Subclinical mastitis (SM) is the most prevalent disease of dairy cows, and is characterized by an increased somatic cell count (SCC), altered milk composition and lower milk yield (Seegers et al., Reference Seegers, Fourichon and Beaudeau2003; Halasa et al., Reference Halasa, Huijps, Osteras and Hogeveen2007). The duration of SM may be short or long-lasting, the latter is usually considered chronic subclinical mastitis (CSM) (Viguier et al., Reference Viguier, Arora, Gilmartin, Welbeck and O'Kennedy2009). Several studies have evaluated milk losses (ML) due to SM based on the assumption that a rise in SCC above a threshold of 200 000 cells/ml causes a concomitant reduction in milk yield (Schukken et al., Reference Schukken, Wilson, Welcome, Garrison-Tikofsky and Gonzalez2003; Bradley and Green, Reference Bradley and Green2005; Hagnestam-Nielsen et al., Reference Hagnestam-Nielsen, Emanuelson, Berglund and Strandberg2009; Hand et al., Reference Hand, Godkin and Kelton2012; Archer et al., Reference Archer, Mc Coy, Wapenaar and Green2013). For example, ML evaluated from milk herd test records were estimated to be 0.3 to 0.7 kg/day for first-lactation cows and 0.6 to 1.9 kg/day for adult-lactation cows per unit increase of Ln SCC above the cutoff of 5.3 (200 000 cells/ml) (Dürr et al., Reference Dürr, Cue, Monardes, Moro-Méndez and Wade2008; Hagnestam-Nielsen et al., Reference Hagnestam-Nielsen, Emanuelson, Berglund and Strandberg2009; Halasa et al., Reference Halasa, Nielen, De Roos, Van Hoorne, de Jong, Lam, van Werven and Hogeveen2009; Hand et al., Reference Hand, Godkin and Kelton2012; Gonçalves et al., Reference Gonçalves, Cue, Botaro, Horst, Valloto and Santos2018a).
Despite previous studies determining ML based on SCC, none have used our approach of evaluating the effects of CSM caused by type of pathogen at the cow-level. How much of the increase of SCC in a cow affected by CSM is reflected in milk yield and quality at the cow-level? We questioned whether CSM could accentuate the effects on milk yield and composition when compared to non-chronic cases since there are results emphasizing that S. aureus-CSM produces virulence factors (cytolytic toxins, exfoliative toxin (TSST-1) and hyaluronidases which cause epithelial damage and mammary gland parenchyma necrosis) that may gradually lead to the replacement of the secretory tissue by a fibrotic one (Gudding et al., Reference Gudding, McDonald and Cheville1984; Barkema et al., Reference Barkema, Green, Bradley and Zadoks2009). Changes in milk yield and quality strongly depend on the pathogen (Coulon et al., Reference Coulon, Gasqui, Barnouin, Ollier, Pradel and Pomiès2002). Thus, we suggest major economic losses might be expected from CSM cases because mammary tissue from non-chronic cases can presumably return to some level of functionality. To our knowledge, few reports consider our comparison (CSM vs. non-chronic) using repeated milk sampling, and only Halasa et al. (Reference Halasa, Nielen, De Roos, Van Hoorne, de Jong, Lam, van Werven and Hogeveen2009) proposed an evaluation of the SM effects on milk composition.
Our hypothesis is that ML and any effects on components yield caused by CSM is higher than for SM, and is differently affected according to pathogen. Therefore, the study aimed to evaluate the effects of CSM caused by type of pathogens on milk and components yield at the cow level.
Materials and methods
Ethics approval was obtained from the Ethical Committee on the Use of Animals of the School of Veterinary Medicine and Animal Science, University of São Paulo (CEUA, FMVZ, USP – Brazil/SP, protocol number 3020/2013).
Data collection: selection of herds and cows, sampling procedures and laboratory procedures
Herds and cow selection
Herds were selected based on (i) the proximity to the milk quality lab (<100 km), (ii) having permanent individual cow identification and data recording systems in place and, (iii) use of a mastitis control program consistent with those established by the National Mastitis Council (NMC; http://www.nmconline.org). This included consistent use of pre- and postmilking teat dipping, application of dry cow therapy, periodic milking machine maintenance, and proper milking and intramammary treatment procedures. For instance, a cow with a previous history of clinical mastitis or a cow that had an episode of clinical mastitis during the experiment had to be eliminated from the study. These criteria led to the selection of eight commercial dairy herds (freestall facilities and herringbone-type milking parlor) located in the Midwest area of São Paulo State, Brazil which were enrolled for a nine-month sampling period. All farms were considered small or medium-sized, with an average of 82 Holstein dairy cows in lactation (range 23 to 165) and a milk yield average of 22.3 kg/cow/day (range 10 to 62.5 kg/cow/day) or approximately 6,792 kg/cow/year.
A total of 790 cows were initially sampled; the intention was to collect three samples from each cow, once every two weeks producing a total of 2087 records. Cow were excluded for clinical mastitis, being dried-off (and hence not having all three samplings) in the study period and excessive days in milk as detailed in Table 1. After all editing there were 388 cows (from six herds) which had provided all three samples. Each milk sample was analyzed for SCC and milk composition (fat, protein, lactose, solids non-fat, and total solids). At the third sampling, composite milk samples were collected aseptically for microbiological culture.
Table 1. Editing criteria and number of records retained and excluded

Bacterial species identification using MALDI-TOF MS
Microbiological analyses of milk samples were performed in accordance with the National Mastitis Council guidelines (NMC, 2017). For bacterial species identification using MALDI-TOF MS, one colony was applied to the steel plate spot with the aid of a wooden stick (Barcelos et al., Reference Barcelos, Martins, Grenfell, Juliano, Anderson, dos Santos and Gonçalves2019). A volume of 1.0 μl of formic acid (70%) was applied to the spot and allowed to dry at room temperature. After drying, 1.0 μl of α-cyano-4-hydroxycinnamic acid (HCCA) matrix solution was applied, and again left to dry at room temperature for 5 to 10 min. A standard protein solution (Bacterial Test Standard, BTS; Bruker) was used for calibration. The analysis employing the MALDI-TOF mass spectrometry methodology was performed in FlexControl 3.4 software (Bruker Daltonik, Bremen, Germany). The spectral data processing was done using the MALDI Biotyper 4.1.70 (Bruker Daltonik, Bremen, Germany) computer software for microorganism identification (MBT version 7311 MPS library) and a score of ≥ 2.0 indicated a species-level identification.
Milk composition analysis
Milk samples (40 ml) were collected into plastic tubes containing the antimicrobial bronopol (2-bromo-2-nitropropane-1,3-diol) as preservative (0.05 g/100 ml milk) according to the International Dairy Federation guidelines (IDF, 2013). Samples were kept refrigerated (4–7°C) until analysis of composition and SCC. Concentrations of milk fat, protein, lactose, total solids and solids non-fat were determined by infrared absorption system using a milk analyzer (Bentley 2000®, Bentley Instruments Inc., Chasca, MN USA). The SCC was determined by flow cytometry using a high capacity somatic cell counter (Somacount 300®, Bentley Instruments Inc., Chasca, MN, USA).
Data preparation and mastitis definitions
Cows were considered healthy if all three samples of SCC were ≤200 000 cells/ml and were culture-negative. Cows with one SCC > 200 000 cells/ml and a culture-positive result were considered non-chronic (SM). Cows with at least two of the three results with a SCC > 200 000 cells/ml were considered CSM. These CSM were then further sorted on the bacteriology to be chronic negative (chronicNC) or chronic positive (chronicPC) cows. Culture-positive results were considered for mastitis definition when milk samples showed an isolation of >10 colonies (1000 cfu/ml) of minor pathogens (Corynebacterium spp. or non-aureus Staphylococci); and ≥1 colony (100 cfu/ml) of other pathogens mastitis-causing. There were therefore four udder health categories: healthy, non-chronic, chronicNC or chronicPC.
The milk yield and compositions were evaluated according to the udder health status (healthy, non-chronic, chronicNC or chronicPC), and to pathogen groups (1 - major pathogens and 2 -minor) and by type of pathogen (Streptococcus uberis, Streptococcus dysgalactiae, Staphylococcus aureus, Streptococcus agalactiae, non-aureus Staphylococci and Corynebacterium spp.). Milk samples with more than one pathogen type detected were not included in the model (n = 10). From four milk samples, two different major pathogens were isolated, and from six milk samples, minor and major pathogens were observed. Milk samples with more than two pathogens detected were considered contaminated (n = 11) and were eliminated from all subsequent analyses (Table 1).
Editing criteria and statistical analysis
The number of records excluded and retained at each step of the editing and the reasons for the exclusions are shown in Table 1. After editing, there were 1164 records retained for the initial analyses of the effects of CSM on milk and components yields. The effects of CSM on milk and components yields were evaluated (Table 2). A linear mixed effects model was used for the analysis; for each of the dependent variables there were three measurements (at days 1, 15 and 30 of sampling, considered as sampling 1, 2 or 3 respectively) on each cow, and cow (random effect) was nested within herd, udder health status and parity. Statistical models were assessed using the SAS MIXED procedure (version 9.4, SAS Institute, Cary, NC, USA). With regard to all of the statistical analyses, P ≤ 0.05 was considered for significance. Thus, the following statistical models were applied:
(1) Yijkmpqr = μ + Hi + Statusj(UdderHealth) + Paritym + Cowijkm + wDIMp + Samplingq + Sampling × Statusjq + eijkmpqr
(2) Yijkmpqr = μ + Hi + Statusj(PathogenType) + Paritym + Cowijkm + wDIMp + Samplingq + Sampling × Statusjq + eijkmpqr
Table 2. Frequency of cow records according to udder health status (healthy, non-chronic, chronicPC and chronicNC) and culture results (N, negative; or P, pathogen isolation) based on three milk samplings

a Chronic positive-culture.
b Chronic negative-culture.
c Streptococcus like-bacteria: Enterococcus spp. (n = 10), Lactococcus spp. (n = 6) and Aerococcus spp. (n = 5).
*Milk samples with more than one pathogen being detected were not included in the model (n = 10); in 4 milk samples, 2 different major pathogens were isolated, and in 6 milk samples, isolation of minor and major pathogens were observed. Milk samples with more than two pathogens being detected were considered contaminated (n = 11) and were eliminated from all analyses.
in which Yijkmpqr was considered as the continuous dependent variable, in turn log SCC, milk yield, fat yield and fat %, total protein yield and %, lactose yield and %, total solids yield and %, and solids not fat yield and % (see Table 3). μ represented the general average. Hi represented the herd (i = 1 to 6), considered as a fixed effect since herds were selected based on their willingness to collaborate in this study. For model 1, Statusj(UdderHealth) represented the presence or absence of infection during the three milk samplings (j = 1 to 4; healthy, non-chronic, chronicNC or chronicPC). For model 2, Statusj(PathogenType) represented the status regarding the type of pathogen during the three milk samplings (o = 1 to 8; major, minor, S. uberis, S. dysgalactiae, S. aureus, S. agalactiae, Non-aureus Staphylococci and, Corynebacterium spp.). Cowijkm represented the cow (within herd, status and parity) which was considered as a random effect (k = 1 to 388). Paritym was considered as the fixed classification effect of the number of calvings (m = 1, 2 and ≥3). wDIMp represents the weeks in milk, included as a fixed classification effect (P = 1 to 69). Samplingq was the effect of time, milk samplings once every two weeks as interval (q = 1 to 3, sampling at 1st, 15th and 30th days of the study). Sampling × Statusjq was the interaction between sampling period and udder health status, and eijkmpqr represented the random residual. Herd was considered fixed, as the selection of the herds cannot completely be considered random; in addition, with only six herds the reliability of any herd variance estimate would be low. Our interest was not to assess the variability amongst herds, rather the focus was on comparing the four cow udder health statuses. To achieve a normal distribution, somatic cell counts (×103) were (natural) log transformed for all statistical analyses, and were back transformed for presentation in tables and figures. Initial analyses, for both Model 1 and 2, included a herd × status interaction, to test whether the effects were the same/similar across herds, or not. The herd interactions were not statistically significant, this term was dropped from the model and is not shown.
Table 3. Effect of non-chronic, chronicPC, chronicNC on milk and components yield in comparison with healthy cows

LnSCC was back transformed and values of SCC were presented within brackets [ × 103 cells/ml].
Standard error was presented within parentheses (se). Different letters mean P < 0.05.
aChronic positive-culture.
b Chronic negative-culture.
c Milk loss estimated by the difference of milk yield from healthy vs. non-chronic, chronicNC and chronicPC cows.
Results
A total of 134 out of 388 cows (34.5%) had chronicPC episodes, 57 cows (14.7%) had chronicNC, 78 cows (20.1%) had non-chronic episodes and 119 cows (30.7%) were considered healthy, which resulted in a grand total of 1,164 cow records included in the statistical model (Tables 1 and 2). The culture-positive cows (n = 330) comprised 47.3% with minor pathogen infections (n = 156/330; Non-aureus Staphylococci, n = 126; and Corynebacterium spp., n = 30), 44.5% with major pathogen infections (n = 147/330; S. uberis, n = 42; S. dysgalactiae, n = 36; S. agalactiae n = 39; and S. aureus, n = 30) and the remainder with Streptococcus like-bacteria (6.4%, n = 21/330) or Klebsiella spp. (1.8%, n = 6/330) infections (Table 2).
Effects of udder health status on milk and components yield
Cows with chronicNC and chronicPC had lower milk and components yields when compared with non-chronic and healthy cows (P < 0.05; Table 3). ChronicPC cows had a higher SCC (756.2 × 103 cells ml, n = 402) in comparison with chronicNC cows (437.1 × 103 cells/ml, n = 171), non-chronic cows (168.8 × 103 cells ml, n = 234), and healthy cows (62.2 × 103 cells/ml, n = 357). The healthy cows produced more milk than non-chronic chronic cows (+2.1 kg/cow/day), chronicNC (+4.1 kg/cow/day) and chronicPC (+5.7 kg/cow/day). Non-chronic mastitis caused a 9% of milk reduction, 17.3% of reduction when cows had chronicNC and 24.1% when cows had chronicPC, but milk reduction was even higher in cows where chronicPC was caused by major pathogens (29.5%).
The milk component yields of the healthy cows were similar to those observed for non-chronic cows, except for lactose (healthy, 108.5 g/cow/day vs. non-chronic, 97.5 g/cow/day: Table 3). ChronicNC cows' component yields were lower than those from healthy cows except for fat (healthy, 93.78 g/cow/day vs. chronicNC, 87.0 g/cow/day). In addition, healthy cows produced more milk components than chronicPC cows (total solids, +69.6; fat +19.4; total protein +15.1; lactose +29.9 and solids nonfat +50.3 g/cow/day)
Effects of type of pathogens causing CSM on milk and components yield
ChronicPC cows infected by both major and minor pathogens cows had lower milk and component yields when compared with healthy cows (P < 0.05; Table 4). The SCC of chronicPC cows caused by major pathogens (792.7 × 103 cells, n = 129) and minor pathogens (644.3 × 103 cells, n = 111) were much higher than for healthy cows (about 60 × 103 cells, n = 357). Consequently, healthy cows produced more milk than cows with chronicPC caused by minor (+5.2 kg/cow/day) and major pathogens ( + 7.1 kg/cow/day). ChronicPC cows had lower milk components yields when infected by minor and major pathogens in comparison with healthy cows (Table 4).
Table 4. Effect of chronic subclinical mastitis caused by major and minor pathogens on milk and components yield in comparison with healthy cows
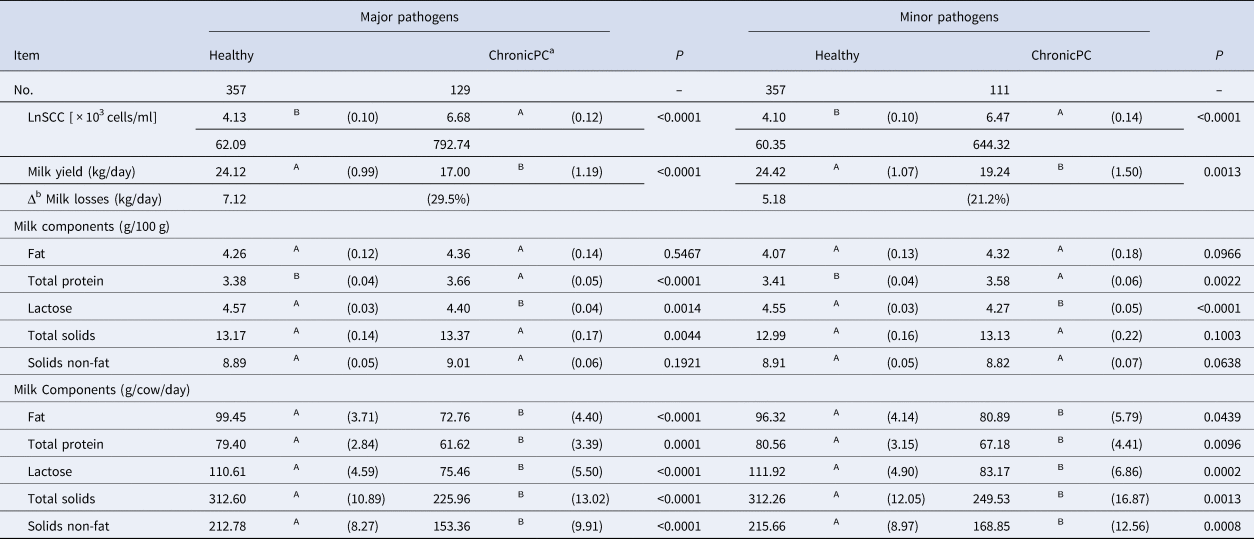
LnSCC was back transformed and values of SCC were presented within brackets [ × 103 cells/ml].
Standard error was presented within parentheses (se). Different letters mean P < 0.05.
a Chronic positive-culture.
b Milk loss estimated by the difference of milk yield from healthy vs. chronicPC cows.
Cows with chronicPC caused by S. uberis, S. dysgalactiae, Streptococcus like-bacteria, S. aureus, S. agalactiae and Non-aureus Staphylococci had lower milk yields when compared with healthy cows (P < 0.05; Fig. 1). Milk losses varied from 5.8 ± 1.5 to 11.8 ± 3.3 kg/cow/day depending on the pathogen. Milk losses per type of pathogens were 24.5% when chronicPC was caused by S. aureus; 26% for S. uberis and non-aureus Staphylococci, 27% for S. dysgalactiae, 35.3% for S. agalactiae and 44.9% for Streptococcus like-bacteria. On the contrary, chronicPC cows infected by Corynebacterium spp. had similar milk yields when compared with healthy cows.

Fig. 1. Effect of chronic subclinical mastitis caused by type of pathogens on milk yield in comparison with healthy cows and, *Δ milk loss estimated by the difference of milk yield from healthy vs. chronicPC cows. ±se and NS, not significant.
Cows with chronicPC (except those infected by Corynebacterium spp.) had lower milk component yields than healthy cows (Fig. 2). Milk protein yield losses of chronicPC cows varied from 17.6% to 33.2%, and equivalent figures for losses of other components were, fat 16.5 to 39.6%, lactose 18.5 to 47.5%, solids non-fat 17.5 to 41.8% and total solids 17.9 to 41.5%, depending on the pathogen (Fig. 2).

Fig. 2. Effect of chronic subclinical mastitis caused by type of pathogens on milk components yield in comparison with healthy cows and, *Δ milk components loss estimated by the difference between healthy vs. chronicPC cows. ±se. *P < 0.05. NS, not significant.
Discussion
We questioned if milk yield and milk composition is altered more by CSM than by non-chronic (SM) in dairy cows, and if any differences are pathogen dependent. It is very well known that ML occurs due to damage caused by pathogens to the secretory tissues of the mammary gland and the breakdown of cell junctions, which may result in the permanent loss of milk synthesis capacity (Forsback et al., Reference Forsback, Lindmark-Mansson, Andren, Akerstedt and Svennersten-Sjaunja2009). ML found in the present study varied from 24.5 to 44.9% in comparison with healthy cows and had a strong dependency on the pathogen type.
Non-chronic cases frequency (20.1%) are consistent with what we find in other dairy herds in Brazil,. but surprisingly higher for chronicPC cases (34.5%). Most of the composite milk samples were culture-negative (>65%) and we believe that culture was positive in 35% of the cases because cows had chronic infection. The interesting results were not only the reduced milk yield of chronicPC cows, it was that the reduction of milk yield was larger in chronicPC cows than in the other SM or SCM cows. Therefore, our obtained results emphasize the importance of the bacteriology results for preventing and controlling pathogens that cause chronic mastitis. ChronicNC had less accentuated effects on milk yield and composition which might be due to the intermittent shedding of bacteria which varies with the type of pathogen. Alternatively, the immune system of the cows may already have solved the problem prior to the milk sampling.
Similar to our study, ML based on results of a single SM (non-chronic) episode caused by S. aureus, S. agalactiae and environmental streptococci had a reduction of 0.6, 1.3 and 0.5 kg/day (Wilson et al., Reference Wilson, Gonzalez and Das1997), respectively, which were lower if we compare them with the observed ML caused by the same pathogens isolated from chronicPC cows. A previous study showed ML of 26.3% by S. aureus, 13.8% by CNS, 11.7% by Streptococcus spp. and 8.4% by Corynebacterium spp. (França et al., Reference França, Del Valle, Campana, Veronese, Nascimento and Morais2017). This ML was higher than we observed from non-chronic mastitis (14% vs. 9%). On the other hand, the ML observed here due to CSM was greater than the estimation of ML based on studies using only SCC evaluation (Hagnestam-Nielsen et al., Reference Hagnestam-Nielsen, Emanuelson, Berglund and Strandberg2009; Hand et al., Reference Hand, Godkin and Kelton2012; Gonçalves et al., Reference Gonçalves, Cue, Botaro, Horst, Valloto and Santos2018a). Hand et al. (Reference Hand, Godkin and Kelton2012) described ML varying from 1.8 to 7.6% for first parity and 2.5 to 10.9% for second parity cows when SCC ranged from 200 to 1000 × 103 cells/ml and Hagnestam-Nielsen et al. (Reference Hagnestam-Nielsen, Emanuelson, Berglund and Strandberg2009) found ML varying from 1.4 to 11.5% depending on the cow parity and the SCC ranging from 100 to 1000 × 103 cells/ml. To summarize, in the present study, we observed that ML in cows with CSM was more intense than cows with SM, which might depend on the persistence of the causative agent in the mammary gland.
We also observed lower milk component yield, but higher milk component concentration when healthy vs. chronicPC cows were compared. The higher concentrations of fat and total protein in chronicPC cows may be partially caused by the compensation effect of lower milk yield. It is known that intramammary infection changes the blood-milk barrier permeability, provoking a concurrent efflux of lactose and K+ into the bloodstream and an increase from the bloodstream of Na+, Cl− and whey proteins in milk (Gonçalves et al., Reference Gonçalves, Kamphuis, Martins, Barreiro, Tomazi, Gameiro, Hogeveen and dos Santos2018b). Consequently, it explains why casein concentration is reduced when SCC is elevated, since there is an increase in proteolytic enzymes (Urech et al., Reference Urech, Puhan and Schallibaum1999; Leitner et al., Reference Leitner, Krifucks, Merin, Lavi and Silanikove2006). Our results suggest that the reduction of milk component yields varied according to the type of mastitis causing pathogens (Table 4 and Fig. 2).
A negative relationship between SCC and content of milk components, similar to what we observed in the present study, was reported by Park et al. (Reference Park, Koo, Kim, Hwang, Jung, Kim, Shin, Kim and Park2007) when they compared cows culture-positive and SCC ≥ 500 × 103 cells. França et al. (Reference França, Del Valle, Campana, Veronese, Nascimento and Morais2017) observed similar fat content between healthy and SM cows, but they reported an increase of 3.15% in total protein content and a decrease of 5.6% in lactose content. Milk component alteration results described by França et al. (Reference França, Del Valle, Campana, Veronese, Nascimento and Morais2017) were much lower than those observed from chronicPC cows in the present study.
Pathogens that elicit a greater somatic cell response are considered ‘major’ ones (e.g. S. agalactiae, S. aureus, S. uberis, and S. dysgalactiae), in contrast to ‘minors’ (Corynebacterium spp. and non-aureus Staphylococci) that are more likely to result in an SCC level below 200 000 cells/ml (Bradley and Green, Reference Bradley and Green2005). We have adopted this classical pathogen grouping based on species identification by MALDI-TOF, which might be considered one limitation of our study since some minor pathogens (e.g. S. chromogenes) have presented similar adhesiveness capacity on the mammary secretory tissue as S. aureus (Taponen and Pyorala, Reference Taponen and Pyorala2009).
Finally, the dairy farms used in the present study were considered to be well managed Brazilian farms. However, based on our results of chronicPC cases we may infer that Brazilian herds had more chronic cases than expected and these facts suggest the adoption of a control plan including preventive measures, e.g. monthly monitoring of SCC and culturing cows with persistent elevated SCC might help in making treatment decisions against the chronic mastitis-causing pathogen. Identifying the responsible pathogens is important, because this might suggest what treatment protocol(s) to use. For example, if the pathogen is S. agalactiae it is known that the use of antibiotics can give a 95% cure rate. However, the presence of an environmental pathogen may indicate the need for a change in bedding management or pre-milking holding area.
In conclusion, lactating cows with CSM had higher SCC, lower milk yield and altered milk composition when compared to cows diagnosed with SM or healthy cows, and this difference was even higher when the CSM case presented as culture-positive. The changes observed in milk yield and compositions were dependent on the type of mastitis (CSM vs. non-chronic) and the type of pathogen causing subclinical mastitis (major and minor pathogens).
Acknowledgments
The authors acknowledge FAPESP for the scholarship (Proc. 2013/23613-8, Proc. 2015/00142-5 and Proc. BEPE 2015/04570-1), and project funding (Proc. 2014/17411-6). We thank ‘Qualileite’, Milk Quality Laboratory (School of Veterinary Medicine and Animal Science – USP, Brazil) team and all milk producers, for their assistance with milk sampling period.








