Introduction
Although a number of calcium-fortified foods and many foods with high calcium (Ca) bioavailability are available, they are not selected in sufficient quantities to correct the calcium shortfall created by excluding dairy products in the diet (Braun & Weaver, Reference Braun and Weaver2006). Milk and dairy products are recommended to be consumed daily at amounts of 750 ml or the equivalent for most energy patterns. Dairy products provide the best package for addressing nutrients limited in the diets. Dairy products provide an important source of nutrients for high-risk groups, including children, young adults, menopausal women, pregnant women and the elderly (Weaver, Reference Weaver2009). Although Ca-supplementation may have negative impacts on organoleptic properties, this nutrient is important and may be needed in local diets. Ca has been shown to reduce the risk of pre-eclampsia and maternal mortality, having a positive role on bone density (Hofmeyr et al. Reference Hofmeyr, Lawrie, Atallah and Duley2011). In addition, scarce information is available in the scientific literature about the dietary calcium intake and lipid profile. In a recent study (Ma et al. Reference Ma, Liang, Chen, Jiang, Kwan, Peng, Jiao, Zuo, Huang and Chen2012), plasma total cholesterol, non-high density lipoprotein cholesterol and triglycerides were decreased in intact female and ovariectomized hamsters, however, different levels of dietary calcium (0, 2 and 8 g/kg diet during 6 weeks) had no effect on lipoprotein profiles in both male and female castrated hamsters.
On the other hand, several environmental factors lead to the formation of cholesterol gallstones. These factors include female sex, reduced physical activity, diet high in energy and low in legumes, a low intake of vegetables and possibly a high polyunsaturated fatty acid diet. The mechanism whereby these factors lead to gallstone formation is as yet poorly understood (Schwesinger et al. Reference Schwesinger, Kurtin and Johnson1988). Other factors which promote gallstone formation include stasis, the concentration of bile, the stratification of bile, the addition of nucleating factors (such as calcium and phospholipids), and possibly alterations in bilirubin metabolism by the gallbladder wall (Van Erpecum et al. Reference Van Erpecum, Van Berge Henegouwen, Stoelwinder, Schmidt and Willekens1990). Some studies show a better effect of a hypocaloric diet when it is associated with dietary calcium supplementation, resulting in a lower hunger score as well as a decrease in plasma resistin levels, demonstrating that added calcium leads to preservation of fat-free mass (Kabrnová-Hlavatá et al. Reference Kabrnová-Hlavatá, Hainer, Gojová, Hlavatý, Kopský, Nedvídková, Kunesová, Parízková, Wagenknecht, Hill and Drbohlav2008), however other studies (Menon et al. Reference Menon, Baxmann, Froeder, Martini and Heilberg2009) reported that increasing calcium intake by CaCO3 supplementation did not contribute to a further reduction of body weight, therefore this would not be a good reason for an increase in calcium intake. In addition, CaCO3 supplementation has been associated with highly increased incidence of cholelithiasis (Powell, Reference Powell1985).
Bile contains biliary acids, bilirubin, cholesterol, metals, steroids and several metabolites of xenobiotics. Among its physiological functions regulating the metabolism of the cholesterol, promoting the absorption of fat and lipophilic vitamins, and the excretion of toxic substances are emphasized. Nevertheless, the biliary acids, which are their major components, are cytotoxic when they accumulate inside or outside the cell, inducing apoptosis and cell necrosis, due to the oxidative damage at the mitochondria generating free radicals (Ferreira et al. Reference Ferreira, Coxito, Sardao, Palmeira and Oliveira2005; Sokol et al. Reference Sokol, Dahl, Devereaux, Yerushalmi, Kobak and Gumpricht2005).
The liver plays a fundamental role in metabolism, toxicity, and elimination of endogenous and exogenous components, and antioxidant enzymes have a major role in protecting tissues from free radicals. Thus, it is of great interest to determine the influence of the consumption of Ca-supplemented diets on the metabolic capacity of the liver. Taking into account the beneficial effects of goat milk on fat metabolism previously reported by us (Alférez et al. Reference Alférez, Barrionuevo, López-Aliaga, Sanz-Sampelayo, Lisbona, Robles and Campos2001; López-Aliaga et al. Reference López-Aliaga, Alférez, Nestares, Ros, Barrionuevo and Campos2005), the present study was carried out to assess the influence of cow or goat milk-based diets, either with normal-Ca content or Ca-supplementation on plasma lipid profile, some aspects of bile physiology and enzymatic antioxidant defence.
Materials and methods
Animals
All animal care procedures and experimental protocols were approved by the Ethics Committee of the University of Granada according to the European Community guidelines. Sixty male albino Wistar breed rats (10 week of age and weighing about 240±6·7 g), purchased from the University of Granada Laboratory Animal Service (Granada, Spain) were used during the study.
Experimental design and diets
At the beginning of the study, the rats were divided into 6 experimental groups (n 10) and fed for 2 weeks with six different types of diets: standard, cow milk-based diet or goat milk based, with normal-Ca content (5·0 g/kg) or double-Ca content (10·0 g/kg).
Table 1 summarises the diets supplied during the experimental period. The standard (non-milk) diet, mineral and vitamin supplements used, were prepared according to the recommendations of the AIN-93 (Reeves et al. Reference Reeves, Nielsen and Fahey1993) except for the fat source (olive oil was used instead of soybean oil, as this more commonly consumed in Spain) and level of fat (10% rather than 5%). The milk-based diets were made with lyophilized cow or goat milk (Holstein and Murciano-Granadina breeds respectively). Cow or goat milk lyophilized samples were formulated to provide 20% of protein in the diets. The Ca content (g/kg diet) measured by analysis in normal-Ca diets were: 5·41±0·98; (standard diet), 5·31±0·85 (cow milk-based diet), 5·20±0·94 (goat milk-based diet) and in double-Ca diets were: 10·42±0·89 (standard diet), 10·56±10·11 (cow milk-based diet) and 10·44±1·00 (goat milk-based diet). The dose of 10 g Ca/kg was chosen, because it has been previously used in supplementation studies (Kenar et al. Reference Kenar, Karayilanoglu, Aydin, Serdar and Kose Erbil2008; Díaz-Castro et al. Reference Díaz-Castro, Alférez, López-Aliaga, Nestares and Campos2009; López-Aliaga et al. Reference López-Aliaga, Diaz-Castro, Nestares, Alférez and Campos2009).
Table 1. Composition of the experimental diets with normal or double Ca content
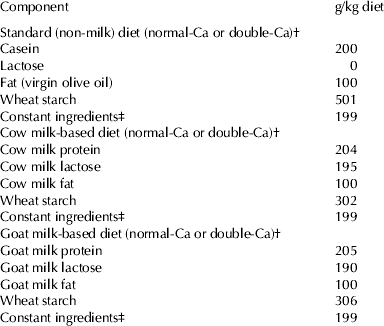
† The diets were prepared according to the recommendations of the AIN-93(11) for normal-Ca (5·0 g Ca/kg diet) (Reeves et al. Reference Reeves, Nielsen and Fahey1993), or double the requirements (10·0 g Ca/kg diet)
‡ The constant ingredients consisted of (g/kg diet): fibre (micronized cellulose) 50, sucrose 100, choline chloride 2·5, L-cystine 1·8, mineral premix 35, vitamin premix 10. The mineral and vitamin premix were prepared according to the recommendations of the AIN (Reeves et al. Reference Reeves, Nielsen and Fahey1993) for standard diet and mineral and vitamin specific supplements for cow and goat milk-based diets were formulated taking into account the mineral content of the lyophilized milks supplied in order to meet these recommendations
During the course of the study, the animals were kept at an automatically controlled temperature (22–23 °C), humidity (55–65%) and a 12-h light-dark cycle (light period 9:00 to 21:00 h). Diet intake was controlled, pair feeding all the animals (80% of the average intake) and bi-distilled water was available ad libitum. On day 15 of the study, 10 rats per group were anesthetized intraperitoneally with sodium pentobarbital (Sigma Diagnostics, St Louis, MO, USA), tested by tail pinch reflex until complete loss of the reflex, and body temperature was maintained at 37 °C with a thermisor-controlled heated pad. To avoid the effect of fasting motor activity on biliary emptying, bile collection for all rats in the 6 experimental groups was carried out on the same day and under the same experimental conditions, by selecting one rat consecutively from each experimental group until all bile extractions were complete. After median laparotomy, the common bile duct was isolated and cannulated with PE-10 non-sterile polyethylene tubing (BD Intra,medic, Ontario, Canada). The volume of bile was determined gravimetrically, assuming a density of 1·0 g/ml, and bile flow was expressed as ml/min per g liver. A bile sample was collected into previously weighed vials for the first 30 min after cannulation and preserved at −80 °C for further analyses of bile composition. After the experiments, the rats were totally bled by cannulation of the abdominal aorta. Blood samples were collected in EDTA tubes (Venoject, Terumo Europe, Leuven, Belgium) and subsequently, was centrifuged (1500 g, 4 °C, 15 min) to separate plasma from cells. Plasma samples were preserved at −80 °C for further analyses of biochemical parameters (cholesterol, triglycerides and transaminases). Finally, the liver was removed weighed and chilled in ice-cold NaCl (0·9% w/v). Hepatic cytosolic fractions were prepared fresh at the same day by successive differential centrifugations with hypotonic haemolysis, preserving these fractions at −80 °C for further analyses of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidise (GPx) and thiobarbituric acid-reactive substances (TBARS).
Bile composition analysis
The following bile parameters were evaluated: total biliary acids (measured enzymatically using 3α-hydroxysteroid dehydrogenase purchased from Sigma (Sigma, St Louis, MO, USA) (Talalay, Reference Talalay1960), total cholesterol (CHOD-PAD method, Spinreact, Girona, Spain) (Deeg & Ziegenhorn, Reference Deeg and Ziegenhorn1983), and phospholipids (Trinder-CHO method, Spinreact, Girona, Spain) (Takeyama et al. Reference Takeyama, Itoh, Nayasaki and Tanimazu1977).
Plasma biochemical parameters
The following plasma parameters were evaluated: total cholesterol (CHOD-PAD method, Boehringer Mannheim GmbH Diagnostica) (Deeg & Ziegenhorn, Reference Deeg and Ziegenhorn1983), triglycerides (GPO-PAP method, Boehringer Mannheim) (Bergmeyer, Reference Bergmeyer and Bergmeyer1974) and alanine and aspartate aminotransferases (Bergmeyer et al. Reference Bergmeyer, Scheibe and Wahlefeld1978).
Lithogenic index
The lithogenic index (cholesterol saturation index) of bile is determined by the molar relation between concentrations of cholesterol, phospholipids, and bile acids, and by the concentration of total lipids. The lithogenic index was calculated from the quotient of the percentage of molar cholesterol in the sample divided by the percentage of molar cholesterol at saturation; the latter value was found with the following third degree polynomial function (Thomas & Hofmann, Reference Thomas and Hofmann1973):
where x=concentration of phospholipids divided by the sum of the concentrations of bile acids+phospholipids, expressed in mol/l.
Calcium determination in the diets
After mineralization by a wet method in a sand bath (J.R. Selecta, Barcelona, Spain), Ca concentrations in different diets were determined by atomic absorption spectrophotometry (PerkinElmer Analyst 1100B spectrometer with WinLab32 for AA software, Überlingen, Germany). To calibrate the measurements, samples of lyophilized skimmed milk powder (certified reference material CRM 063 R; Community Bureau of References, Brussels, Belgium) were used to check the Ca recovery (Ca value=13·88±0·10 mg/g, mean values with sem of five determinations. Certified value: Ca=13·49±0·10 mg/g).
Hepatic cytosolic preparations
The liver samples were thawed and homogenates were prepared in a Potter homogenizer after the addition of 4·0 ml phosphate buffer (pH=7·4) per 1 g liver. Successive differential centrifugations (Beckman Coulter Inc., CA, USA) were performed to separate cytosolic fractions, according to a procedure reported previously (DeSandro et al. Reference DeSandro, Chevrier, Boddaert, Melcion, Cordier and Richiert1991). The final fractions were aliquoted, snap-frozen in liquid nitrogen, and stored at −80 °C until analysis. Cytosolic protein content was measured as described by Lowry et al. (Reference Lowry, Rosenburgh, Farr and Randall1951).
Catalase (CAT) activity
CAT activity was determined following the method described by Aebi (Reference Aebi1984), monitoring at 240 nm spectrophotometrically (Thermo Spectronic, Rochester, USA) the H2O2 decomposition, as a consequence of the catalytic activity of CAT. The activity was calculated from the first-order rate constant K (1/s).
Superoxide dismutase (SOD) activity
SOD activity was determined according to the method of Crapo et al. (Reference Crapo, McCord and Fridovich1978), based on its inhibition in the reduction of cytochrome c, measured spectrophotometrically (Thermo Spectronic, Rochester, USA) at 550 nm. One unit of the SOD activity is defined as the amount of enzyme required to produce 50% inhibition of the rate of reduction of cytochrome c.
Glutathione peroxidase (GPx) activity assay
GPx activity was measured by the method of Flohé & Gunzler (Reference Flohé and Günzler1984). That method is based on the instantaneous formation of oxidized glutathione during the reaction catalysed by glutathione peroxidase. That oxidized glutathione is continually reduced by an excess of glutathione reductase and NADPH present in the cuvette. The subsequent oxidation of NADPH to NADP+ was monitored spectrophotometrically (Thermo Spectronic, Rochester, USA) at 340 nm. During the reaction, cumen hydroperoxide was used as substrate.
Thiobarbituric acid–reactive substances (TBARS) measurement
The extent of lipid peroxidation was evaluated in liver cytosolic fractions by measuring the concentration of thiobarbituric acid–reactive substances (TBARS) according to the methods of Yagi (Reference Yagi1976) and Ohkawa et al. (Reference Ohkawa, Ohishi and Yagi1979). A 0·5 ml cytosolic fraction was mixed with 1 ml 15% trichloroacetic acid (Sigma-Aldrich) and centrifuged at 80 g for 10 min. One milliliter of supernatant was mixed with 1 ml TBA reagent (0·67%) and the mixture was kept in a boiling water bath for 20 min. The reaction product was extracted and measured by spectrophotometric analysis (Thermo Spectronic, Rochester, USA) at 532 nm. The assay procedure was calibrated using tetraethoxypropanone (Sigma-Aldrich) as a malodialdehyde source.
Statistical analysis
Statistical analyses were performed using the SPSS computer program (version 18.0, 2010, SPSS Inc., Chicago, IL). Differences between groups fed with normal-Ca or double Ca-content diets were tested for statistical significance with Student's t test. Variance analysis by one-way ANOVA methods was used to compare the different diets supplied to the animals. Individual means were tested by pair-wise comparison with Tukey's multiple comparison test, when main effects and interactions were significant. The level of significance was set at P<0·05. All data are reported as mean values with their standard errors.
Results
Bile compositions are shown in Table 2. Bile flow was not statistically different among the three diets with normal-Ca content, however it was higher in the standard diet compared with the milk-based diets with double-Ca content (P < 0·001). Cholesterol output was higher in rats fed goat milk in comparison with those fed with standard and cow milk-based diet with normal or double-Ca content (P < 0·001). In addition, cholesterol concentration was also higher in the animals fed goat milk with normal-Ca content. Phospholipids concentration was higher in the milk-based diets compared with the standard diet with normal-Ca content (P < 0·001). Ca supplementation led to a reduction in the phospholipid concentration in the milk-based diets. It is noteworthy that lithogenic index of bile did not significantly differ between the three experimental diets with normal-Ca content, however Ca-supplementation increased this parameter in the standard and cow-milk based diet (P < 0·05), this change was not observed with goat milk (Table 2). The phospholipids output/bile acid output ratio was similar in all the diets assayed (Fig. 1), however cholesterol output/bile acid output+phospholipids output ratio was higher in the goat milk-based diets compared with the standard and cow-milk diets (P < 0·05) (Fig. 2).

Fig. 1. Effect of diets and Ca-supplementation on phospholipid output/bile acid output ratio.

Fig. 2. Effect of diets and Ca-supplementation on cholesterol output/bile acid output+phospolipid output ratio. *Different from standard and cow milk-diet, P<0·05.
Table 2. Bile compositions of rats fed on standard nonmilk or milk-based diets (cow or goat) either with normal or double Ca content. Values are means±sd for n=10

a–cMeans in the same row followed by different superscript are significantly different (P < 0·05)
*Mean values from the corresponding group of rats with normal-Ca content were significantly different (P < 0·05)
Table 3 shows the results of the plasma biochemical parameters. Cholesterol values were similar in the animals fed goat milk-based and standard diets and lower than those fed cow milk diet (P<0·01) with normal-Ca content. Triglyceride plasma concentrations were lower in the animals fed goat milk compared with those fed cow milk and standard diets (P<0·05) with normal or double-Ca content. Alanine and aspartate aminotransferases levels were lower in the animals fed goat milk-based diet, in comparison with the other two Ca-supplemented diets assayed (P<0·001). Ca- supplementation led to a reduction in the activities of alanine and aspartate aminotransferases (P<0·001) in the animals fed goat milk.
Table 3. Cholesterol and triglyceride contents and transaminase activities in plasma of rats fed on standard nonmilk or milk-based diets (cow or goat) either with normal or double Ca content. Values are means±sd for n=10

a–cMeans in the same row followed by different superscript are significantly different (P < 0·05)
* Mean values from the corresponding group of rats with normal-Ca content were significantly different (P < 0·05)
Enzymatic antioxidant status and lipid peroxidation in hepatic cytosol fractions are shown in Table 4. TBARS levels were lower in the goat milk-based diet with normal-Ca content compared with the standard and cow milk-based diet (P < 0·01 for standard diet and P < 0·05 for cow milk diet), however, although goat milk has a different lipid profile than cow milk, we did not observe any difference regarding lipid peroxidation (TBARS) in the milk-based diets with double Ca-content. SOD activity was higher in the animals fed standard and cow milk-based diet compared with goat milk (P < 0·001 in the animals fed with normal-Ca content and P < 0·01 for the diets with double-Ca content). The CAT activity values were also significantly higher in rats fed standard diet in comparison with the milk-based diets (P < 0·001). GPx activity was lower in the animals fed goat milk-based diet compared with the other diets assayed with normal-Ca content (P < 0·001 for standard diet and P < 0·01 for cow milk-based diet). Another noteworthy result obtained in the present study is that Ca-supplementation in the diet led to an increase in the oxidative damage, as shown by the raise in the activities of the antioxidant enzymes studied in the standard and cow milk diets (P<0·001), however this increase was not apparent with goat milk, except for GPx activity which was also increased by Ca-supplementation (P < 0·001).
Table 4. Lipid peroxidation and antioxidant enzymes in cytosolic fractions of liver of rats fed on standard nonmilk or milk-based diets (cow or goat) either with normal or double Ca content. Values are means±sd for n = 10

a–cMeans in the same row followed by different superscript are significantly different (P < 0·05)
* Mean values from the corresponding group of rats with normal-Ca content were significantly different (P < 0·05)
Discussion
The current study shows that goat milk consumption induces beneficial effects in the hepatobiliary function, increasing the biliary excretion of cholesterol, reducing the lithogenicity and oxidative damage induced by Ca-supplementation. The current results are in agreement with those reported in previous studies of our research group (Alférez et al. Reference Alférez, Barrionuevo, López-Aliaga, Sanz-Sampelayo, Lisbona, Robles and Campos2001; López-Aliaga et al. Reference López-Aliaga, Alférez, Nestares, Ros, Barrionuevo and Campos2005), showing that goat milk consumption diminishes the concentrations of plasma cholesterol. This effect can be explained by several factors. Firstly, the higher levels of medium chain triglycerides (MCT) in goat milk (34%) compared with the cow milk (21%), as the presence of MCT in the diet reduces the synthesis of endogenous cholesterol and its intestinal absorption (García Unciti, Reference García Unciti1996). Secondly, goat milk fat has higher monounsaturated fatty acid content than cow milk, which could induce a hypocholesterolemic effect (Kris-Etherton & Yu, Reference Kris-Etherton and Yu1997; López-Aliaga et al. Reference López-Aliaga, Alférez, Nestares, Ros, Barrionuevo and Campos2005). It is also interesting that higher cholesterol concentrations and cholesterol output were found in the bile of animals fed the goat milk diet compared with the other diets assayed, findings that can be explained by the different quality of the fats in these experimental diets. Bravo et al. (Reference Bravo, Flora, Cantafora, De Luca, Tripodi, Avella and Botham1998) demonstrated the influence of dietary fat on the biliary excretion of cholesterol, indicating that feeding monounsaturated fatty acids compared with saturated fatty acid results in a more rapid excretion of dietary cholesterol via the bile. On the other hand, Lafont et al. (Reference Lafont, Lairon, Vigne, Chanussot, Chabert, Portugal, Pauli, Crotte and Hauton1985) reported no significant variation in the biliary secretion of phospholipids and an increase in the lithogenic index of bile, as occurred under our experimental conditions, in which there were no significant differences concerning the phospholipids output. In spite of these findings, the lithogenic index was similar for both milk-based diets, result which lies within the range of normal values previously reported in the scientific literature consulted (Bravo et al. Reference Bravo, Flora, Cantafora, De Luca, Tripodi, Avella and Botham1998; López-Aliaga et al. Reference López-Aliaga, Alférez, Nestares, Ros, Barrionuevo and Campos2005).
Feeding the standard and cow milk-based diets supplemented with Ca during 14 d led to an increase in the lithogenic index, that surprisingly was not observed with the goat milk. In spite of the fact that we have not detected precipitates in the bile, other authors (Muller et al. Reference Muller, Grace and Pitt1986) found Ca gallstones in animal models receiving diets supplemented with Ca, since Ca constitutes one of the main factors involved in nucleation of biliary gallstones. In addition, it has been also reported that Ca promotes the precipitation of the biliary acids and fatty acids in the bile, stimulating the formation of biliary gallstones (Van Der et al. Reference Van Der, Lapre, Govers and Kleibeuker1997). The lower biliary lithogenicity of goat milk can be explained by its better Ca bioavailability compared with the cow milk and standard diet. Goat milk has a major content of vitamin D (Alférez et al. Reference Alférez, López-Aliaga, Nestares, Díaz-Castro, Barrionuevo, Ros and Campos2006), which increases Ca energy-dependent transcellular transport. Moreover, goat milk has higher lysine content and a greater quantity of cysteine, nutrients that improve Ca absorption via passive transport (Campos et al. Reference Campos, López-Aliaga, Alférez, Nestares and Barrionuevo2003; Alférez et al. Reference Alférez, López-Aliaga, Nestares, Díaz-Castro, Barrionuevo, Ros and Campos2006). Finally, Díaz-Castro et al. (Reference Díaz-Castro, Ramírez, ópez-Frías, Campos, López-Frías, Alférez, Nestares, Ortega and López-Aliaga2011a) have reported that goat milk provokes a decrease in the rate of Parathormone (PTH) secretion, improving Ca utilization and its deposit in target organs. All these factors would lead to a lower excretion of Ca in the bile, diminishing its lithogenicity, even under conditions of Ca-supplementation.
Goat milk consumption led to a reduction in plasma triglycerides concentrations compared with the cow milk, with lower values to the animals fed standard diet (in which the fat source is virgin olive oil). This fact can be explained by the favourable fatty acids profile of goat milk compared with cow milk fat, with higher mono – and polyunsaturated fatty acids content (Haenlein, Reference Haenlein2001). On the other hand, Alférez et al. (Reference Alférez, López-Aliaga, Barrionuevo and Campos2003) and Díaz-Castro et al. (Reference Díaz-Castro, Alférez, López-Aliaga, Nestares and Campos2009) have demonstrated that goat milk consumption increases Zn bioavailability, a mineral with antioxidant capacity. Furthermore, caprine milk has a better lipidic quality than cow milk, improving the nutritive utilization of goat milk fat (Alférez et al. Reference Alférez, Barrionuevo, López-Aliaga, Sanz-Sampelayo, Lisbona, Robles and Campos2001), providing lower substrate for lipid peroxidation and consequently reducing free radicals production (Díaz-Castro et al. Reference Díaz-Castro, Pérez-Sánchez, Ramírez, ópez-Frías, López-Aliaga, Nestares, Alférez, Ojeda and Campos2011b). These findings that can be linked to the better plasma lipid profile found in the current study and, in this sense, Gorinstein et al. (Reference Gorinstein, Leontowicz, Lojek, Leontowicz, Ciz, Krzeminski, Gralak, Czerwinski, Jastrzebski, Trakhtenberg, Grigelmo-Miguel, Soliva-Fortuny and Martin-Belloso2002) have also reported a positive influence on plasma lipids of rats fed oil with high antioxidant potential.
Activities of both transaminases (ALT and AST) studied were lower in the animals fed Ca-supplemented goat milk. When the liver is injured or inflamed, the levels of transaminases rise indicating for signs of liver damage (Tolman & Rej, Reference Tolman, Rej, Burti and ER1999). The improvement in fat metabolism (Alférez et al. Reference Alférez, López-Aliaga, Nestares, Díaz-Castro, Barrionuevo, Ros and Campos2006) and the higher biliary excretion of cholesterol can explain the decrease of these transaminases, indicating a hepatoprotective effect in the animals fed goat milk-based diet.
CAT activity values were significantly higher in rats fed standard diet (containing virgin olive oil as the fat source). These results are in agreement with those reported by other authors (Quiles et al. Reference Quiles, Huertas, Battino, Ramírez-Tortosa, Casinello, Mataix, Lopez-Frias and Mañas2002), indicating that the intake of a particular type of dietary fat indirectly affects the susceptibility of the organism to oxidation; thus, Quiles et al. (Reference Quiles, Huertas, Battino, Ramírez-Tortosa, Casinello, Mataix, Lopez-Frias and Mañas2002) observed that CAT activity increases with virgin olive oil vs. sunflower oil intake.
The SOD is the first line of enzymatic defence against free radicals, later the intermediate metabolites produced are converted into harmless compounds by the activities of the CAT and GPx (Ghone et al. Reference Ghone, Kumbar, Suryakar, Katkam and Joshi2008). The increased activity of SOD in the groups fed cow milk-based diet, reveals that these animals feature a higher generation of radicals O2•– than those fed goat milk-based diet, indicating an increase in the production of H2O2, fact directly linked with the rate of neutralization of radicals O2•– (Sahin & Gümüşlü, Reference Sahin and Gümüşlü2007). In addition, the previously mentioned better Zn bioavailability (Alférez et al. Reference Alférez, López-Aliaga, Barrionuevo and Campos2003; Díaz-Castro et al. Reference Díaz-Castro, Alférez, López-Aliaga, Nestares and Campos2009) and the better lipid quality (Alférez et al. Reference Alférez, Barrionuevo, López-Aliaga, Sanz-Sampelayo, Lisbona, Robles and Campos2001) would led to a lower generation of free radicals when goat milk is supplied.
Another interesting result is that Ca-supplementation induces an increase in the liver oxidative damage, as shown by the increase in the antioxidant enzymes in hepatic cytosolic fractions. The mechanism proposed by Kristian & Siesjö (Reference Kristian and Siesjö1998), reported that cell damage is induced by a massive Ca2+ influx into cells through receptors and voltage-operated channels. This massive Ca load, is sequestered by the mitochondria, inducing an enhanced production of reactive oxygen species. In addition, Ca2+ activates phospholipases, endonucleases, and proteases, since it affects protein phosphorylation by altering the activity of protein kinases and phosphatases, and since it activates enzymes that give rise to the production of free radicals. Moreover, Kenar et al. (Reference Kenar, Karayilanoglu, Aydin, Serdar and Kose Erbil2008) reported high and significant levels of SOD and GPx in rats receiving Ca-supplemented drinking water (10 g water soluble Ca/l water). The effect of a high Ca intake on increasing SOD and GPx activity could be explained by an allosteric mechanism (Stefan et al. Reference Stefan, Edelstein and Le Novere2008); these authors have demonstrated the differential activation of various enzymes depending on extracellular Ca concentration. Thus, enzymes may adopt distinct conformations, active or not, in the presence or absence of Ca. Moreover, Ca may react as an allosteric modulator of GPx, inducing a change in the configuration of the enzyme active site. In consequence, there would be a greater affinity between the enzyme and the substrate. All these findings explain the increases in the enzymatic activity of SOD, CAT and GPx observed in animals fed Ca-supplemented diets, revealing that Ca supplementation led to a higher generation of free radicals and the increase in the enzymatic activity would be focused to neutralize these harmful species. Nevertheless, we suppose that these antioxidant enzyme inductions seem to constitute a compensatory mechanism enough to neutralize and scavenge the excess of free radicals induced by the Ca excess, since the lipid peroxidation does not suffer modifications by the Ca-supplementation.
Conclusions
Under our experimental conditions in animal models, Ca-supplemented goat milk consumption has positive effects on the bile composition and secretion, leading to a reduction in the transaminases, triglycerides and stimulating the excretion of cholesterol via bile. In addition, the consumption of this type of milk does not increase the lithogenic index, or hepatic oxidative damage. These favourable effects cannot be observed in animals consuming cow milk.
Funding was provided by the Inter Ministerial Commission of Science and Technology (CICYT) Research Project No. AGL-2006-02301/ALI. The authors are grateful to Lactalis Iberia, S.A. (Lugo, Spain) for providing the dairy products used in this study, to the University of Granada for the personal support of J. Díaz-Castro and to Ms. Elisa Alcover for her efficient administrative support.