Whey is a major co-product of cheese-making and casein manufacture in the dairy industry. Across the world, volumes of whey are growing at about the same rate as milk volumes (>2% per year) (Smithers, Reference Smithers2008). While having excellent nutritional properties, whey represents a rich and heterogeneous mixture of secreted proteins with wide range of biological and food functional attributes. The main constituents are β-lactoglobulin (β-lg), representing about 53% of the whey proteins, and α-lactalbumin (α-la) about 20–25% of the whey protein. These proteins have found use in functional foods and beverages, infant formulas, sport diets as well as being a very good source of bioactive peptides (Chatterton et al. Reference Chatterton, Smithers, Roupas and Brodkorb2006; Smithers, Reference Smithers2008; Cheison et al. Reference Cheison, Schmitt, Leeb, Letzel and Kulozik2010).
Since β-lg is the most common type of food allergen in humans especially in infant formula, there is a considerable interest in its removal (Lönnerdal & Lien, Reference Lönnerdal and Lien2003). Monaci et al. (Reference Monaci, Tregoat, Hengel and Anklam2006) also reported on the allergenicity of β-lg and showed that there are many allergenic epitopes spread all over the β-lg structure. Second major whey protein, α-la, is very important as one of the two components of the lactose synthase system which catalyses the final step in the lactose synthesis in the lactating mammary gland (Permyakov & Berliner, Reference Permyakov and Berliner2000; Kamau et al. Reference Kamau, Cheison, Chen, Liu and Lu2010). In addition, owing to its high content of essential amino acids, especially tryptophan, cysteine and lysine it is has nutraceutical and therapeutic applications. The amino acid composition of bovine α-la shows a 72% sequence identity to human α-la, which makes it an ideal protein for use in human infant nutrition (Monaci et al. Reference Monaci, Tregoat, Hengel and Anklam2006; N'Negue et al. Reference N'Negue, Miclo, Girardet, Campagna, Mollé and Gaillard2006). Accordingly, there is a considerable technical interest in isolation of pure and native α-la, and for use in novel milk products in the production of nonallergenic health and nutritional products.
A number of reports on the separation and purification of α-la and on its applications were reviewed in our earlier paper (Kamau et al. Reference Kamau, Cheison, Chen, Liu and Lu2010). Applications for large scale production of isolated α-la in the dairy industry are based mainly on membrane technology (Cheang & Zydney, Reference Cheang and Zydney2004; Konrad & Kleinschmidt, Reference Konrad and Kleinschmidt2008). Its separation with membrane techniques can be achieved through microfiltration (MF) to remove β-lg or ultrafiltration (UF) using a 50 or 100 kDa membrane, thereby passing α-la into the permeate (Chatterton et al. Reference Chatterton, Smithers, Roupas and Brodkorb2006; Konrad & Kleinschmidt, Reference Konrad and Kleinschmidt2008). More common is the use of a two membrane cascade filtration or a two stage UF process combining 30 and 100 kDa membranes for isolation of α-la from whey protein isolate (Cheang & Zydney, Reference Cheang and Zydney2004). Salting-out procedure (Mailliart & Ribadeau-Dumas, Reference Mailliart and Ribadeau-Dumas1988), exploitation of the selective thermal stability of α-la in acidic conditions (Bramaud et al. Reference Bramaud, Aimar and Daufin1997; Gésan-Guiziou et al. Reference Gésan-Guiziou, Daufin, Timmer, Allersma and Van Der Horst1999; Alomirah & Alli, Reference Alomirah and Alli2004) and separation by a combination of UF and anion-exchange chromatography under very gentle conditions (Kristiansen et al. Reference Kristiansen, Otte, Ipsen and Qvist1998) and ion-exchange chromatography (Outinen et al. Reference Outinen, Tossavainen and Syväoja1996) were also used. In addition, α-la was purified through selective denaturation of β-lg and use of membrane filtration to remove caseinomacropeptide (CMP) (Kiesner et al. Reference Kiesner, Clawin-Rädecker, Meisel and Buchheim2000; Tolkach et al. Reference Tolkach, Steinle and Kulozik2005). Gel-filtration chromatography has also been used to isolate α-la from other milk proteins (Manji et al. Reference Manji, Hill, Kakuda and Irvine1985). High yields of α-la were obtained with ultrafiltration (UF) of whey using 100 kDa membranes at pH 8·0 (Mehra & Donnelly, Reference Mehra and Donnelly1993). The main weak points of these techniques are low purification and yield as well as obtaining α-la with various degrees of denaturation.
On the other hand, enzymes hold great potential for the production of proteins with minimum denaturation. It is already known that bovine β-lg is selectively susceptible to trypsin (Schmidt & Poll, Reference Schmidt and Poll1991) in a genetic-variant-dependent manner (Creamer et al. Reference Creamer, Nilsson, Paulsson, Coker, Hill and Jimenez-Flores2004). Trypsin was therefore used to selectively hydrolyse β-lg as a novel approach in order to obtain isolated native α-la (Cheison et al. Reference Cheison, Leeb, Toro-Sierra and Kulozik2011) since the difference in enzyme selectivity for a defined substrate can be used to obtain the desirable product. It is reported that trypsin has enzymatic activity that selectively digests β-lg while α-la remains more or less in its native state (Schmidt & Poll, Reference Schmidt and Poll1991; Galvão et al. Reference Galvão, Silva, Custódio, Monti and Giordano2001; Custodio et al. Reference Custodio, Goulart, Marques, Giordano, Giordano and Monti2005; Konrad & Kleinschmidt, Reference Konrad and Kleinschmidt2008; Cheison et al. Reference Cheison, Leeb, Toro-Sierra and Kulozik2011). The enzyme α-chymotrypsin is a serine protease of the peptidase S1 family consisting of 241 amino acid residues. It is the predominant form of active enzyme produced from its zymogen; chymotrypsinogen A. The α-chymotrypsin from bovine pancreas selectively catalyses the hydrolysis of peptide bonds on the C-terminal side of amino acids tyrosine, phenylalanine, tryptophan, and leucine with extraordinary catalytic efficiency. A secondary hydrolysis also occurs on the C-terminal side of methionine, isoleucine, serine, threonine, valine, histidine, glycine, and alanine (Sweeney & Walker, Reference Sweeney, Walker and Burrell1993). Since chymotrypsin shares around 40% of trypsin amino acid sequence homology and a conservation of the same catalytic triad (His57, Asp189 and Ser195) it was intended to explore whether the hydrolysis was similar to the protein selectivity obtained during trypsin hydrolysis. In previous studies, it was shown that working outside the optimum conditions for trypsin hydrolysis resulted in enhanced selectivity for β-lg (Cheison et al. Reference Cheison, Leeb, Toro-Sierra and Kulozik2011).
The aim of the present study was to examine the applicability of the chymotryptic digestion to whey proteins; to investigate any selective protein resistance in order to develop a process for selective removal; to consider the effect of different enzyme reaction stoppage methods on protein recovery. Also, optimise the hydrolysis conditions by variations of the working temperature, pH, substrate concentration and the enzyme-to-substrate (E/S) ratio which would give the highest recovery of native and pure α-la. These results should provide new insights into the possibility of using chymotrypsin instead of trypsin owing to cost differences. In this case, efficacy, but not speed would be the decisive criteria in milieu selection for the hydrolysis process.
Materials and Methods
Materials
Whey protein isolate (WPI, 93·84% (w/w) protein); obtained from Fonterra Co-operative Group Ltd (Auckland, New Zealand) was used as the substrate for hydrolysis. The enzyme, α-chymotrypsin (EC 3.4.21.1) from bovine pancreas with a declared activity of ⩾40 units/mg as well as bovine whey protein calibration standards: calcium depleted α-la (L6010, ⩾85%), β-lg genetic variant A, β-lg A (L7880, ⩾90%) and β-lg B (L8005, ⩾90%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Trypsin–chymotrypsin inhibitor from Glycine max (soybean), Bowman–Birk inhibitor and trypsin inhibitor from chicken egg white were purchased from Sigma-Aldrich (St. Louis, MO, USA). Additional reagents are mentioned under respective methods.
Methods
Hydrolysis of whey protein isolate
WPI was dissolved in 100 ml MilliQ (MilliQ System, Millipore Corporation, Bedford, USA) purified water to 5 or 10% w/v. The pH of the solutions was adjusted using 0·5 m NaOH. The hydrolysis temperature was maintained by a thermostatic bath with circulation (Haake CH, Berlin, Germany) to a Schott Duran jacketed-beaker glass batch reactor (HLL Landgraf Laborsysteme, Langenhagen, Germany) which contained a magnetic stirrer. The pH of the reaction was kept constant during the hydrolysis process with the addition of 0·5 m NaOH using manual titration dispensed through a burette. The amount of NaOH used to maintain the pH was used for the degree of hydrolysis (DH) calculation using the relationship in Eq. (1) as reported earlier by Adler-Nissen (Reference Adler-Nissen1986) and Cheison et al. (Reference Cheison, Wang and Xu2007):
where V b is the volume of NaOH required to maintain constant pH in ml; N b, the normality of the NaOH (0·5 m in this study); α, the average degree of dissociation of the α-NH2 groups; W p, the mass of protein in sample (defined as Kjeldahl N(%) 6·38) in g; and h tot, the total number of peptide bonds in the protein substrate (8·8 meqv/g for whey protein).
Hydrolysis experiments were performed using WPI (5% and 10% w/v) at different pH values (pH 7·0, 7·8 and 8·5) and different temperatures (25, 37 and 50 °C) according to the randomised experimental design. The enzyme-to-substrate ratios (E/S) used in the experiments were 0·1, 0·5 and 1% (w/w). The experiments were designed and randomised using Statgraphics Plus Version 5.0 (Statpoint Technologies Inc., Warrenton, VA, USA) and hydrolysis performed for various durations ranging between 60 and 120 min, depending on hydrolysis conditions. All the experiments were carried out in duplicate and average values for the determinations of the DH and the corresponding standard deviations were calculated.
After pH and temperature stabilisation in the reactor a 430 μl aliquot of the reaction mixture solution was drawn out before the addition of the enzyme, mixed with 70 μl 1 m HCl and marked as blank sample (t=0). Then around 9·4, 47 or 94 mg chymotrypsin (corresponding to the enzyme required to achieve the respective E/S for the experiment) was weighed out and dissolved in MilliQ water just before addition to commence the reaction. During hydrolysis the sample aliquots were drawn out at variable time intervals (0, 0·5, 1, 3, 5, 10, 20, 30, 60, 90 and 120 min) and mixed with acid. In the experiments when reaction was stopped by inhibitors or heat, procedure as described in the next section.
Stopping the enzyme reaction
To stop the enzyme activity, three methods were used. To test the efficacy of the inhibitors, samples from the reaction at 25 °C and pH 8·5 with 5% WPI (w/v) and 1% (E/S) were used as these were the conditions which yielded the highest recovery of α-la. Thus, samples were (i) immediately mixed with either HCl (1 m) to adjust the pH to around 3·0 to stop the chymotrypsin activity; (ii) heated at 65 °C/10 min in water bath; or (iii) the enzyme reaction stopped using inhibitors. Prior to addition in samples, lyophilised inhibitor powders were diluted in MilliQ purified water in an amount corresponding to the ratio of chymotrypsin to be inhibited (1 mg Bowman–Birk inhibitor inhibits 0·5 mg chymotrypsin while 1 mg trypsin inhibitor inhibits 0·3 mg enzyme). Therefore, solutions of 93·84 and 156·4 mg Bowman–Birk inhibitor and trypsin inhibitor from chicken egg white, respectively, were dissolved in 1 ml MilliQ water and used. Reactor aliquots (1250 μl) were drawn and mixed with 300 μl of the inhibitor solution (already in the cuvettes) in order to stop the enzyme reaction.
Determination of residual protein concentration (RP-HPLC)
The aliquots drawn out of the reactor at the stated time intervals were further diluted with MilliQ water (with progressive hydrolysis time sample dilution was decreased to cater for declining protein content in the hydrolysates). The pH of the diluted samples was adjusted to 4·6 with 0·01 m NaOH or 0·01 m HCl, filtered through RC-45/25 Chromafil® Xtra Φ 0·45 μm syringe filters (Macherey-Nagel GmbH & Co. KG, Düren, Germany) and 1 ml placed into 1·5 ml HPLC vials (Macherey-Nagel GmbH & Co. KG, Düren, Germany). The quantitative determination of individual protein fractions in whey was done using RP-HPLC with an Agilent 1200 (Santa Clara, CA, USA) as described by Cheison et al. (Reference Cheison, Leeb, Toro-Sierra and Kulozik2011). The whole analysis process was optimised for a short analysis time of 18 min. Injection volumes were maintained at 20 μl throughout.
Electrophoresis
Sodium-dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was performed for samples produced at 50 °C, in order to determine whether there was any likelihood of the failure of RP-HPLC to detect denatured proteins. For SDS-PAGE analysis, the blank samples and samples produced at 30 and 60 min (50 °C, pH 7·0), 10 and 20 min (50 °C, pH 7·8) and 5 and 10 min (25 °C, pH 8·5) were used. The procedure was performed as described earlier (Cheison et al. Reference Cheison, Leeb, Toro-Sierra and Kulozik2011).
Statistical analysis
All experiments were performed in duplicate. Data were tested for significance by the one-way analysis of variance (ANOVA) at P < 0·05 using SPSS 13.0 for Windows® (SPSS Inc., Chicago, IL, USA). Post-hoc analysis for significance was done using Tukey's honestly significant difference (HSD) test. Curves were fitted using SigmaPlot for Windows Version 11 Build 11.0.0.75 (Systat Software Inc., Chicago, IL, USA).
Results and Discussion
The declared pH and temperature optimum of α-chymotrypsin are 50 °C and pH 7·8. It was intended to investigate if operating the hydrolysis, at below or above optimum conditions of pH and temperature could contribute to hydrolysis of β-lg and resistance of native α-la when compared with the process at optimum conditions.
Influence of temperature, pH and E/S ratio on the degree of hydrolysis
The degree of hydrolysis (DH) is an important index for protein hydrolysis which gives a measure of the extent of protein hydrolysis and approximate peptide size composition. The highest DH recorded in this hydrolysis experiment was 7·56 ± 0·04%, which was reached during hydrolysis at 50 °C and pH 8·5 using 0·5% E/S. Other DH values to which whey proteins were hydrolysed by chymotrypsin, depending on hydrolysis conditions, are shown in Table 1. These values correspond to the total DH of total WPI hydrolysates and not individual proteins which make up whey proteins. Generally, increasing reaction temperature, pH and E/S ratio resulted in higher final DH.
Table 1. The average the degrees of hydrolysis reached in the chymotrypsin hydrolysis of the whey protein isolate with its associate standard deviations (n = 2)

a,b,c,d,e,f,g,h,i,j,k,l,m,n,o Same letter superscripts denote no significant difference between means (P < 0·05)
As the reaction temperature was raised from 25 to 50 °C, an increase of DH was observed at pH 7·0 with E/S ratio of 0·1%. Also, an increase of DH was recorded at a five-fold E/S ratio increase to 0·5%. The DH increased at pH 7·8 with a temperature increase from 25 to 37 °C at E/S ratio 0·1% as well as at E/S ratio 0·5%. However, at 50 °C the DH was marginally lower than at 37 °C and both 0·1 and 0·5% E/S ratio.
At pH 8·5, the same trend on the DH change occurred. By increasing pH of the reaction from 7·0 to 8·5 the DH was also increased except at 50 °C where the DH decreased. When the E/S ratio was increased from 0·1 to 1%, the DH also increased concomitantly. Pintado et al. (Reference Pintado, Pintado and Malcata1999) reported similar results during trypsin hydrolysis of bovine whey proteins, showing that by increasing the enzyme concentration the hydrolysis DH was also increased.
Influence of temperature, pH and E/S on hydrolysis of whey protein
According to the Tanford transition (Tanford et al. Reference Tanford, Bunville and Nozaki1959), at pH values higher than 7·5, β-lg exists as monomers and its attack by an enzyme could be improved. Influence of the enzyme to substrate ratio, reaction temperature, pH and hydrolysis duration on 10% WPI (w/v) hydrolysis by chymotrypsin is shown in Table 2. After RP-HPLC analysis for residual proteins, a strong dependence of the susceptibility to chymotrypsin hydrolysis on protein type during different reaction temperature at the same pH and E/S ratio was noticeable. It is observed that, at 25 °C, chymotrypsin hydrolysed the proteins in the order β-lg A > β-lg B > α-la. Cheison et al. (Reference Cheison, Leeb, Toro-Sierra and Kulozik2011) reported the same trend during the trypsin hydrolysis of WPI where after only 40 min at pH 8·5, a complete depletion of β-lg A was witnessed, and after 2 h of hydrolysis only α-la could be detected with no more intact β-lg. In conditions where reaction temperature was higher, enzyme selectivity was lost and the enzyme depleted both β-lg and α-la. Hence, by increasing reaction temperature from 25 to 50 °C, the amount of native residual proteins decreased. Therefore, α-la showed the highest resistance against chymotrypsin hydrolysis at lower temperatures, and better selectivity for β-lg hydrolysis at higher pH. Chymotrypsin was able to completely hydrolyse β-lg A and B and 99% of α-la at 50 °C after only 10 min at pH 8·5 and 0·5% E/S implying a loss of enzyme selectivity and low protein resistance.
Table 2. Average values of the residual proteins in the chymotrypsin hydrolysis at different enzyme to substrate ratio, temperature, pH, residual proteins and time of hydrolyses with its associate standard deviations (n = 2)

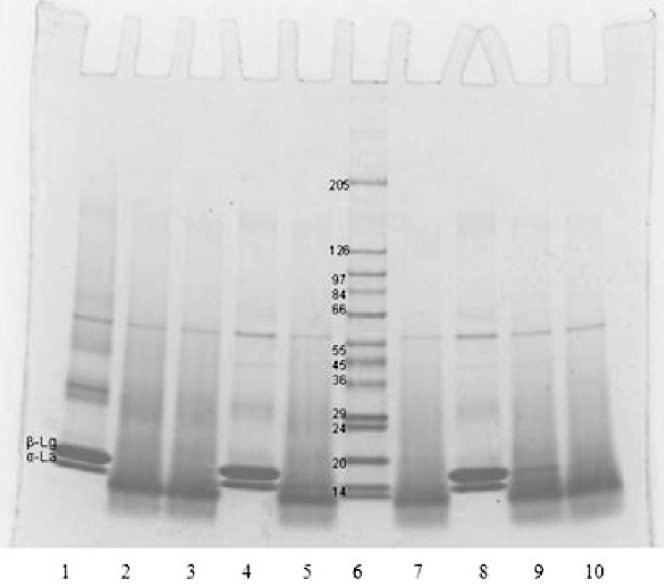
At 50 °C chymotrypsin has optimum activity as per manufacturer declaration. Besides, at temperatures above 40 °C and pH >7·5, according to Tanford transition, β-lg undergoes a dimer-to-monomer transition, which increases protein hydration, protein molecular separation and enzyme penetration leading to better enzyme access to the hydrolytic peptide bonds. It is assumed that dimers are generally less easily hydrolysed because dimerisation results in the masking of the substrate. In addition, β-lg denaturation starts at 50 °C and that probably influenced the enzyme attack of peptide bonds. In most cases, the hydrolysis sequence suggests that chymotrypsin preferentially hydrolyses β-lg over α-la, except at 50 °C where the enzyme apparently attacked both α-la and β-lg with little or no selectivity, implying less controlled hydrolysis. To exclude the inability of the RP-HPLC to detect heat-denatured proteins, SDS-PAGE was performed for hydrolyses at 50 °C (Fig. 1). In each case (pH 7·0, 7·8 or 8·5), SDS-PAGE electrophoretograms show that there were no visible bands of whey proteins after chymotryptic digestion, with only peptides that matched to RP-HPLC analysis. This confirms that no denatured residual proteins that could not be detected by RP-HPLC were present at 50 °C and pH 7·0, 7·8 and 8·5 after hydrolyses, but all proteins were already converted to peptides.
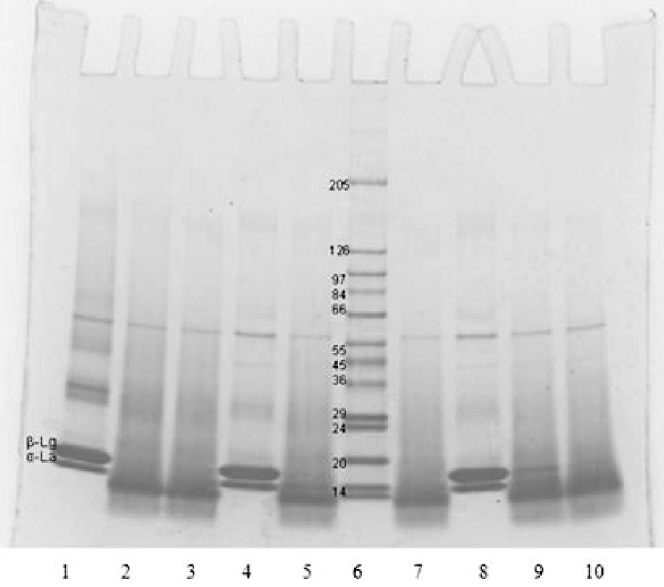
Fig. 1. SDS-PAGE electrophoretogram of chymotryptic hydrolysates of whey protein isolate. Lanes 1–3: 50 °C, pH 8·5, 0·5% E/S (lane 1, 0 min; lane 2, 5 min; lane 3, 10 min); lanes 4, 5, 7: 50 °C, pH 7·8, 0·5% E/S (lane 4, 0 min; lane 5, 10 min; lane 7, 20 min); lane 6: protein calibration standards; lanes 8–10: 50 °C, pH 7·0, 0·5% E/S (lane 8, 0 min; lane 9, 30 min; lane 10, 60 min)
Regarding the pH influence (Table 2) on hydrolysis at pH 7·0, 25 °C and 0·5 E/S for 120 min, residual proteins were detected with 91·91% α-la, 96·64% β-lg B and 89·80% β-lg A remaining, which implies almost total protein resistance to hydrolysis at this pH. Hydrolysis at pH 7·8, 25 °C and 0·5 E/S after 120 min yielded marginal reductions to 86·77, 66·31 and 30·78% of residual α-la, β-lg B and β-lg A, respectively. Hydrolysis at pH 8·5 improved β-lg genetic variants A and B depletion compared with hydrolysis at lower pH values. For example, after 120 min of hydrolysis at 25 °C, 72·66% of α-la, 11·66% of β-lg B and 1·46% of β-lg A, remained. At higher pH the selectivity of the enzyme for β-lg was enhanced due to β-lg dimer separation leading to a better cleavage of peptide bonds, a property we noticed with trypsin in our earlier work (Cheison et al. Reference Cheison, Leeb, Toro-Sierra and Kulozik2011). Therefore, the results suggest that higher pH, not temperature; was good for enzyme selectivity and removal of β-lg. These results show that use of pH higher than 7·5 to break the dimeric forms of β-lg, combined with temperatures far below its optimum (<37 °C), drives the process to favour hydrolysis of β-lg. Increasing the E/S ratio (Table 2) at 25 °C and pH 8·5 resulted in enhanced enzyme selectivity for β-lg although the susceptibility of β-lg was still genetic-variant dependent. Thus the enzyme in all cases (each of three E/S ratios used) hydrolysed the proteins in the order β-lg A > β-lg B while attacking α-la the least. When hydrolysis was performed with an E/S ratio of 0·1% for 120 min at pH 8·5 and 25 °C, 93·63% of α-la, 42·52% of β-lg B and 13·43% β-lg A remained unhydrolysed. In contrast, hydrolysis with E/S ratio of 0·5% for 120 min, 72·66%, 11·66% and 1·46% of α-la, β-lg B and β-lg A, respectively, remained undigested. Meanwhile, hydrolysis with an E/S ratio of 1% at the same conditions led to a rapid depletion of β-lg A, such that after 90 min, 63·29% of α-la and only 0·8% of β-lg B could be detected with no more β-lg A. When hydrolysis was performed with higher E/S ratios, the interaction between the enzyme and proteins was also higher and led to better digestion. A compromise of the use of high enzyme amounts could be weighted against the cost. Use of low enzyme amounts is also known to lead to substrate inhibition. Thus, selection of the optimum enzyme to substrate ratio was guided by the cost, considering likely industrialisation, and enzyme selectivity.
Influence of substrate concentration on hydrolysis of whey protein
Influence of substrate concentration on residual proteins at fixed conditions (25 °C, pH 8·5, 1% E/S) showed that when hydrolysis was performed with 5% WPI (w/v), after 90 min recovery of α-la was better compared with hydrolysis of 10% WPI (w/v). Thus, with 5% WPI (w/v) for 90 min (DH 5·88%), 72·67% of α-la remained unhydrolysed and only 0·78% of β-lg B with no more β-lg A being detected. Hydrolysis of 10% of WPI (w/v) for 90 min to a DH of 6·09% on the other hand left 63·29% of α-la and 0·8% of β-lg B.
For all hydrolysis conditions, the combination of 25 °C; pH 8·5; at an E/S of 1% gave the best results for maximal recovery of α-la (72·67 and 63·29% for 5 and 10% of WPI (w/v), respectively) with less than 1% of β-lg B remaining in both cases. No more β-lg A could be detected at the end of hydrolysis (120 min). Similar conditions for recovery of α-la by tryptic digestion were obtained in our recent work (Cheison et al. Reference Cheison, Leeb, Toro-Sierra and Kulozik2011), whereas in 120 min with hydrolysis DH of 7·11 and 10% WPI (w/v), recovery of 67·87% of α-la with no residual β-lg was recorded. From obtained results it is revealed for the first time that chymotrypsin shares the same hydrolysis similarities with trypsin with regard to the way it attacked the whey proteins. To the best of our knowledge, this work is the first attempt that demonstrates the selective susceptibility of WPI to chymotrypsin hydrolysis. Thus, chymotrypsin hydrolysis of whey proteins could be a cheaper alternative to trypsin owing their price difference based on $/g.
Influence of different enzyme inhibition methods
The enzyme inactivation method plays a significant role in the post hydrolysis process. It is always desired to arrest the enzyme activity immediately; hence pH adjustment is a common method. Heat-inactivation poses a challenge because it is slow and where the temperature for hydrolysis is lower than the enzyme optimum, the temperature increase passes through the optimum region which may activate the enzyme further, resulting in enhanced reaction kinetics and altered products by the time the enzyme is inactivated. It was desired in our work to avoid pH and heat-inactivation because these two methods were likely to influence the level of native protein which was to be obtained in its pure and native form. Thus, the influence on different enzyme inhibition methods was investigated under the best hydrolysis conditions found for the highest possible recovery of native α-la (25 °C, pH 8·5, 1% E/S, 5% WPI) and compared with the results obtained when chymotrypsin activity was arrested by either pH adjustment using HCl or heat-inactivation. The results obtained by different enzyme inhibition methods are shown in Fig. 2. Since chymotrypsin is inactive at pH less than 3·5 it was intended to arrest the enzyme activity by adding 1 m HCl to aliquots drawn from the reactor. With this method, α-la recovery was 72·67% and less than 1% β-lg B and without β-lg A. Earlier, Cheison and et al. (2011) used 1 m HCl to inactivate trypsin activity.

Fig. 2. Influence of the different enzyme inhibition methods during chymotrypsin hydrolysis at 25 °C, pH 8·5, 1% E/S ratio and 5% WPI, showing the residual proteins α-lactalbumin (α-la), β-lactoglobulin A and B (β-lg A and β-lg B). The hydrolysis time to achieve the levels of residual proteins was 90 min. (Error bars show the standard deviation, n = 2).
Chymotrypsin activity can also be inactivated by heating. Heating of aliquots was performed in a water bath at 65 °C for 10 min. After 10 min of heating there was only around 17% of residual α-la and no residual β-lg A and B. This was a significant drop from the instantaneous inactivation using pH adjustment. One possible explanation for this low α-la recovery could be because of protein denaturation. Konrad & Kleinschmidt (Reference Konrad and Kleinschmidt2008) also stopped the enzyme (trypsin) activity by heating at 65 °C for 10 min. The most significant explanation for the dramatic protein reduction is that as the temperature rose from 25 to 65 °C and passed the optimum for chymotrypsin (50 °C), the enzyme was thermally activated and the raised activity led to further and rapid hydrolysis of the proteins. It has already been shown in this study that at 50 °C, enzyme selectivity was lost with α-la being highly susceptible to chymotrypsin activity. For instant stoppage of the enzyme, therefore, heat-inactivation would not be a good option unless steam injection was used with the risks of protein denaturation.
The Bowman–Birk inhibitor from soybeans was also used. This inhibitor has a well-characterised ability to inhibit trypsin and chymotrypsin (Kennedy, Reference Kennedy1998). It was used in an amount (1 mg of inhibitor on 0·5 mg of enzyme) that inhibited the amount of chymotrypsin used in the experiment. When Bowman–Birk inhibitor was used to stop the enzyme activity around 81% of α-la was recovered and less than 1% of β-lg B and no residual β-lg A were detectable. This was a significant saving on the protein compared with the recovery obtained with the use of pH adjustment and heat-inactivation. The second inhibitor was trypsin inhibitor from chicken egg white. Since chymotrypsin shares around 40% of trypsin amino acid sequence and has the same catalytic triad it was intended to investigate if this inhibitor could be appropriate to stop chymotrypsin activity. The results show that around 74% of α-la remained with no residual β-lg. This was lower than the amounts recoverable with enzyme inhibition using the Bowman–Birk inhibitor but higher than the amounts of α-la recoverable with both pH adjustment and heat-inactivation. The added advantage that the inhibitors offer is the possibility to obtain native α-la although this introduces another challenge of inhibitor removal, a challenge which must be considered with membrane filtration and/or affinity chromatography in downstream processing. It was indeed expected that upon interacting with the inhibitors, the enzyme could become big enough to be excluded by membrane filtration, a process whose trial is under study currently.
The highest α-la recovery was therefore reached when the enzyme reaction when the enzyme reaction was stopped using the Bowman–Birk inhibitor. The Bowman–Birk inhibitor offers potential to avoid the heat-denaturation and further losses due to continued hydrolysis during heat-denaturing. In addition, the method avoids the likely pH effects on levels of native protein. However, the cheapest way to stop the enzyme activity would be achieved through pH adjustment with HCl acid. It is likely that the lower amounts of residual α-la recorded with pH adjustment as compared with inhibitor may be attributed to formation of molten-globule-state, or leakage of Ca2+ at low pH resulting in the formation of the holo-form of α-la under acid pH, a property which RP-HPLC was not able to detect.
These materials are based on work co-financed by the Croatian Science Foundation and a DAAD scholarship for author Katarina Lisak wishes to acknowledge their kind assistance for the sponsorship during her stay in Germany at the ZIEL-NFG Bioactive Peptides and Protein Technology and ZIEL-Abteilung Technologie for hosting. This research project was supported by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e. V., Bonn). Project AiF 15834 N.