Unlike the clinical form, subclinical mastitis does not cause significant changes in milk appearance, nor does it show the typical signs of inflammation in infected udders. However, in addition to increasing somatic cell counts, milk production decreases, making this form of mastitis responsible for most of the economic losses in the dairy industry, due to its high prevalence (Busanello et al., Reference Busanello, Rossi, Cassoli, Pantoja and Machado2017). Studies have shown that the prevalence of subclinical mastitis in dairy cows is close to 48% on Brazilian farms (Acosta et al., Reference Acosta, Silva, Medeiros, Pinheiro-Júnior and Mota2016; Busanello et al., Reference Busanello, Rossi, Cassoli, Pantoja and Machado2017).
Staphylococcus aureus is one of the main causes of this disease (Gomes et al., Reference Gomes, Saavedra and Henriques2016) and the severity of mastitis caused by this bacterium is related to the production of several virulence factors involved in tissue adhesion, evasion of host defenses and injuring host cells (Cote-Gravel and Malouin, Reference Cote-Gravel and Malouin2019). The frequency of S. aureus in Brazilian dairy farms is over 70% (Mesquita et al., Reference Mesquita, Rocha, Bruhn, Custódio, Braz, Pinto, Silva and Costa2019) and bacteria of this genus can represent 1 to 90% of the microorganisms causing both clinical and subclinical mastitis. However, coagulase negative staphylococci (CoNS) exceed the frequency of S. aureus causing the disease in all regions of Brazil (Acosta et al., Reference Acosta, Silva, Medeiros, Pinheiro-Júnior and Mota2016).
The persistence of CoNS is related to the high levels of antimicrobial resistance genes carried by them, and to the fact that many strains remain in the form of biofilms, making infections difficult to eradicate and, therefore, recurrent (Becker et al., Reference Becker, Heilmann and Peters2014; Marsilio et al., Reference Marsilio, Di Francesco and Di Martino2018). The dense matrix-embedded communities of biofilms makes it difficult for antibiotics to reach their targets and provides an environment of increased availability of mobile genetic elements, through which antibiotic resistance can be exchanged between staphylococci (Gomes et al., Reference Gomes, Saavedra and Henriques2016; Rossi et al., Reference Rossi, Pereira and Giambiagi-deMarval2020).
The development of biofilms by S. aureus is influenced by the production of the multi-domain and cell-surface anchored biofilm-associated protein (Bap) and is dependent on the synthesis of the polysaccharide intercellular adhesin (PIA), encoded by the icaADBC operon (Cucarella et al., Reference Cucarella, Solano, Valle, Amorena, Lasa and Penades2001; O'Gara, Reference O'Gara2007). Even though CoNS are the main cause of bovine subclinical mastitis in Brazil, the vast majority of studies still focus on the clinical form of the disease and on S. aureus isolates. In this study we aimed to characterize biofilm formation by different Staphylococcus species, mainly CoNS, isolated from bovine subclinical mastitis, analyzing the genetic diversity between the strains, the distribution of the bap and icaA genes and the effect of biofilm establishment on antimicrobial resistance.
Materials and methods
Microorganisms and culture conditions
We studied a diverse collection of Staphylococcus strains (n = 44) isolated from 22 different farms that comprise the main milk-producing areas of southeastern Brazil (online Supplementary Table S1), provided by EMBRAPA Dairy Cattle (Juiz de Fora, MG, Brazil). All strains were isolated from the milk of cows with subclinical mastitis, according to recommendations of the National Mastitis Council (Oliver et al., Reference Oliver, Gonzalez, Hogan, Jayarao and Owens2004). These strains belong to eight species, identified by RFLP-PCR of the groEL gene (Santos et al., Reference Santos, Barros, Brito, Bastos, Santos and Giambiagi-deMarval2008): the coagulase-positive S. aureus (n = 5) and S. hyicus (n = 1), and the coagulase-negative S. capitis (n = 3), S. caprae (n = 1), S. chromogenes (n = 27), S. epidermidis (n = 4), S. haemolyticus (n = 2), and S. xylosus (n = 1). Culture stocks maintained in Brain Heart Infusion (BHI, Difco, USA) and glycerol 30% at −20°C were activated on blood agar plates and inoculated in BHI and incubated at 37°C for 24 h before the experiments.
Pulsed-field gel electrophoresis
The clonal diversity of the strains was evaluated by means of their chromosomal restriction band patterns, produced after digestion with the restriction enzyme SmaI, separated by pulsed-field gel electrophoresis (PFGE), as suggested by Nunes et al. (Reference Nunes, Teixeira, Bastos, Silva, Ferreira, Fonseca and Santos2005). Restriction band patterns were analyzed visually, as recommended by van Belkum et al. (Reference Van Belkum, Tassios, Dijkshoorn, Haeggman, Cookson, Fry, Fussing, Green, Feil, Gerner-Smidt, Brisse and Struelens2007), and with the Gel Compar II software version 4.01 (Applied Maths, Belgium) using the unweighted pair-group method with arithmetic mean (UPGMA) algorithm and the Jaccard coefficient. Strains that were the only ones in their species were not included in the analysis.
Biofilm formation assay
Biofilm formation by the strains was evaluated by the crystal violet method, as described by Ahn et al. (Reference Ahn, Lemos and Burne2005). Briefly, overnight cultures were diluted (1:50) in fresh BHI and grown at 37°C to an OD600 nm of 0.4. Then, the cultures were diluted (1:100) in BHI supplemented with 1% glucose in polystyrene microplates to a final volume of 200 μl. After incubation at 37°C for 24 h, planktonic cells were removed, cells that were adhered to the wells were gently washed three times with distilled water and the plates were dried at 60°C for 1 h. The adherent bacteria were stained for 15 min with 0.1% crystal violet, and the microtiter plates were gently immersed in water to rinse the wells, followed by drying at 60°C for 30 min. Stained cells were resuspended in 200 μl of ethanol and the OD570 nm was measured. Tests were performed in triplicate. Strains were classified as non, weak, moderate or strong biofilm producers, as suggested by Stepanovic et al. (Reference Stepanovic, Vukovic, Hola, Di Bonaventura, Djukic, Cirkovic and Ruzicka2007).
Detection of bap and icaA genes by PCR
Total DNA was extracted from all strains by the guanidinium thiocyanate method (Pitcher et al., Reference Pitcher, Saunders and Owen1989). The genes bap and icaA were amplified with the oligonucleotides bap2F/bap2R and icaAF/icaAR, respectively (Potter et al., Reference Potter, Ceotto, Giambiagi-Demarval, dos Santos, Nes and Bastos Mdo2009). Amplification was performed with 100 ng of DNA, 0.2 mm of each primer, 0.5 mm of each dNTP, 1.5 mm MgCl2, and 1 U of Taq DNA polymerase in amplification buffer (50 mm KCl, 20 mm Tris-HCl, pH 8.4, Biotools, USA). Amplicons (598 bp for bap and 287 bp for icaA) were observed after electrophoresis on 1.5% agarose gel and staining with ethidium bromide 0.5 μg/ml. S. epidermidis ATCC 35 984 was used as a control for icaA amplification (Arciola et al., Reference Arciola, Baldassarri and Montanaro2001), and S. aureus V329, as a control for bap amplification (Cucarella et al., Reference Cucarella, Solano, Valle, Amorena, Lasa and Penades2001).
Detection of bap and icaA genes by dot blotting
The detection of the bap and icaA genes was complemented by dot blotting. The probe consisted of PCR products obtained for each gene from their respective positive controls (described above), which were purified with the QIAquick PCR purification kit (QIAgen, Germany), and tagged with digoxigenin using the Dig DNA Labeling and detection kit (Roche, Switzerland), according to the manufacturer's instructions. Total DNA of all strains were applied separately as dots to a Hybond N+ nylon membrane (GE Healthcare Life Sciences, USA) and fixated by UV radiation, following the procedures suggested by Sambrook and Russell (Reference Sambrook and Russell2001). For icaA probes, hybridization was performed at 56°C and washes were carried out under high-stringency conditions; for bap probes, hybridization was performed at 46°C and under low-stringency conditions. The same positive controls mentioned above were used in this procedure.
Antimicrobial resistance tests
The antimicrobial susceptibility of the strains was tested in triplicate by the disk diffusion method, against the following antimicrobial agents (Cecon, Brazil): ampicillin (10 μg), cefoxitin (30 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), clindamycin (2 μg), erythromycin (15 μg), gentamicin (10 μg), oxacillin (1 μg), penicillin G (10 UI), rifampicin (5 μg), trimethoprim-sulfamethoxazole (23.75/1.25 μg), tetracycline (30 μg) and mupirocin (200 μg). The minimum inhibitory concentrations (MIC) of erythromycin and tetracycline for selected strains were determined by the broth microdilution method. Tests were performed and interpreted according to the Clinical and Laboratory Standards Institute guidelines (CLSI, 2016).
Effects of minimum inhibitory concentrations on preformed biofilms
Strong biofilm-producing strains were selected to evaluate the effect of the antibiotics erythromycin and tetracycline on biofilm suppression, following the procedures suggested by Flemming et al. (Reference Flemming, Klingenberg, Cavanagh, Sletteng, Stensen, Svendsen and Flaegstad2009). Biofilms were preformed as described above, and incubated for 24 h and 48 h. After incubation, the culture medium was removed from the wells and replaced by 200 μl of fresh Mueller Hinton broth (Difco, USA) containing concentrations of each antibiotic equivalent to their respective MIC, 5xMIC and 10xMIC. Microplates were incubated again for 24 h at 37°C. Then, the supernatant was removed, and wells were carefully washed twice with sterile PBS. To quantify viable cells, we added 200 μl of BHI supplemented with 1% glucose and 5% of the redox indicator AlamarBlue (Invitrogen, USA). After 1 h of incubation at 37°C, the OD570 nm was measured. The negative control (NC) of these experiments consisted only of culture medium, while the positive control (PC) consisted of biofilms that were not subjected to antimicrobial treatment. Tests were performed in triplicate and results were interpreted as follows: strong biofilm suppression if the antimicrobial caused a ≥75% reduction in OD570 nm when compared to the PC; complete suppression if the antimicrobial caused a reduction ≤ the NC, and no suppression if none of the previous criteria were met.
Statistical analysis
The Spearman's correlation and the χ2 tests were performed to investigate if there was a correlation between the presence of bap or icaA genes and biofilm formation. All analyses were done using Graphpad Prism 6 (available at https://www.graphpad.com/) and the degree of statistical significance was 95% (P < 0.05).
Results
Clonal diversity of strains
The strains studied here comprise a genetically diverse group, with 24 different genotypes and 18 subtypes obtained by PFGE (Fig. 1). S. chromogenes, the largest group, included nine pulsotypes and 16 subtypes. Considering them, only two strains (4496 and 4497, possibly siblings) isolated from the same farm (S) were classified with the same pulsotype (20C). Two strains from the N farm were classified with pulsotypes 18d and 18e, and showed a similarity closer to 80%. Pulsotype 18 was the most common among S. chromogenes. Considering the other species, only two isolates of S. aureus, classified as pulsotypes 1a and 1b, obtained from different farms, showed approximately 80% similarity (strains 3264 and 5475).

Fig. 1. Pulsed-field gel electrophoresis (PFGE) profiles of the genomic DNA of Staphylococcus strains causing subclinical mastitis, digested with the SmaI restriction enzyme.
Phenotypic and genetic characteristics of biofilms
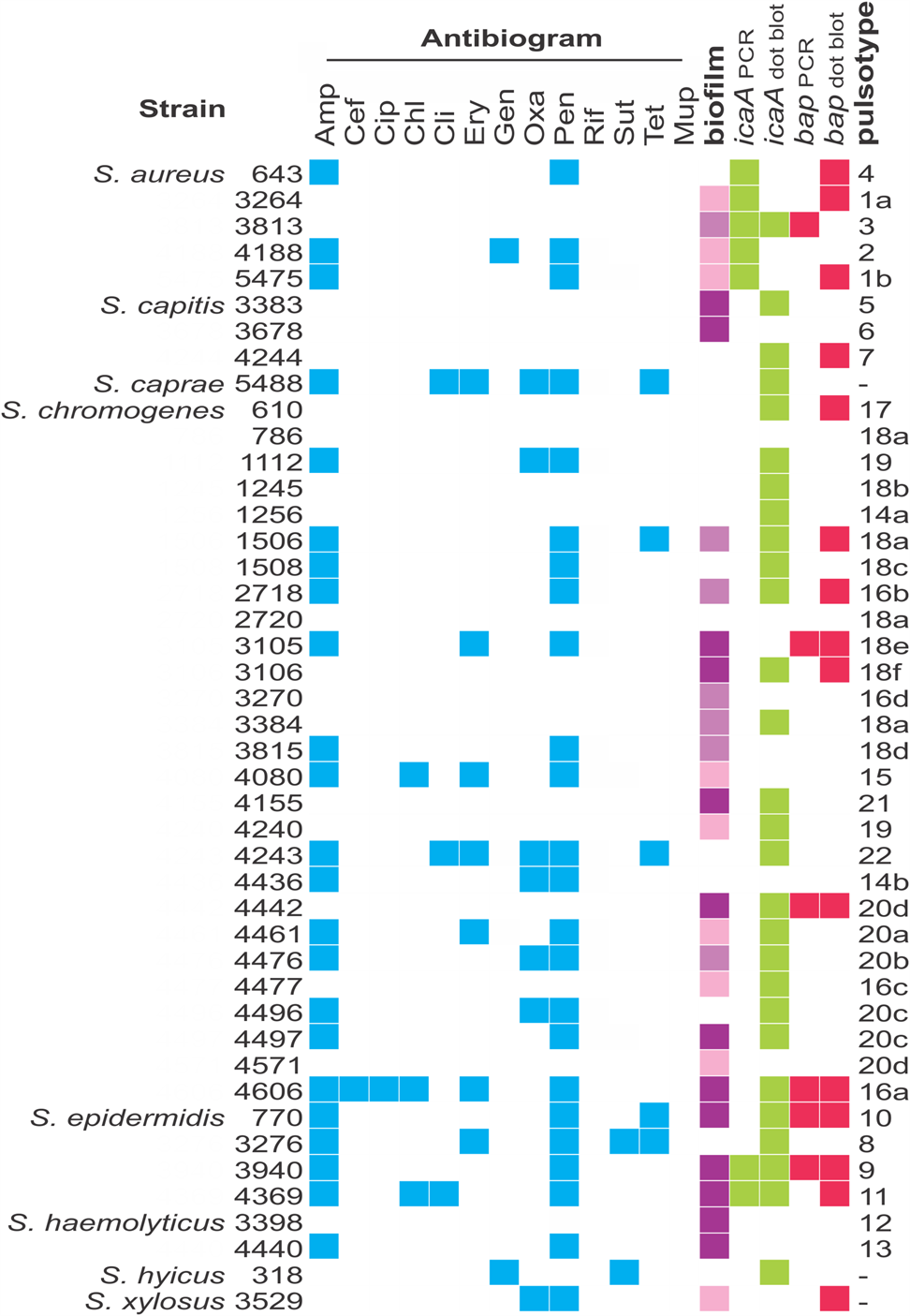
Most strains studied (65.9%) formed biofilms in the conditions tested, but at different levels (Fig. 2). They were classified as strong (29.5%), moderate (15.9%), weak (20.5%) or non-biofilm producers (34.1%). Regardless of their ability to form biofilms, most strains harbored the icaA gene (72.7%), while 36.3% contained the biofilm-associated bap gene. PCR results were confirmed by dot blotting, but the second was more sensitive (Fig. 2); while 15.9% of strains were PCR-positive for icaA, 63.6% were positive by dot blotting. The same was observed for the bap gene; 13.6% were PCR-positive, 34.1% were positive by dot blotting. There was no statistically significant (P < 0.05) correlation between biofilm formation and the presence of bap and icaA gene in these strains, as non-biofilm producers, such as S. aureus 643, S. capitis 4244 and S. chromogenes 610 were positive for both genes, while strong biofilm producers, like S. haemolyticus 3398 and 4440 were negative for both.
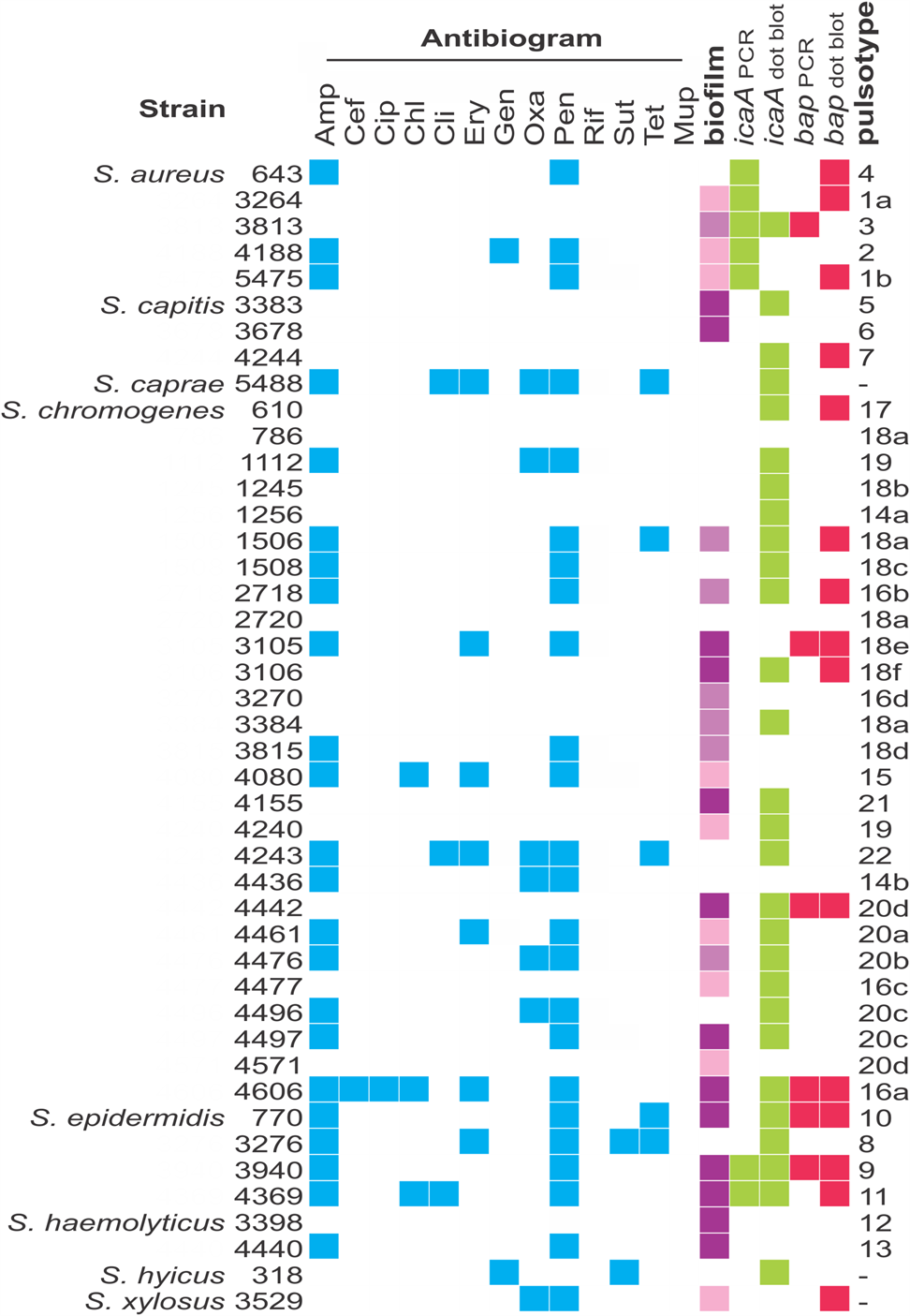
Fig. 2. Characterization of the Staphylococcus strains used in this work. Blue squares show resistance to the antimicrobials used; green and pink squares designate positive results for the presence of icaA and bap genes, respectively. Classification of biofilm formation is displayed in different shades of purple, which represent, from the lightest to the darkest tone, weak, moderate and strong biofilm formation. Pulsotypes were defined based on PFGE patterns, analyzing each species separately. Antimicrobials: ampicillin (Amp), cefoxitin (Cef), chloramphenicol (Chl), ciprofloxacin (Cip), clindamycin (Cli), erythromycin (Ery), gentamicin (Gen), oxacillin (Oxa), penicillin G (Pen), rifampicin (Rif), trimethoprim-sulfamethoxazole (Sut) and tetracycline (Tet).
Antimicrobial resistance profile
More than half (56.8%) of the strains were resistant to at least one of the 13 antimicrobials tested (Fig. 2). Resistance to penicillin (54.5%) and ampicillin (52.3%) were the most common. Additionally, 13.6% of the strains were classified as multidrug resistant due to resistance to at least three classes of antibiotic. All strains were susceptible to rifampicin.
Based on their strongly attached biofilms and antimicrobial resistance profiles, we chose four strains to conduct the subsequent experiments: S. capitis 3383, S. chromogenes 4442 and S. haemolyticus 3398, which were susceptible to all antimicrobials tested, and S. epidermidis 770, resistant to ampicillin, penicillin G and tetracycline. For trials with preformed biofilms and drugs, erythromycin and tetracycline were chosen given the low resistance rate among the strains, 15.9% and 11.3%, respectively. Erythromycin MIC ranged from 0.25 to 0.5 μg/ml and tetracycline MIC ranged from 0.03125 to 0.125 μg/ml for susceptible strains; for the tetracycline-resistant S. epidermidis 770, MIC was 64 μg/mL (Table 1).
Table 1. Minimum inhibitory concentration and biofilm suppression by erythromycin (Ery) and tetracycline (Tet) in Staphylococcus strains

Effects of MIC, 5MIC and 10xMIC on preformed biofilms
We did not observe any complete or strong suppression (over 70%) of the viability of bacterial cells in their biofilms using inhibitory concentrations of erythromycin or tetracycline (Table 1). In biofilms, all strains tested were able to grow at the concentrations of their respective MICs and concentrations of 5x and 10x their MICs. The highest rates of biofilm suppression were observed in younger (24 h) biofilms of S. epidermidis 770 using different concentrations of tetracycline (35% for MIC, 52% for 5xMIC and 55% for 10xMIC). In general, suppression rates were higher as the concentrations of antibiotics increased, when considering biofilms of the same age (Table 1).
Discussion
Although subclinical mastitis is highly prevalent in dairy herds and is caused mainly by coagulase-negative staphylococci, most studies still focus on S. aureus and the clinical form of the disease, with many of them not even addressing the species of CoNS involved in the infectious processes (Acosta et al., Reference Acosta, Silva, Medeiros, Pinheiro-Júnior and Mota2016; Busanello et al., Reference Busanello, Rossi, Cassoli, Pantoja and Machado2017). While this must be related to the fact that CoNS have historically been referred to as less pathogenic, since they lack the most of the virulence factors of S. aureus, the realization that CoNS can cause several diseases suggests the opposite may be true (Becker et al., Reference Becker, Heilmann and Peters2014).
In addition to being the main cause of subclinical mastitis, studies have shown that CoNS species can act as reservoirs of antimicrobial resistance genes, which can be transferred to more pathogenic species, such as S. aureus, thus increasing their potential for resistance to drug therapy. This, therefore, represents a major threat (Coimbra-e-Souza et al., Reference Coimbra-e-Souza, Rossi, Jesus-deFreitas, Brito, Laport and Giambiagi-deMarval2019; Rossi et al., Reference Rossi, Pereira and Giambiagi-deMarval2020). The exchange of genetic material is greatly facilitated by bacterial adhesion to biotic and abiotic surfaces, forming biofilms, that is, dense communities embedded in a self-produced matrix of exopolysaccharides (Madsen et al., Reference Madsen, Burmolle, Hansen and Sorensen2012). The high cell density of biofilms leads to increased concentration of exogenous DNA, enhanced cell competence to receive mobile genetic elements, and stabilization of cell-cell contact by the matrix, which may facilitate horizontal gene transfer and dispersion of antimicrobial resistance, while protecting bacteria from hosts' immune defenses and environmental stresses (Flemming et al., Reference Flemming, Wingender, Szewzyk, Steinberg, Rice and Kjelleberg2016).
In this study, we analyzed the phenotypic and genotypic characteristics of biofilm production by a collection of staphylococcal isolates causing subclinical mastitis, most of which are CoNS. Their diversity was verified by PFGE, and is explained by the fact that small independent dairy farmers are responsible for most of the milk produced in Brazil, which increases diversity, while restricting strains to the animals of the same farm (Balcão et al., Reference Balcão, Longo, Costa, Uller-Gómez, Filho and Hötzel2016). Many of the strains studied in this work are resistant to multiple drugs, as they have acquired non-susceptibility to at least one agent in three or more categories of antimicrobials. This may be a consequence of the prophylactic use of antimicrobial drugs, a common practice in animal production in several countries, with the aim of promoting growth or preventing the development of diseases when animals are subjected to stressful conditions (Aarestrup, Reference Aarestrup2015). Resistance to oxacillin and cefoxitin is particularly alarming, since it indicates that strains are resistant to methicillin and cannot be combatted with last-resort antibiotics. In addition, resistance to most of the other antibiotics is frequently associated with the acquisition of genes located in mobile genetic elements, as recently reviewed by Schwarz et al. (Reference Schwarz, Febler, Loncaric, Wu, Kadlec, Wang and Shen2018), which can be exchanged in biofilms (Rossi et al., Reference Rossi, Pereira and Giambiagi-deMarval2020).
As noted here, most Staphylococcus involved with subclinical mastitis can form biofilms. While biofilm formation is known to be a multifactorial feature, it is strongly related to the production of two of the most studied factors: (i) the surface protein Bap (biofilm-associated protein) and (ii) the products of the ica operon, which coordinate the synthesis and export of the most important adhesive molecule of staphylococcal biofilms, the (exo)polysaccharide intercellular adhesion (PIA), also called poly-N-acetylglucosamine or PNAG (Otto Reference Otto2013; Salina et al., Reference Salina, Guimarães, Richini Pereira, Menozzi, Rall and Langoni2020). Even though bap and icaA are widespread among staphylococci, we found no significant correlation between genotype (the presence of these genes) and phenotype (biofilm production). This lack of correlation has been reported before for CoNS causing mastitis in dairy animals (Coimbra-e-Souza et al., Reference Coimbra-e-Souza, Rossi, Jesus-deFreitas, Brito, Laport and Giambiagi-deMarval2019), and indicates that other factors must be involved in biofilm formation by non-aureus staphylococcal species. These factors can include extracellular DNA (eDNA) released from lysed bacteria and several other adhesive proteins, such as the accumulation-associated protein (Aap), protein A, fibrinogen-binding proteins FnbpA and FnbpB, among others (Otto, Reference Otto2013). In addition, discrepancies between the detection of bap and icaA by PCR and dot blotting indicate that these genes may present polymorphisms among staphylococcal species, and the lack of sequences available for understudied CoNS makes it difficult to elaborate universal molecular markers for screening these genes. Most of the dozens of icaA sequences currently available on Genbank were obtained from S. aureus, S. epidermidis and S. haemolyticus and have only 75% of identity (data not shown), and there are only a few complete bap sequences available, mostly from S. aureus.
Although we still do not fully understand the nature of biofilms formed by non-aureus Staphylococcus species, here we showed that its production is an important protective barrier for CoNS, interfering with the activity of erythromycin and tetracycline, since a concentration equivalent to 10 × of each antibiotic MIC was not enough to suppress cell viability. Biofilms can act as a barrier to prevent antibiotics from reaching their molecular targets by various mechanisms, including drug chelation and precipitation, modification and degradation of the antibiotic, and tolerance to due to slower growth (Flemming et al., Reference Flemming, Wingender, Szewzyk, Steinberg, Rice and Kjelleberg2016).
In conclusion, our study shows that a variety of Staphylococcus species are involved in subclinical mastitis, most of which can form biofilms and carry a high rate of resistance to ampicillin and penicillin G, but also to several other antibiotics commonly associated with mobile genetic elements in Staphylococcus. This work also reinforces the concern that high concentrations of antimicrobials may not be sufficient to eradicate biofilm cells, so their use in dairy animals may not only may be ineffective in controlling infections, but also may support selection of resistant microorganisms. Although biofilms do play an important role in the development of infections in mammary glands (Gomes et al., Reference Gomes, Saavedra and Henriques2016), in vivo studies with pathogens causing bovine mastitis are challenging and limited, therefore, we do not know to what extent the results obtained here in vitro translate in vivo. Nevertheless, new studies and searching for alternative therapies capable of disrupting biofilms are needed and should be encouraged.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029921000285
Acknowledgements
The authors thank the financial support from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, grants E-26/203.037/2016 and E-26/201.451/2014), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 304.506/2014-1 and 304.318/2013-2) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Programa de Excelência Acadêmica – FinanceCode 001 (CAPES ProEx, grant 23038.002486/2018-26). We are thankful for Olinda C. S. Santos, for her helpful assistance.