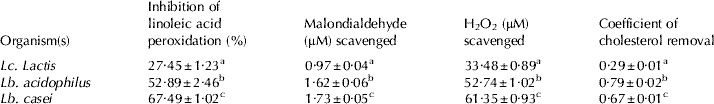The prevalence of cardiovascular diseases (CVD), diabetes, cancer and bowel inflammatory syndrome are rapidly increasing, due to lack of successful curative or preventing strategies available in the market (DeCoster, Reference DeCoster2008; Maramaldi et al. Reference Maramaldi, Dungan and Poorvu2008; Willcox et al. Reference Willcox, Curb and Rodriguez2008; Zisman & Rubin, Reference Zisman and Rubin2008). Hence, development of new strategies to inhibit the increasing prevalence of these diseases is urgently warranted. The consumption of fermented milk products containing health beneficial probiotic lactic acid bacteria (LAB) may turn out to be a successful solution to prevent such diseases by eliminating various risk factors. Oxidative stress and hypercholesterolemia are hallmark risk factors for cardiovascular diseases (CVD) and closely related to various diseases such as diabetes, cancer and bowel inflammatory syndrome (Sinclair et al. Reference Sinclair, Barnett and Lunec1991; Roessner et al. Reference Roessner, Kuester, Malfertheiner and Schneider-Stock2008). Numerous strains of LAB and their fermented milk products have been studied for various health beneficial effects including anti-oxidant and hypocholesterolemic effects (Gorbach, Reference Gorbach1990; Grajek et al. Reference Grajek, Olejnik and Sip2005; Pham et al. Reference Pham, Lemberg and Day2008). These biofunctional properties of LAB are strictly strain dependent (Fujiwara et al. Reference Fujiwara, Inoue, Wakabayashi and Fujii2004; Sonnenburg et al. Reference Sonnenburg, Chen and Gordon2006). Therefore, search for new biologically functional strains of LAB with anti-oxidant and hypocholesterolemic potential and development of fermented milk products with similar functions are current interests of dairy scientific community and industries. Present study was an attempt to examine the antioxidant and cholesterol removal potential of three selected strains of LAB i.e. Lactobacillus acidophilus, Lactobacillus casei and Lactococcus lactis in-vitro. Further milk was fermented with these LAB strains and tested for in-vivo anti-oxidant and hypocholesterolemic effects in mice. It is always a considerable issue for probiotic products that the oral consumption of functional bacteria as dietary adjunct must survive the extremely harsh conditions of the gut before colonizing the intestine and providing health beneficial effects (Klaenhammer, Reference Klaenhammer1982). Therefore, in the present study we also determined the success of transmission and colonization of these microorganisms into mice gastrointestinal tract (GIT) and effects on commensal bacteria such as coliforms in GIT of mice.
Materials and Methods
Bacterial strains
All cultures used in this study were obtained from the National Collection of Dairy Cultures (NCDC), of the National Dairy Research Institute (NDRI), Karnal, India. The lactobacilli and lactococci were grown in MRS-broth (Hi-Media Laboratories Pvt. Ltd, Bombay, India) and M17 broth (Terzaghi & Sandine, Reference Terzaghi and Sandine1975) by incubating for 18–20 h at 37 and 30°C, respectively. The cultures were maintained by sub-culturing weekly with 1% inoculum in 10 ml portions of sterile respective media and stored at 4°C. For all the estimations ~108–109 colony forming units per millilitre (CFU/ml) bacterial counts were used.
Antioxidant activity measurements
The free radical scavenging activity of cultures was measured according to the method described by Shibata et al. (Reference Shibata, Natori, Nishihara, Tomisaka, Matsumoto, Sansawa and Nguyen2003). The hydrogen peroxide scavenging activity of cultures was determined by the method described by Lin & Yen, (Reference Lin and Yen1999). Thiobarbituric acid method was used for measurement of linoleic acid (which was chosen as the source of unsaturated fatty acid) peroxidation (Halliwell & Chirico, Reference Halliwell and Chirico1993) and iron-ascorbate system was used for the catalysis of oxidation (Decker & Faraji, Reference Decker and Faraji1990). t-Butylhydroperoxide (TBH) and malondialdehyde (MDA) were chosen as representative of the primary and secondary product of lipid peroxidation, respectively for determining the scavenging activity of the selected LAB cultures. For determination of TBH scavenging activity, the method described by Wolfe (Reference Wolfe1962) was adopted and for determination of MDA scavenging activity the method reported by Lin & Yen (Reference Lin and Yen1999) was used.
Cholesterol assimilation activity
The cholesterol removal activity of bacterial strains was performed according to the procedure described by Gilliland et al. (Reference Gilliland, Nelson and Maxwell1985). Freshly prepared MRS thio-broth (MRS broth with 0·2% sodium thioglycollate) was supplemented with 0·3% oxgall and an amount of pleuropneuminia-like organism (PPLO) serum to give a concentration of 0·24% cholesterol. The broth was inoculated with 1% of each culture for 24 h at 37°C and cholesterol content in the growth medium was determined using the method described by Rudel & Morris (Reference Rudel and Moris1973).
Preparation of fermented milk
Bacterial cultures were revitalized three times in reconstituted and autoclaved skim milk prior to use for preparation of fermented milk samples. Skim milk was obtained from the Institute experimental dairy and heated at 90°C for 15 min. After cooling, the milk samples were inoculated (1%) with the corresponding stock cultures of selected LAB and incubated at respective temperature for 14–15 h and subsequently stored at 4°C until use. The pH of fermented milk product was measured directly by digital pH meter (LabIndia Pvt. Ltd. Mumbai, India). The final bacterial counts in fermented milk product was determined by the method described elsewhere (Terzaghi & Sandine, Reference Terzaghi and Sandine1975; Dave & Shah, Reference Dave and Shah1996; Ravula & Shah, Reference Ravula and Shah1998) and were found to be approximately 108−109 CFU/ml.
Animal study
Animals:
Male Swiss albino mice (30–40 g body weight) were obtained from small animal house of the NDRI. The animals were housed (3 animals/cage) in plastic polypropylene cages. They were divided into 5 groups (n=6) i.e. 1). Control; fed with synthetic diet (Table 1), 2). Skim milk fed; fed with skim milk supplemented diet, 3). Lc. lactis fed; fed with diet supplemented with Lc. lactis fermented milk, 4). Lb. casei fed; fed with diet supplemented with Lb. casei fermented milk and 5). Lb. acidophilus fed; fed with diet supplemented with Lb. acidophilus fermented milk. Animals were allowed access free intake of diet and water and consumed approximately 5–6 g/d per mice of diet for 8 consecutive days.
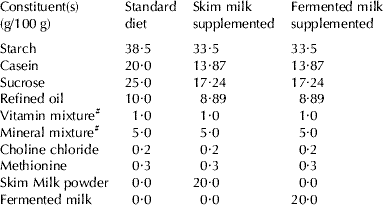
Table 1. Composition of different diets of treatment
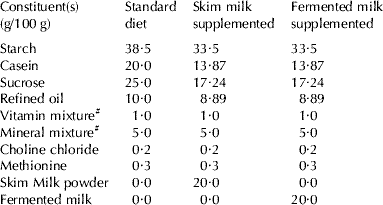
# Vitamin and mineral mixture were prepared and mixed according to AOAC (1995)
Blood collection and plasma cholesterol estimation:
On the last day of experiment blood was collected from the orbital venous plexus of 12 h fasted mice and drawn in a vial containing heparin solution (2 U/μl). The blood samples were centrifuged at 5000 g for 5 min and plasma was separated out. Plasma total cholesterol was estimated using enzymatic kits procured from Bayer Diagnostics Pvt. Ltd, Baroda, India and thiobarbituric acid reactive species (TBARS) were measured by the method of Ohkawa et al. Reference Ohkawa, Ohishi and Yagi1979.
Determination of microbial counts:
For the assay of faecal lactobacilli and coliforms, fresh samples of faeces were collected before and after 2, 5 and 8 d of feeding by gently squeezing rectal part of mice. Faecal materials were put into sterile tightly caped tubes. Animals were sacrificed by cervical dislocation and, small and large intestinal tissues collected aseptically. One gram of faecal sample or intestinal tissue was gently homogenized to a final volume of 5 ml in 0·1% peptone water. The cell suspensions were serially diluted in peptone water and aliquot plated (in triplicate) on M17 agar for enumeration of total lactococci, on lactobacilli MRS agar for total lactobacilli and on violet red bile agar (Hi-Media Laboratories Pvt Ltd., Mumbai, India) for coliforms, and plates were incubated at 30, 37 and 37°C for 72, 48 and 24 h, respectively. Numbers of colonies (in CFU) were counted.
Statistical analysis
The results were expressed as mean ± standard error of means (sem). Significant differences were tested by employing analysis of variance (ANOVA) and for individual parameters and groups; student t-test was performed by using Statistical Package for the Social Sciences (version 10.0) for computation of data.
Results and Discussion
Antioxidant property
Antioxidant potential of all three strains was screened using DPPH, which is a free radical and shows a characteristic purple colour, but when it encounters any proton radical scavengers then the colour fades. In the present study all three LAB strains had shown potent dose dependent free radical scavenging activity in terms of DPPH scavenging potential (Fig. 1A, B). Furthermore, results for antioxidant potential in terms of Trolox equivalents (μM; Fig. 1C) clearly shown that, Lb. casei exhibited highest Trolox equivalents (48·7 μM) followed by Lb. acidophilus (46·3 μM) and Lc. lactis (23·4 μM). Our findings are in line with many other studies (Kaizu et al. Reference Kaizu, Sasaki, Nakajima and Suzuki1993; Zommara et al. Reference Zommara, Takagi, Sakono, Suzuki and Imaizami1994; Lin & Yen, Reference Lin and Yen1999). Lin & Chang (Reference Lin and Chang2000) also reported that both intact and intracellular cell free extracts of intestinal LAB i.e. Bifidobacterium longum (ATCC 15708) and Lb. acidophilus (ATCC 4356) of 109 cells exhibited a potent anti-oxidant activity by inhibiting linoleic acid peroxidation. Therefore we also measured antioxidant activity using this approach (Table 2) and found that inhibition of linoleic acid peroxidation followed similar trend as observed in DPPH and Trolox equivalent method i.e. Lb. casei showed highest inhibitory activity (67%) followed by Lb. acidophilus (52%) and Lc. lactis (27%). Results of present study clearly indicate that 108−109 cells of Lb. casei, Lb. acidophilus and Lc. lactis have potent antioxidant activity.

Fig. 1. Curves of dose dependent DPPH scavenging activity (A) and antioxidant activities as Trolox equivalents (B) of Lc. lactis, Lb. acidophilus and Lb. casei. Values are means of triplicate tests. The results were obtained ED50 values from 2A.
Table 2. Antioxidant activity cholesterol assimilation activities of Lc. lactis, Lb. acidophilus and Lb. casei Footnote 1
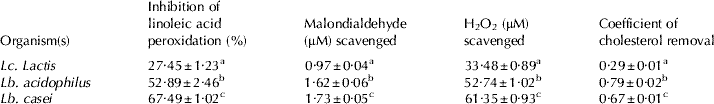
1 The values are means ± SEM of three trails in triplicates
a,b,c Values with different superscripts in a column are significantly different at the level of p<0·05
TBH, MDA and hydrogen peroxide scavenging activity
Oxidative stress is the result of excess formation and/or insufficient removal of reactive oxygen species (ROS). Increased production of ROS oxidizes unsaturated fatty acids of cell membranes and produces lipid hydroperoxides by initiating a chain reaction (Halliwell & Chirico, Reference Halliwell and Chirico1993), this leads to changes in cell membrane and genotoxicity which result in tissue damage (Baker & He, Reference Baker and He1991). Interestingly, all three strains exhibited potent scavenging ability of cellular toxic substances i.e. lipid peroxidation end products (MDA) as well as H2O2 radicals (Table 2), indicating detoxification effects by ROS removal potential of these strains.
In present study, TBH was used as a primary product of lipid peroxidation and the selected bacterial strains were unable to scavenge this lipid hydroperoxide. The results of present study were in line with Lin & Yen (Reference Lin and Yen1999) study, in which they reported that the cell free extract of Streptococcus thermophilus and Lb. delbrueckii ssp. bulgaricus were unable to scavenge TBH. Indeed, MDA is a secondary end product of lipid peroxidation and a highly reactive substance which has ability to deteriorate structural and functional characteristics of cellular proteins and nucleic acids (Aubourg, Reference Aubourg1993). In the present study, the whole cells of the selected strains had ability to scavenge low concentration (μM) of MDA also. However, the milk fermented with these three strains i.e. Lc. lactis, Lb. acidophilus and Lb. casei did not cause significant differences in plasma TBARS levels in mice fed with different diets for 8 days (data not shown), while dahi (Indian yogurt) prepared with these selected strains had shown a potent antioxidant activity in-vivo in diabetic rats fed for 56 days (Yadav et al. Reference Yadav, Jain and Sinha2008). This indicates that, short term feeding of fermented milk in the present study may not be able to show potential anti-oxidant activity in normal mice.
Cholesterol lowering activity
All three strains grew well in the presence of cholesterol and decreased cholesterol concentration in the culture broth (Table 2). However, the cholesterol assimilation ability was different from antioxidant activity among cultures i.e. Lb. acidophilus had shown highest (79%) cholesterol removal activity followed by Lb. casei (67%) and Lc. lactis (29%). During the feeding trial, feeding milk fermented with Lb. acidophilus and Lb. casei exhibited decreased plasma total cholesterol levels (~47 and ~49 mg/dl, respectively) after 8 days compared with those of control group (~63 mg/dl). However, no significant changes were observed in plasma total cholesterol levels by non-fermented skim milk- and Lc. lactis fermented milk-fed animals (~63 and ~62 mg/dl, respectively) relative to control. Indeed mean values did not differ significantly among all the groups of animals (P>0·05). Similarly, several workers also reported that LAB and their fermented milk products have an ability to reduce media as well as blood cholesterol (Kimoto et al. Reference Kimoto, Ohmomo and Okamoto2002; Taranto et al. Reference Taranto, Fernandez, Murga and deValdez2003; Liong & Shah, Reference Liong and Shah2005). This might be due to the binding of dietary cholesterol with the bacterial cell wall in the food and/or intestinal tract before cholesterol can be absorbed into the body (Gilliland et al. Reference Gilliland, Nelson and Maxwell1985; Gilliland & Walker, Reference Gilliland and Walker1990). Hosono & Tono-oka (Reference Hosono and Tono-oka1995) demonstrated that binding of dietary cholesterol by LAB cells in different fermented milk products varied among strains and species. They also suggested that differences in binding abilities might be due to chemical and structural properties of peptidoglycan layer of bacterial cell wall. In the present study, cholesterol removal ability also differed in different cultures such as Lb. acidophilus had shown highest cholesterol assimilation ability followed by Lb. casei and Lc. lactis. In addition dahi prepared with these bacterial species had shown a potent antidiabetic as well as anti-hypercholesterolemic effect in high fructose diet-fed rats (Yadav et al. Reference Yadav, Jain and Sinha2006, Reference Yadav, Jain and Sinha2007).
Successful transmission and colonization of LAB into the gut
In order to act as a successful probiotic dietary adjunct, a bacterial strain must survive and transmit successfully to GIT. We confirmed the successful transmission of live bacteria in GIT by analysing faecal samples of mice fed with different fermented milk supplemented diets and found that total lactobacilli and lactococci counts were significantly increased in faeces of lactobacilli and lactococci fermented milk fed animals, respectively (Fig. 2E, F) The ability of micro-organisms to adhere to intestinal epithelium is often considered one of the main selection criteria for potential probiotics (Ouwehand et al. Reference Ouwehand, Kirjavainien, Shortt and Salminen1999). Adhesion to the intestinal mucosa is thought to be an important property for colonization by preventing wash-out (Wadstrom, Reference Wadstrom1998), especially in the small intestine where flow rates are relatively high (Sanford, Reference Sanford1992). The counts of total lactobacilli were significantly increased on intestinal mucosa by feeding Lb. acidophilus and Lb. casei fermented milks (Fig. 2A–D), which demonstrates that the selected lactobacilli strains are able to adhere to the intestinal epithelium after crossing from the harsh conditions of GIT.

Fig. 2. The total counts of lactococci (A, C, E) and lactobacilli (B, D, F) in small (A, B) and large intestinal mucosa (C, D) as well as faecal samples (E, F) of mice fed with control, skim milk and milk fermented with Lc. lactis, Lb. acidophilus and Lb. casei supplemented diets analysed on 0, 2, 5 and 8 d of experiments.
Removal of coliforms from intestinal tract
Several studies have also suggested that adhesive probiotic bacteria could prevent the attachment of pathogens, such as coliform bacteria and Clostridia, and stimulate their removal from the infected intestinal tract (Lidbeck et al. Reference Lidbeck, Gustafasson and Nord1987; Saxelin et al. Reference Saxelin, Pessi and Salminen1995). A significant decline in the counts of intestinal and faecal coliforms in mice fed with milk fermented by Lb. casei, Lb. acidophilus and Lc. lactis supplemented diets was observed, whereas no changes were observed in control and skim milk supplemented diet fed animals (Fig. 3). However, the maximum reduction of coliform counts was observed in animals fed with Lb. casei fermented milk supplemented diet (39%), followed by Lb. acidophilus and Lc. lactis, which have shown 32 and 25%, reduction, respectively (Fig. 3). These results clearly indicate that feeding of milk fermented with selected LAB significantly reduced the burden of commensal bacteria i.e. coliforms in the intestinal tract of mice.

Fig. 3. The total coliform counts in small (A) and large intestinal mucosa (B) as well as faecal samples (C) in mice fed with control, skim milk and milk fermented with Lc. lactis, Lb. acidophilus and Lb. casei supplemented diets analysed on 0, 2, 5 and 8 d of the experiment.
In conclusion, selected LAB strains exhibited potent antioxidant activity by inhibiting linoleic acid peroxidation, scavenging hydrogen peroxide and malondialdehyde, in addition to cholesterol assimilation activities and ability to successfully transmit into GIT of consumer animals. On the basis of these results it can be suggested that, these selected LAB stains may be used for the preparation of biotherapeutic fermented milk products with potential health beneficial properties. The consumption of a food with these properties may play a vital role in the prevention of highly prevalent diseases like CVD, diabetes, colon cancinogenesis and inflammatory bowel diseases.