation. In addition, β-Lg was separated from whey proteins at pH 2.0 in the presence of 70 g/l NaCl (Mailliart and Ribadeau-Dumas, Reference Mailliart and Ribadeau-Dumas1988; Maté and Krochta, Reference Maté and Krochta1994) or low pH and either NaCl or sodium hexametaphosphate (Alomirah and Alli, Reference Alomirah and Alli2004), a precipitation process which yielded various levels of purity and protein recovery levels. These isolation methods were mainly based on the selective precipitation of either β-Lg or α-La aggregates under variation of different environmental or process parameters followed by ultra-filtration (UF). The methods, which exploit selective thermal precipitation, yield a product that is variably denatured, an undesirable side effect if applications that require native proteins are targeted. β-Lg is responsible for most of the functional properties in whey proteins (Foegeding et al., Reference Foegeding, Davis, Doucet and McGuffey2002). Potentially, the functional properties of β-Lg are less variable if compared to whole whey proteins and it is desirable to have sufficient quantities of this important protein in as pure and native form as possible. Alternative methods are, therefore, of interest in order to develop processes that yield pure and native β-Lg fraction.
Enzymes hold great potential for the production of individual protein fractions from substrate with the minimum denaturation of desirable protein while other proteins from the substrate are fully denatured. It is already known that bovine β-Lg is susceptible to trypsin (Schmidt and Poll, Reference Schmidt and Poll1991) and chymotrypsin (Lisak et al., Reference Lisak, Toro-Sierra, Božanić, Kulozik and Cheison2013) in a genetic-variant-dependent manner (Creamer et al., Reference Creamer, Nilsson, Paulsson, Coker, Hill and Jimenez-Flores2004). Trypsin was therefore used to selectively hydrolyse β-Lg as a novel approach in order to obtain isolated native α-La (Galvão et al., Reference Galvão, Silva, Custódio, Monti and Giordano2001; Custodio et al., Reference Custodio, Goulart, Marques, Giordano, Giordano and Monti2005; Konrad and Kleinschmidt, Reference Konrad and Kleinschmidt2008; Cheison et al., Reference Cheison, Leeb, Toro-Sierra and Kulozik2011). In our previous work (Lisak et al., Reference Lisak, Toro-Sierra, Božanić, Kulozik and Cheison2013) we reported that chymotrypsin shares hydrolysis similarities with trypsin with regard to the way it attacks the whey proteins. During chymotrypsin digestion of WPI, higher resistance of α-La to hydrolysis compared to β-Lg was demonstrated. Different hydrolysis behaviour for the two genetic variants of β-Lg (A and B) was observed, where β-Lg B was more resistant than β-Lg A (Lisak et al., Reference Lisak, Toro-Sierra, Božanić, Kulozik and Cheison2013). Acid Protease A is an acid proteolytic enzyme obtained from Aspergillus niger. It is stable in an acid pH range of 3.0–6.0 and temperature range 30–55 °C. Its optimum is pH around 2.5 and 55 °C. Acid Protease A has a molecular weight 19 kDa. Protease M is as well acid proteolytic enzyme obtained from Aspergillus sp. It is stable in pH range of 3.0–6.0 and temperature range 30–55 °C. Optimal conditions are pH around 4.5 and temperature 50 °C. Acid Protease A and Protease M are derived from microbial sources and thus their price is lower, making them attractive at industrial scale.
The main goal of this research was to investigate whether usage of the microbial enzymes rather than pepsin would give the same or similar reaction properties in terms of hydrolysis of whey proteins. The hypothesis of the presented research goes in three directions: (i) is the pH influencing the susceptibility of α-La while conferring resistance to β-Lg?, (ii) is it a property of the enzymes that they are selective irrespective of the pH and (iii) a combination of both the inherent enzyme selectivity and the milieu? Thus, this work investigates whether proteases with commercial names Acid Protease A and Protease M could be applied as an alternative to pepsin hydrolysis.
Materials and methods
Materials
Whey protein isolate (WPI, 938.4 g/kg protein); obtained from Fonterra Co-operative Group Ltd. (Auckland, New Zealand) was used as the substrate for hydrolysis. The enzyme, pepsin (EC 3.4.23.1) from porcine gastric mucosa was purchased from Sigma-Aldrich (St. Louis, MO, USA). The enzymes, Acid Protease A and Protease M were purchased from Amano Enzyme Inc. (Nagoya, Japan). Bovine whey protein calibration standards: calcium depleted α-La (L6010, ≥850 g/kg), β-Lg genetic variant A (L7880, ≥900 g/kg) and β-Lg genetic variant B (L8005, ≥900 g/kg) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Additional reagents are mentioned under respective methods.
Methods
Calculation of the degree of hydrolysis (DH)
At acid pH values 1.5–4.0, at which the experiments were performed, amino acids are in the zwitterion state. During hydrolysis, the pH increased and addition of acid was necessary to keep it constant. HCl (0.5 mol/l) was used to keep the pH constant. The volume of HCl consumed in the reaction was used to calculate the degree of hydrolysis (DH) according to Diermayr and Dehne (Reference Diermayr and Dehne1990) using Equation (1).
where, F pH = 1/(1 − α) is a correcting factor and is influenced by the pH value between 30 and 45 °C. The value depends on the dissociation constant of the carboxylic acid group (COOH). V HCl [L] presents volume of HCl consumed during hydrolysis, N HCl [mol/l] presents normality of HCl used to maintain the pH at constant experimental value; M p [g] is mass of protein in the substrate (nitrogen content); h tot is total number of available peptide bonds (in 8.8 meq/g for WPI) and α is the dissociations degree of the COOH group defined by α = 10pH−pKA/(1 + 10pH−pKA).
Hydrolysis of whey protein isolate
WPI was dissolved in 100 mL MilliQ (MilliQ System, Millipore Corporation, Bedford, USA) purified water to 100 g/l. The pH of the solutions was adjusted using 0.5 or 1 mol/l HCl. The hydrolysis temperature was maintained by a thermostatic bath with circulation (Haake CH, Berlin, Germany) to a Schott Duran jacketed-beaker glass batch reactor (HLL Landgraf Laborsysteme, Langenhagen, Germany) which contained a magnetic stirrer. The pH of the reaction was kept constant during the hydrolysis process with the addition of 0.5 mol/l HCl using manual titration dispensed through a burette with a tap at the bottom to control the added volumes. The amount of HCl used to maintain the pH was used for the calculation of the degree of hydrolysis (DH) using the relationship in Eq. (1). Hydrolysis experiments were performed using WPI (100 g/l) at different pH values (pH 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0 and 5.0) and different temperatures (30, 35, 40 and 45 °C). The enzyme to substrate concentration (E/S) investigated was 10 g/l. Durations of hydrolyses were ranged between 60 to 120 min. Performed hydrolyses conditions are shown in Table 1.
Table 1. Average values of residual α-lactalbumin (α-La), β-lactoglobulin B (β-Lg B), β-lactoglobulin A (β-Lg A) and the degree of hydrolysis (DH) of hydrolysed 100 g/l WPI by Acid Protease A, Protease M and pepsin at 10 g/l E/S at different temperatures (30, 35, 40 and 45 °C), pH values (1.5, 2.0, 2.5, 3.0, 3.5 and 4.0) and time of hydrolysis (60 and 120 min). (Error bars show the standard deviation, n = 2).

After pH and temperature stabilization in the reactor, a 430 µl aliquot of the reaction mixture solution was drawn out before the addition of the enzyme, mixed with 70 µl of 1 mol/l NaOH and marked as blank sample (t = 0). Then, around 93.84 mg of enzyme (pepsin, Acid Protease A or Protease M) was weighed out and dissolved in 1 mL of MilliQ water just before addition to commence the reaction. During hydrolysis, the pH was kept constant with manual titration of 0.5 mol/l HCl and the sample aliquots of 1 mL were drawn out at variable time intervals (0, 0.5, 1, 3, 5, 10, 20, 30, 60, 90 and 120 min), depending on the reaction conditions, during 60 or 120 min.
Determination of residual protein concentration (RP-HPLC)
From the aliquots drawn out at the time intervals, a definite amount was drawn out and further diluted with MilliQ water (with hydrolysis time progress sample dilution was decreased to cater for declining protein content). The samples were filtered through RC-45/25 Chromafil® Xtra Φ 0.45 µm syringe filters (Macherey-Nagel GmbH & Co. KG, Düren, Germany) and 1 mL placed into 1.5 mL HPLC vials (Macherey-Nagel GmbH & Co. KG, Düren, Germany). The quantitative determination of individual protein fractions in whey was done using RP-HPLC with an Agilent 1200 (Santa Clara, CA, USA) as described by our previous work Lisak et al. (Reference Lisak, Toro-Sierra, Božanić, Kulozik and Cheison2013). The whole analysis process was optimized for a short analysis time of 18 min. Injection volumes were maintained at 20 µl throughout.
Statistical analysis
All the experiments were carried out in duplicate and average values and standard deviations were calculated. The degree of hydrolysis was tested for significance by the one way analysis of variance (ANOVA) at P < 0.05 using SPSS 13.0 for Windows® (SPSS Inc., Chicago, IL, USA).
Results and discussion
WPI hydrolysis by pepsin
Pepsin (EC 3.4.23.1) is an acid enzyme and its optimal conditions are at pH 2.0–4.0 and it is stable to the temperature of 60 °C. Literature cites that native β-Lg is resistant to peptic hydrolysis (Kinekawa and Kitabatake, Reference Kinekawa and Kitabatake1996., Otte et al., Reference Otte, Zakora, Qvist, Olsen and Barkholt1998., Konrad et al., Reference Konrad, Lieske and Faber2000). Table 1 shows the average values of the residual proteins (α-lactalbumin, β-lactoglobulin B, β-lactoglobulin A) and the degree of hydrolysis (DH) of hydrolysed 100 g/l WPI by pepsin at 10 g/l E/S, different temperatures (40 and 45 °C), pH values (1.5, 2.0, 2.5, 3.0, 3.5 and 4.0) and time of hydrolysis (60 min).
The degree of hydrolysis was calculated according to Diermayr and Dehne (Reference Diermayr and Dehne1990) (Eq. 1). By increasing the pH value from 2.0 to 4.0 at the 40 °C the DH was also increased from 2.01 ± 0.03 to 5.88 ± 0.11%. The same trend occurred when reaction temperature was 45 °C. By increasing the pH value from 1.5 to 4.0 the DH was also increased from 2.13 ± 0.02 to 6.37 ± 0.12%. When the temperature was raised from 40 to 45 °C at the same pH the DH was as well increased. The highest DH was noticed at 45 °C and pH 4.0 and amounted 6.37 ± 0.12%, and the lowest DH was at the 40 °C and pH 2.0 and was 2.01 ± 0.03%. A higher DH values indicates that the hydrolysis condition was favourable for the enzymatic process. Proteins and enzymes are sensitive to pH and temperature due to their activation and inactivation effects as well as their solubility profiles. The enzyme hydrolysis was influenced by temperature and pH.
When amount of the residual proteins was assessed at pH 2.5 and 40 °C or at the pH 3.0 and 40 °C all of the α-La was hydrolysed while both genetic variants of β-Lg A and B remained unhydrolysed. When the pH value is low (pH ≤ 3.0) tertiary structure of α-La is completely disrupted, and the secondary is partially retained and α-La binds up to 270 molecules of water and takes up the so called ‘molten globule state’ (Permyakov and Berliner, Reference Permyakov and Berliner2000), At these conditions β-Lg is a monomer and as well suitable substrate for hydrolysis, but pepsin selectivity was enhanced for α-La, which offers great potential for the native and pure β-Lg isolation. At pH 4.0 and 40 °C or pH 4.0 and 45 °C enzyme selectivity is lost and after 60 min of hydrolysis around 19.98% of α-La, 72.61% of β-Lg B and 79.06% of β-Lg A remains unhydrolysed. Otte et al. (Reference Otte, Zakora, Qvist, Olsen and Barkholt1998) also performed hydrolysis of β-Lg with pepsin at pH 4.0 for 22 h in order to determine the extent of degradation and concluded that native β-Lg is a poor substrate for pepsin where only 10% of β-Lg was hydrolysed.
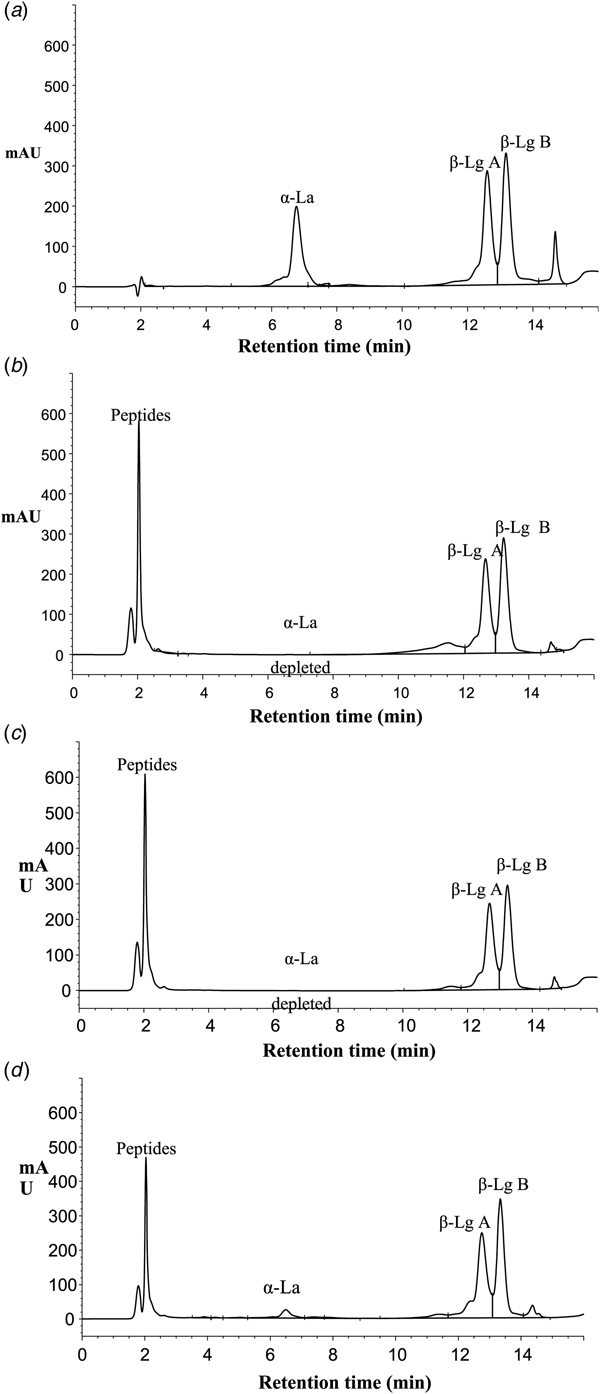
For this research, desirable hydrolysis conditions should be that enzyme hydrolyses the α-La and β-Lg remains native and unhydrolysed. It can be concluded that the best conditions for the isolation of native and pure β-Lg by pepsin were at 45 °C and pH 2.5 and after 1 min (Fig. 1a) total hydrolysis of the α-La was obtained whereas β-Lg A and B remained intact. Similar results were reported by Kinekawa and Kitabatake (Reference Kinekawa and Kitabatake1996) who purified β-Lg from WPI at 37 °C and pH 2.0 and they concluded that native β-Lg was not susceptible to the peptic digestion. Fig. 2 depicts a typical RP-HPLC chromatogram obtained for WPI before hydrolysis (Fig. 2a) and after 60 min hydrolysis (Fig. 2b) by pepsin. Fig. 2b shows an increase in peptide accumulation and disappearance of α-La while β-Lg remains largely intact. The same trend was found for Acid protease A (Fig. 2c) and Protease M (Fig. 2d).

Fig. 1. Hydrolysis flow of 100 g/l WPI solution hydrolysed by: (a) pepsin (45 °C, pH 2.5, 10 g/l E/S, 60 min); (b) Acid Protease A (40 °C, pH 2.0, 10 g/l E/S, 120 min) and (c) Protease M (45 °C, pH 3.0, 10 g/l E/S, 120 min) on the residual proteins α-lactalbumin (◊), β-lactoglobulin B (□) and β-lactoglobulin A (Δ) (n = 2).

Fig. 2. Typical RP-HPLC chromatograms of whey proteins before hydrolysis at (a) time t = 0 and after: (b) pepsin hydrolysis, t = 60 min; (c) Acid Protease A hydrolysis, t = 120 min and (d) Protease M hydrolysis, t = 120 min showing α-lactalbumin (α-La), β-lactoglobulin B (β-Lg B) and β-lactoglobulin A (β-Lg A).
WPI hydrolysis by acid protease A
Table 1 shows the average values of the residual proteins (α-lactalbumin, β-lactoglobulin B, β-lactoglobulin A) and the degree of hydrolysis (DH) of hydrolysed 100 g/l WPI by Acid Protease A at 10 g/l E/S concentration, different temperatures (30 and 40 °C), pH values (2.0 and 4.0) and time of hydrolysis (120 min). By increasing the pH value from 2.0 to 4.0 at 30 °C the DH was also increased from 1.87 ± 0.04 to 2.99 ± 0.09%. The same trend was observed at 40 °C. By increasing the pH value from 2.0 to 4.0 the DH was also increased from 2.17 ± 0.11% to 4.24 ± 0.07%. When temperature was raised from 30 to 40 °C at the same pH the DH was as well increased. The highest DH was noticed at 40 °C and pH 4.0 and amounted to 4.24 ± 0.07%, and the lowest DH was at the 30 °C and pH 2.0 and was 1.87 ± 0.04%. A higher DH values indicates that the hydrolysis condition was favourable for the enzymatic process. The same DH trend was observed when hydrolysis was performed with pepsin. The obtained results are similar to findings published by Cheison et al. (Reference Cheison, Bor, Faraj and Kulozik2012). They reported that under low pH (2.5) and low temperature (30 °C), the degree of hydrolysis (DH) was lowest (1.90%), with limited hydrolysis and incomplete depletion of α-La. At higher pH of 3.5 and 45 °C, a DH of 7.35% was reached and α-La was depleted. Hydrolysis progress of WPI by Acid Protease A at 40 °C and pH 2.0 is shown in Fig. 1b. Majority of α-La (about 88%) was hydrolysed during the first 5 min of reaction. At the end of hydrolysis (120 min) all of the α-La was hydrolysed. Amounts of β-Lg A and B during the 120 min of the reaction remained unchanged, Acid Protease A did not hydrolyse β-Lg at all. By increase of the pH value, the enzymes are outside of their optimal pH milieu and the proteins, specifically α-La, are not in the hydrated molten globule state like at pH ≤3 and, thus, the result is the higher amount of unhydrolysed proteins.
WPI hydrolysis by Protease M
Protease M is an acidic proteolytic enzyme obtained from Aspergillus sp. It is stable in the pH range between 3.0–6.0 and temperatures between 30–55 °C. However, the declared optimum conditions for enzyme activity are pH 4.5 and temperature 50 °C. By increasing the pH value from 3.0 to 5.0 at 35 °C the DH was also increased from 2.23 ± 0.02% to 4.47 ± 0.09% (Table 1). When the temperature was raised from 35 to 45 °C at the same pH (3.0) the DH was again increased. The highest DH was obtained at 35 °C and pH 5.0 and amounted to 4.47 ± 0.09%, and the lowest DH was at the 35 °C and pH 3.0 and was 2.23 ± 0.02%. Increasing the temperature by 10 °C whilst keeping other environment conditions the same achieved residual α-La of 0.48% while amounts of residual β-Lg B and β-Lg A were 5.08 and 8.49% respectively (Table 1). By increasing the pH for 2.0 pH units at the same temperature (35 °C) the amount of residual α-La increased by 11.36% while amounts of residual β-Lg B and β-Lg A decreased by 26.31% and 41.20%, respectively. The same trend was observed when hydrolysis was performed by pepsin and Acid Protease A, since all three enzymes optimally works under acidic pH.
Hydrolysis progress of WPI by Protease M at 45 °C and pH 3.0 is shown in Fig. 1c. The majority of α-La (about 92%) was hydrolysed during the first 15 min of reaction. At the end of hydrolysis (120 min) less than 1% of α-La remains unhydrolysed. Amounts of β-Lg A and B during the 120 min of the reaction remained unchanged, Protease M did not hydrolyse β-Lg at all. Thus Protease M has a great potential for isolation of β-Lg and it shows the same selectivity to proteins as pepsin and Acid Protease A. The best conditions for the isolation where β-Lg remains completely unhydrolysed and remains less than 1% α-La were at 45 °C and pH 3.0.
Comparison of WPI hydrolysis by different enzymes
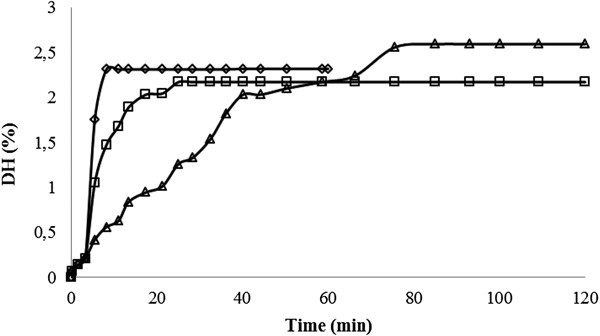
Similarity between enzymes preference in protein hydrolysis order was evident. Pepsin, Acid Protease A and Protease M shows the same selectivity according to proteins when environment conditions are similar (pH 2.0–3.0 and 40–45 °C). First, pepsin, Acid Protease A and Protease M hydrolyses α-La in a short time, for 1, 20 or 30 min, respectively. Both genetic variants of β-Lg A and B remain unhydrolysed during the whole period of reaction (60 or 120 min). Influence on enzyme type on the α-La depletion over hydrolysis time is shown in Fig. 3. Fig. 3 shows that pepsin hydrolyses α-La very fast, followed by Acid Protease A and Protease M. The best environmental conditions for β-Lg isolation were in pH range 2.0–3.0 and 40–45 °C, dependent on the enzyme used. Influence of the enzyme type on the DH for the best chosen conditions for potential β-Lg isolation is shown in Fig. 4. The DH of hydrolyses were 2.32 ± 0.09, 2.17 ± 0.11 and 2.59 ± 0.08% for pepsin, Acid Protease A and Protease M, respectively. The DH flow corresponded with the α-La depletion. The DH line for the pepsin hydrolysis had a biggest increase in first few min of hydrolysis and it achieved its maximum value 2.32% at less than 10 min. The course of reaction for Acid Protease A is very similar to the one for pepsin hydrolysis. The biggest increase in DH was in the first 10 min and the maximum value 2.17% was achieved after around 25 min of reaction. DH value for Protease M had the slowest rise until it reached its maximum value of 2.59%. The biggest increase in the DH was in the first 40 min of the reaction. The DH line of Protease M hydrolysis (Fig. 4) follows the α-La depletion (Fig. 3).

Fig. 3. Influence of the enzyme type on the α-lactalbumin depletion over hydrolysis time (60 or 120 min) at the best obtained hydrolysis conditions for pepsin (◊, 45 °C, pH 2.5), Acid Protease A (□, 40 °C, pH 2.5) and Protease M (Δ, 45 °C, pH 3.0).

Fig. 4. Influence of the enzyme type (pepsin (◊, 45 °C, pH 2.5), Acid Protease A (□, 40 °C, pH 2.5) and Protease M (Δ, 45 °C, pH 3.0)) on the degree of hydrolysis (DH) at the best hydrolysis conditions for potential β-lactoglobulin isolation.
In conclusion, during the hydrolysis of 100 g/l WPI with three different acidic enzymes (pepsin, Acid Protease A and Protease M) and different milieu conditions, higher resistance of the β-Lg to hydrolysis compared to α-La was demonstrated. The enzymes of microbial origin, Acid Protease A and Protease M, showed similar selectivity as pepsin. The obtained results confirm our hypothesis and, therefore, it can be concluded that the pH value had great influence on the susceptibility of α-La, while β-Lg stayed unhydrolysed under low pH values (2.0–3.0). Furthermore, by pH change, enzyme selectivity was also changed due to the different protein susceptibilities to proteolysis at different pH values and the enzymes operating outside of their optimal conditions. Resistance to hydrolysis of β-Lg was higher at lower pH (2.0–3.0) and higher temperatures (40 and 45 °C) while at the same time depletion of α-La was better.
Acknowledgements
These materials are based on work co-financed by the Croatian Science Foundation and a DAAD scholarship for author Katarina Lisak Jakopović to study in Germany at the ZIEL-NFG Bioactive Peptides and Protein Technology and ZIEL- Abteilung Technologie, who are thanked for hosting. This research project was supported by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e. V., Bonn). Project AiF 15834 N.