Mycobacterium avium subsp. paratuberculosis (MAP) causes Johne's disease (JD) or paratuberculosis, a chronic granulomatous enteritis primarily in ruminants (Merkal et al. Reference Merkal, Larsen and Booth1975). Clinically as well as subclinically infected shedders are the main source of infection, which is derived especially in calves via the faecal/oral route. Environmental MAP-contamination is an important factor in the persistence of infection in dairy farms (Whittington et al. Reference Whittington, Marshall, Nicholls, Marsh and Reddacliff2004; Eisenberg et al. Reference Eisenberg, Nielen, Santema, Houwers, Heederik and Koets2010).
Diagnosis of MAP infection in a herd level depends mainly on serological examination of individual milk or blood samples using commercially available enzyme-linked immunosorbent assays (ELISA), sometimes together attempts at bacterial culture in individual or pooled faecal samples (Whitlock et al. Reference Whitlock, Wells, Sweeney and Van Tiem2000). According to previous studies the examination of environmental samples such as manure, soil and pastures are suitable alternative screening matrices for MAP detection facilitating efficiency of JD control strategies (Raizman et al. Reference Raizman, Wells, Godden, Bey, Oakes, Bentley and Olsen2004; Berghaus et al. Reference Berghaus, Farver, Anderson, Jaravata and Gardner2006; Lombard et al. Reference Lombard, Wagner, Smith, McCluskey, Harris, Payeur, Garry and Salman2006; Aly et al. Reference Aly, Anderson, Whitlock, Fyock, McAdams, Adaska, Jiang and Gardner2009; Eisenberg et al. Reference Eisenberg, Nielen, Santema, Houwers, Heederik and Koets2010; Donat et al. Reference Donat, Schau and Soschinka2011; Smith et al. Reference Smith, Schukken, Pradhan, Smith, Whitlock, Van Kessel, Wolfgang and Grohn2011). However, these samples do not necessarily reflect an appropriate average sample for the respective area. Therefore, the aim of the present study was to evaluate boot swabs as an easy accessible matrix for MAP diagnostics in dairy herds that correlates well with environmental contamination. In addition to that, the further intention was to investigate whether these boot swab samples are useful in continuous MAP-detection during a longitudinal study. This would provide a standardisable and economical tool in herd screening programmes for MAP as can be extrapolated from, for example, Salmonella detection in poultry holdings (Arnold et al. Reference Arnold, Carrique-Mas and Davies2010). The present study represents a first report on a proof of concept.
Material and methods
Dairy herds
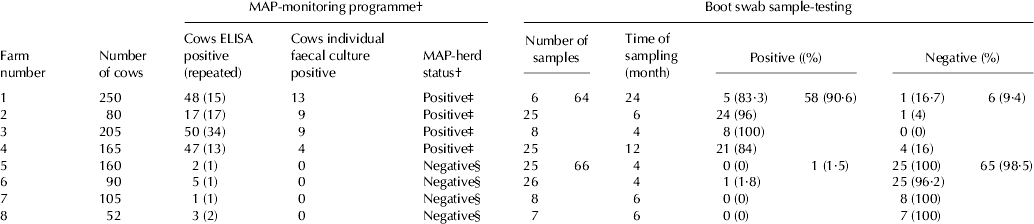
Eight clinically healthy (no signs of paratuberculosis, such as diarrhoea, emaciation etc.) Holstein dairy herds in Hesse, Germany with 52–250 (average 138) cows/herd were selected for this study (Table 1). The total number of lactating cows was 1107. All included herds were housed in freestall. Health records from these dairy herds were available according to the results of a MAP-monitoring programme conducted since 2006 (Table 1). Within this programme herds were sampled twice a year using milk, blood and individual faecal samples. In all herds, no clinical symptoms of JD have ever been recorded before. According to a repeatedly performed ELISA-test (Pourquier®, Montpellier, France) and the culture investigation of individual faecal samples, four herds could be considered as truly positive. There was at least one MAP-shedder in each herd because the MAP pathogen was isolated repeatedly from these individual faecal samples. Many cows showed positive or repeated positive ELISA reactions. The four remaining herds were regarded as presumably negative. The MAP pathogen was never isolated by individual faecal culture and nearly all cows showed negative ELISA reactions (Table 1).
Table 1. Details regarding farms (n=8) and results of a previously performed MAP-monitoring programme and cultural test results for Mycobacterium avium ssp. paratuberculosis (MAP) detection in boot swab samples (n=130)

† According to results of repeated serological Pourquier®-ELISA testing and individual faecal culture within a MAP-monitoring programme (previously conducted)
‡ Surely positive
§ Presumably negative
Boot swab samples
One hundred-and-thirty pairs of boot swab samples were collected repeatedly in total on eight different dairy farms during 7–26 visits/farm (Table 1) within a 3-year study (2007–2010). Boot swabs are commercially available adsorptive polyethylene overboots (Kolmi, St.-Barthélémy-d´Anjou, France) which are pulled over both feet. Prior to use and directly after entering the stable a new pair of plasic overboots is pulled over the shoe or rubber boot before preparing a fresh pair of boot swabs for sampling. Any contamination prior to use or any contact with disinfectant was avoided. The sampling staff moved around the stable especially on sectors with high cow traffic areas such as walk ways, feeding areas and the milking parlour entrance and exit alleyways in a meandering pattern – no pens were walked through. At least 100 and not more than 200 steps were conducted with the pair of boot swabs. In principal the normal route of a lactating cow in the stable was walked. The main paths were meandering expired. Especially areas with much slurry and faeces were preferred. The number of steps depends on the stable size. A minimum of 100 steps should be walked.
On completion of sampling in the respective sectors boot swabs were carefully removed in order to avoid any dislodgement of adherent environmental material. Boot swabs were then inverted to retain the sampling material and placed in a sterile plastic bag for transportation to the laboratory. All samples reached the laboratory on the day of sampling and were immediately further processed. The swabs were transported at room temperature and not especially cooled or transported on ice.
Culture methods
Inoculated boot swab samples were processed according to a method for faecal MAP-culture (Anonymous, 2007) with some modifications. Briefly, boot swab samples were cut into small pieces using sterile scissors and thereafter suspended in 100 ml sterile NaCl in a glass bottle and shaken at 200 rpm for 10 min. Using 10-ml cut-off pipette tips, supernatant of the boot swab wash solution was transferred to 50-ml plastic tubes and then centrifuged at 2600 g for 10 min. Supernatants were discarded and sediments were resuspended in a ten-fold volume of freshly prepared 0·75% hexadecylpyridinium chloride (HPC) solution (Merck, Darmstadt, Germany) for decontamination. Tubes were stored for 5 min at room temperature for sedimentation of the coarse ingredients. Supernatants were removed again in a new 50-ml plastic tube and shaken at 200 rpm for 30–60 min at room temperature. Thereafter tubes were incubated for 48 h at room temperature in the dark. Following decontamination, samples were centrifuged at 2600 g for 15 min. Finally, supernatants were decanted and pellets were resuspended in 0·75% HPC solution and inoculated onto slant agar surfaces of three tubes of HEYM (Herrold's egg yolk medium) containing mycobactin J and ampothericin B, nalidixic acid, vancomycin (ANV) and one tube of HEYM containing ANV but not mycobactin J (both Becton Dickinson, Heidelberg, Germany). These tubes were then incubated at 37 °C for at least 16 weeks. Bacterial growth was monitored once every other week. Colonies suspected to be mycobacteria (diameter 1–2 mm, waxy appearance and white colour) were processed for Ziehl-Neelsen staining (ZN) according to the manufacturer's instruction (Merck). Respective colonies exclusively from mycobactin J-containing agar with microscopic features consistent with rod-shaped acid fast bacilli in clumps were selected for further investigations. Single colonies were subcultured until final molecular confirmation.
Identification of MAP isolates
Suspension of MAP colony material in sterile 0·9% NaCl solution was heated for 10 min at 100 °C and then stored at 4 °C until further processing. Nucleic acid preparations were carried out using DNeasy® Tissue Kit according to the manufacturer's instructions (Qiagen, Hilden, Germany). MAP-specific IS900 and f57 pcr were applied using the primer sets TJ1/TJ2 and F57/R57, respectively (Bull et al. Reference Bull, McMinn, Sidi-Boumedine, Skull, Durkin, Neild, Rhodes, Pickup and Hermon-Taylor2003; Vansnick et al. Reference Vansnick, De Rijk, Vercammen, Geysen, Rigouts and Portaels2004). PCR conditions were identical for both primer sets: 1 cycle of 95 °C for 10 min, 40 cycles of 95 °C for 1 min, 58 °C for 1 min, and 72 °C for 3 min, followed by 1 cycle of 72 °C for 7 min in a Biometra T3000 thermocycler (Biometra, Göttingen, Germany). PCR products (12 μl) were mixed with 2 μl loading dye solution and separated by 2% agarose gel electrophoresis (Biozym, Hessisch Oldendorf, Germany) in 1×TAE buffer (0·04 mol/l Tris, 0·001 mol/l EDTA, pH 7·8) and a 100 bp DNA ladder marker (Roche, Mannheim, Germany) followed by staining for 5 min with ethidium bromide solution (Sigma-Aldrich, Steinheim, Germany).
Results
From 130 pairs of boot swab samples from eight dairy farms, MAP could be cultured from 59 (45·4%). The remaining 71 boot swab sample pairs gave negative culture results. All obtained culture isolates proved to be positive for IS900 and f57, respectively. The 64 boot swab samples from dairy herds with known history of positive MAP-status (surely MAP positive) were in the vast majority culture-positive [58 (90·6%) vs. 6 (9·4%)]. In contrast, from the four presumably negative herds, all but one of the overall investigated 66 boot swab samples (1·5%) remained culture-negative (Table 1).
Details regarding distribution of cultural MAP results within different herds are given in Table 1. In the MAP-positive herds the investigation of 5–24 boot swab sample pairs gave positive culture results. While in one MAP-positive farm all 8 swabs gave positive results (100%) another positive-livestock had only 21 of 25 samples (84%) positive. In the remaining 2 farms all but one boot swab sample each was positive. In 3 out of 4 presumably MAP-negative dairy farms, 7–25 taken boot swab samples gave only negative culture results. In only one herd MAP could be isolated from one boot swab sample.
Discussion
Utilisation of environmental samples is an attractive alternative for monitoring MAP prevalence at state or region level (Pillars et al. Reference Pillars, Grooms and Kaneene2009). The objective of this study was to determine whether mycobacterial culture of boot swab samples can provide a cost-effective means of screening herds for the presence of MAP using environmental samples. A prerequisite for testing is to obtain highly sensitive results at reasonable cost. Recently, the role of environmental sampling for herd classification has been evaluated in several studies (Raizman et al. Reference Raizman, Wells, Godden, Bey, Oakes, Bentley and Olsen2004; Anonymous, 2005; Fyock et al. Reference Fyock, Smith, Hoving, Schukken and Whitlock2005; Berghaus et al. Reference Berghaus, Farver, Anderson, Jaravata and Gardner2006; Lombard et al. Reference Lombard, Wagner, Smith, McCluskey, Harris, Payeur, Garry and Salman2006; Aly et al. Reference Aly, Anderson, Whitlock, Fyock, McAdams, Adaska, Jiang and Gardner2009; Donat et al. Reference Donat, Schau and Soschinka2011; Smith et al. Reference Smith, Schukken, Pradhan, Smith, Whitlock, Van Kessel, Wolfgang and Grohn2011). Obvious advantages compared with serology and individual faecal culture of this approach include lower costs compared with serology, individual faecal culture, ease of sampling, no additional handling of individual animals as well as high specificity of cultural examination (Raizman et al. Reference Raizman, Wells, Godden, Bey, Oakes, Bentley and Olsen2004). The goal of the present study was principally to show the usefulness of boot swab sampling, which is successfully employed to detect other bacteria (Arnold et al. Reference Arnold, Carrique-Mas and Davies2010), as an adequate and simple method for MAP detection. Despite previously performed individual MAP testing in the investigated herds, the MAP-prevalence in the herds from this study is not known exactly. We could only state that 4 herds were definitely MAP-positive and the remaining herds were presumably negative. From 64 boot swab samples taken from the 4 MAP-positive herds at different sampling times and intervals more than 90% were positive in culture. Only 6 boot swab samples of which 4 were from the same herd gave negative results. Compared with remaining positive herds in this study, this herd contained the lowest number of ELISA-positive and MAP-shedding cows according to the previously conducted MAP-monitoring programme (Table 1). As stated in an earlier environmental study (Pillars et al. Reference Pillars, Grooms and Kaneene2009) this suggests a positive relationship between MAP-positive environmental samples and herd prevalence also in boot swab samples. Furthermore the proportion of positive environmental samples on individual farms was statistically significantly correlated with proportion of sero-reagents (Berghaus et al. Reference Berghaus, Farver, Anderson, Jaravata and Gardner2006). Lombard et al. (Reference Lombard, Wagner, Smith, McCluskey, Harris, Payeur, Garry and Salman2006) received 71·4–76% positive environmental sample results depending of the classification methods (culture vs. ELISA) used as reference. Smith et al. (Reference Smith, Schukken, Pradhan, Smith, Whitlock, Van Kessel, Wolfgang and Grohn2011) reported a direct but not statistically significant correlation of positive environmental samples with the number of animals shedding more than 30 cfu/g faeces. Beyond that, the method of environmental sampling could vary in sensitivity based on the stage of disease and the shedding level of animals.
Environmental samples containing only small numbers of viable mycobacteria (<10 cfu/g) may fall below the detection limit as a consequence of the lack of sensitivity of the culturing procedure (Raizman et al. Reference Raizman, Wells, Godden, Bey, Oakes, Bentley and Olsen2004). Boot swab sampling ensures the most diverse technique with respect to different locations in the stable. Thus it is conceivable that the sensitivity of MAP-detection in environmental material could be increased by this sampling technique. Another study found relatively consistent correlations of estimated proportions of MAP-infected herds while comparing environmental samples with faecal culture or ELISA testing (Berghaus et al. Reference Berghaus, Farver, Anderson, Jaravata and Gardner2006).
However, MAP-prevalence of herds in the present study is not known exactly in general as well as the number of MAP-shedding cows at each time point of sample collection. Further investigations with an exact determination of herd prevalence are necessary, and are under way, to determine marginal prevalence and limits for this technique. Yet, we think that in environmental sampling repeated collection of boot swab samples does increase the probability for MAP detection thereby representing a cost-efficient tool for MAP-herd diagnostics. Although a few negative boot swab test results occurred in 3 of 4 surely MAP positive herds (6 samples). The repeated collection of boot swab samples and the longitudinal reflection, all according to our surveillance programme, surely positive herds would be recognised as being MAP positive.
Nevertheless one MAP-positive boot swab could be detected in one presumably MAP-negative herd. MAP-positive samples most probably occur because of a shedding cow. Another explanation could be that a currently unidentified MAP-shedder was introduced into that herd. An intake of MAP through other animals such as wild birds (Corn et al. Reference Corn, Manning, Sreevatsan and Fischer2005) or miscellaneous materials is also conceivable. Even invertebrates like flies and worms can become colonised with MAP (Pawlik et al. Reference Pawlik and Yayo Ayele2002). All these other sources, though possible, are less likely to be the cause. On the other hand, this result highlights the putative sensitivity of our sampling method even under low prevalence. Berghaus et al. (Reference Berghaus, Farver, Anderson, Jaravata and Gardner2006) and Lombard et al. (Reference Lombard, Wagner, Smith, McCluskey, Harris, Payeur, Garry and Salman2006) also obtained positive results in putative MAP-negative herds.
Contaminated target areas within the farm environment, especially those with high cow traffic such as cow alleyways, walk ways, feeding areas and milk parlour entrance represent good locations for boot swab sampling and thus are promising alternatives compared with faecal pool samples for herd screening and assessment of MAP infection status. The combination of boot swab sampling with real time-PCR techniques performed directly from the environmental samples would accelerate and maybe sensitise the diagnostic procedure.
Conclusions
Based on the results of this study the boot swab sampling technique offers the possibility to sensitise the detection of MAP in environmental samples in a herd/pen as many different locations are being sampled, especially those where manure from adult cattle accumulates. Nevertheless preliminary results indicate a good sampling technique and a sensitive diagnostic tool for MAP-herd status assessments. Especially, its use in low level prevalence herds may require further studies with exactly known intra herd prevalence.
This study was financed by a grant from the Hessian Ministry of Environment, Nutrition, Agriculture and Consumer protection. We thank U. Kling for excellent technical assistance and Hossnia Gutmann for an improvement of the English manuscript.