Lactobacilli are commonly retrieved in fermented foods and as probiotics in functional foods. Adhesion to the intestinal mucosa is, among several factors, one of the main selection criteria for a potentially successful probiotic as it will assure the colonisation of mucosal surfaces, at least transiently, thus interfering with pathogen binding and allowing the interaction with the immune cells (Muñoz-Provencio et al. Reference Muñoz-Provencio, Llopis, Antolín, de Torres, Guarner, Pérez-Martínez and Monedero2009).
Since mucus is the first barrier that covers the epithelial cells of the gastrointestinal (GI) tract, a possible mechanism for bacterial adherence and colonisation involves the binding of microbial cell-surface molecules to this protective mucus layer. Thus, adhesion to this matrix could be considered a prerequisite for colonisation of the gut (Tallon et al. Reference Tallon, Arias, Bressollier and Urdaci2007). The layer of mucus bound to GI epithelium is a continuous gel matrix composed primarily of complex glycoproteins (mucins) and glycolipids that acts as a barrier to protect the host from harmful antigens and promote luminal motility. However, the secretion of mucins, among other products, differs significantly along the gut leading to different components profiles in each compartment (McGuckin et al. Reference McGuckin, Lindén, Sutton and Florin2011). Several difficulties involved in the study of bacterial adhesion in vivo, especially in humans, have led to the development of different in vitro models such as colonocyte-like epithelial cell lines (Caco-2, HT-29, HT-29 MTX) or mucin/mucus-binding systems (Deepika & Charalampopoulos, Reference Deepika and Charalampopoulos2010).
Several studies showed that surface proteins of some lactobacilli participate in adhesion to epithelial cell lines, GI mucins, or extracellular matrix proteins (Rojas et al. Reference Rojas, Ascencio and Conway2002; Granato et al. Reference Granato, Bergonzelli, Pridmore, Marvin, Rouvet and Corthesy-Theulaz2004; Wang et al. Reference Wang, Wei, Yuan, Li, Li, Li and Li2008). However, some unidentified sugar moieties and lipoteichoic acid have also been reported to be involved (Chan et al. Reference Chan, Reid, Irvin, Bruce and Costerton1985; Henriksson & Conway, Reference Henriksson and Conway1996). Until now, several lactobacilli mucus lycolns have been identified and functionally characterized (Roos & Jonsson, Reference Roos and Jonsson2002; Buck et al. Reference Buck, Altermann, Svingerud and Klaenhammer2005; Pretzer et al. Reference Pretzer, Snel, Molenaar, Wiersma, Bron, Lambert, de Vos, van der Meer, Smits and Kleerebezem2005; Miyoshi et al. Reference Miyoshi, Okada, Uchimura and Satoh2006). Among them, surface layers (S-layers), planar arrays of (glyco)-proteinaceus subunits non-covalently linked to each other covering the cell envelope of many bacteria species (Sára & Sleytr, Reference Sára and Sleytr2000), are good candidates to be involved in adhesion to different matrixes (Schneitz et al. Reference Schneitz, Nuotio and Lounatma1993; Antikainen et al. Reference Antikainen, Antón, Sillanpaa and Coronen2002; Avall-Jääskeläinen et al. Reference Avall-Jääskeläinen, Lindholm and Palva2003; De Leeuw et al. Reference De Leeuw, Li and Lu2006).
A few years ago, Garrote et al. (Reference Garrote, Delfederico, Bibiloni, Abraham, Pérez, Semorile and De Antoni2004) described the presence of S-layer proteins in Lactobacillus kefiri isolated from kefir, a probiotic fermented product obtained by fermentation of milk with kefir grains (Garrote et al. Reference Garrote, Abraham and De Antoni2001; Farnworth, Reference Farnworth2005). It has been demonstrated that these S-layer proteins are involved in several probiotic properties of Lb. kefiri strains. In particular, they are capable of inhibiting the invasion of Salmonella enterica serovar enteritidis to Caco-2 cells (Golowczyc et al. Reference Golowczyc, Mobili, Garrote, Abraham and De Antoni2007) and the cytotoxic effect of Clostridium difficile toxins (Carasi et al. Reference Carasi, Trejo, Pérez, De Antoni and Serradell2012). Besides, it has been described that S-layer proteins mediate the interaction of Lb. kefiri bacterial cells with yeasts present in kefir grains (Golowczyc et al. Reference Golowczyc, Mobili, Garrote, Serradell, Abraham and De Antoni2009).
In this study, we investigated the mucus-binding properties of Lb. kefiri strains, a group of potentially probiotic microorganisms isolated from kefir, and the effect of different treatment on the degree of bacterial adherence, in order to analyse the role of surface components in the adhesion properties of these bacteria.
Materials and methods
Bacterial strains and growth conditions
Lactobacillus kefiri CIDCA 83111, 83113, 83115, 8321, 8345, and 8348 isolated from kefir grains (Garrote et al. Reference Garrote, Abraham and De Antoni2001), and Lb. kefiri JCM 5818 obtained from the Japanese Collection of Microorganisms (Reiken, Japan) were used. Previously, Lb. kefiri CIDCA 83115, 8321, 8345 and 8348 were characterized as aggregating strains, meanwhile Lb. kefiri CIDCA 83111, 83113 and JCM 5818 were described as strains without aggregative capability (Mobili et al. Reference Mobili, Serradell, Trejo, Avilés-Puigvert, Abraham and De Antoni2009). All strains were identified by 16S rDNA sequencing (Weisburg et al. Reference Weisburg, Barns, Pelletier and Lane1991) and they were characterized by Random amplified polymorphic DNA-PCR (RAPD-PCR) using three 10-nucleotide single-strand primers OPA3 (5′-AGTCAGCCAC-3′), OPH3 (5′-AGACGTCCAC)-3′ and OPL12 (5′-GGGCGGTACT) (Bioprobe, Montrevils-sous-Bois, France). The PCR was performed as follows: one step at 95 °C for 5 min; 45 steps 94 °C for 30 s, 36 °C for 60 s and 72 °C for 60 s; and a final step at 72 °C for 10 min. Lactobacilli were cultured in MRS-broth (DIFCO, Detroit, USA) 37 °C for 48 h in aerobic conditions. Frozen stock cultures were stored at −80 °C in skim milk.
Biochemical and enzymatic pre-treatment of bacterial cells
Bacterial cells were subjected to different pre-treatments in order to investigate the involvement of surface structures in the adhesion to different mucus sources. One hundred ml of MRS culture of lactobacilli was harvested at stationary phase, collected by centrifugation (10 000 g at 10 °C for 10 min), and washed three times with phosphate buffered saline (PBS, KH2PO4 0·144 g/l, NaCl 9 g/l, Na2HPO4 0·795 g/l, pH 7·2).
For some experiments, bacteria were resuspended in 15 ml of 5 m LiCl (J.T. Baker, Mallinckrodt Baker S.A., Edo de Mexico, Mexico) giving a bacterial suspension of OD550=25·0 (around 5×1010 CFU/ml). The mixture was incubated in a shaking incubator (Environ Shaker, Lab-line Instruments Inc., Melrose Park, IL, USA) at 300 g and 4 °C for 60 min. Then, it was centrifuged (16 000 g at 10 °C for 30 min) and bacteria were washed three times with PBS.
For other experiments, bacterial cells suspensions (OD550=25·0) were treated with proteinase K (SIGMA, 0·1 g/l, 30 min at 37 °C) or NaOH (ANEDRA®, 0·01 m, 30 min at 4 °C), then centrifuged and finally washed as described above.
For SDS-PAGE, both treated and untreated bacteria were resuspended in buffer Laemmli and incubated for 5 min in boiling water. Finally, 20 μl of each sample were run.
Preparation of surface protein (S-layer) extracts
Surface protein extracts from bacterial cells were obtained as described by Carasi et al. (Reference Carasi, Trejo, Pérez, De Antoni and Serradell2012). Briefly, after bacteria were treated with 5 m LiCl and centrifuged as described above, the supernatant was filtered through a membrane of 0·45 μm diameter pore. The solution containing the S-layer protein was dialysed against PBS plus 0·5 ml/l Tween 20 (Sigma–Aldrich, Inc., St. Louis, MO, USA) for 24 h at room temperature, making 3 changes of 5 l each, using a cellulose membrane (SpectraPor membrane tube, MWCO 6000–8000, Spectrum Medical Industries, Rancho Dominguez, California, US). Protein concentration was determined by the Bradford method (Bradford, Reference Bradford1976).
Extraction of intestinal mucus from piglets
Mucus extraction was based in the experimental protocol described by Conway et al. (Reference Conway, Welin and Cohen1990) with some modifications. Briefly, mucus was isolated from pig small intestinal walls (hereafter referred to as SIM) or pig colon walls (hereafter referred to as CM) by gentle scraping into PBS. Epithelial cells and large cellular components were removed by centrifugation at 11 500 g for 15 min. The protein concentration of mucus preparations was determined by the Bradford method. The extract concentration was fixed in 2 g/l.
Adhesion assays
Plate preparation for binding
Partially purified type III porcine gastric mucin obtained from Sigma-Aldrich (hereafter referred to as MUCIN) was dissolved in phosphate-buffered saline (PBS) pH 7·0. Solutions of 3 g MUCIN/l, 2 g SIM/l or 2 g CM/l were bound to 96-well sterile polystyrene plates (Maxisorp Nunc, Roskilde, Denmark), according to Sánchez et al. (Reference Sánchez, Arias, Chaignepain, Denayrolles, Schmitter, Bressollier and Urdaci2009). Plates were incubated at 37 °C for 1 h, followed by an overnight incubation at 4 °C. A second incubation for 2 h at 37 °C was performed with the same solution in order to minimise the number of empty binding sites in the polystyrene microtitre plates. Finally, the wells were washed twice with 200 μl PBS. A minimum of four replicates were used to estimate the adhesion of a given strain or protein.
Bacterial binding assays
Bacterial preparation was performed according to Tallon et al. (Reference Tallon, Arias, Bressollier and Urdaci2007). Lb. kefiri was grown for 48 h at 37 °C in MRS-broth (DIFCO, Detroit, USA). Aliquots of 1 ml were sampled and centrifuged at 10 000 g at 10 °C for 5 min and the pellets were washed twice with sterile PBS, resuspended in the same buffer and adjusted to the optical density OD550=1·0 (around 108 CFU/ml). One hundred microlitres of the bacterial suspension were added to each well. The plates were incubated 2 h at 37 °C. The wells were washed 6 times with 200 μl of sterile PBS to remove unbound bacteria. The wells were then treated with 200 μl of a 5 ml/l Triton X-100 solution for 30 min at 37 °C to desorb the bound bacteria. One hundred microlitres of the content of each well were removed, diluted in PBS and plated on MRS agar plates. The concentration of Triton X-100 and the temperature of contact used were tested on all strains in order to determine the influence on bacterial viability. Some adhesion experiments were performed in presence of different sugars (lactose, galactose, sucrose, xylose, fructose, glucose, mannose, rhamnose) (SIGMA) at final concentration of 0·08 m or in presence of soluble S-layer proteins at 15 μg/ml.
Protein binding assays
Protocol 1 (immunochemical detection using anti-S-layer antibodies): 100 μl of surface protein extracts (concentration 15 μg/ml) were added to coated MUCIN, SIM or CM wells and incubated 2 h at 37 °C. The wells were washed five times with 200 μl PBS plus 0·5 ml Tween 20/l (Sigma–Aldrich) to remove unbound proteins. Afterwards, 100 μl of polyclonal rabbit antibody anti-S-layer Ac21 diluted 1 : 100 (Garrote et al. Reference Garrote, Serradell, Abraham, Añon, Fossati and De Antoni2005) were applied to each well and incubated at 37 °C for 1 h. Washing was repeated as described above, and then peroxidase-conjugated anti-rabbit IgG (sc-2004, Santa Cruz Biotechnology, Inc., USA) diluted 1 : 2000 was added to each well. The plates were incubated at 37 °C for 1 h. After another cycle of washing, the reaction was visualised by adding 100 μl of 3,3′,5,5′-Tetramethylbenzidine (TMB) Liquid Substrate System for ELISA (Sigma-Aldrich, France). The reaction was stopped with 1 m HCl and the yellow reaction product was read at 450 nm. Assays were performed at least in triplicate, being done each time in triplicate, and results are expressed as the mean±sd.
Protocol 2 (SDS-PAGE and silver staining): 100 μg of surface protein extracts in PBS at pH 7, were added to coated MUCIN wells and incubated at 37 °C for 2 h. After the incubation period, wells were washed five times with 0·020 m NH4HCO3 in order to eliminate unbound proteins (Sánchez et al. Reference Sánchez, Arias, Chaignepain, Denayrolles, Schmitter, Bressollier and Urdaci2009). Then 60 μl of 10 g SDS/l was added to each well and incubated at 37 °C for 2 h with gentle agitation. Wells were then dried and proteins were solubilised in Laemmli buffer without SDS (Laemmli, Reference Laemmli1970). Proteins were analysed by SDS-PAGE and visualised by standard silver staining.
Statistical analysis
One-way ANOVA with Dunnett's post-test was performed using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
Results
Adhesion of Lb. kefiri strains to stomach mucin, small intestinal mucus and colon mucus from piglets
Before adhesion assays, we confirmed that the seven Lb. kefiri strains used in this work were different strains of the same species. By using OPL12 we obtained different amplification patterns for all tested strains (Fig. 1).
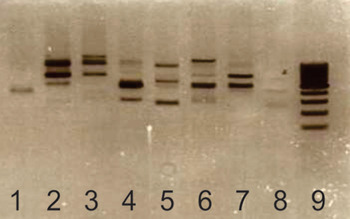
Fig. 1. RAPD profile of different Lb. kefiri strains using primer OPL12. The picture shows a 1,5% w/v agarose gel; DNA was stained using ethidium bromide. Lanes 1: Lb. kefiri CIDCA 8321, 2: CIDCA 8345, 3: CIDCA 8348, 4: CIDCA 83115, 5: CIDCA 83111, 6: CIDCA 83113, 7: JCM 5818, 8: blank, 9: molecular weight marker.
The analysis of the bacterial adhesion assays to MUCIN, SIM and CM showed that all strains were able to interact with these substrates (Fig. 2a–c). Strains adhered to MUCIN and SIM in a similar manner, nevertheless some differences were observed concerning CM adhesion. Overall, some strains (CIDCA 8321, 83115 and 83115) seem to adhere less to CM compared with the other substrates (MUCIN or SIM). Moreover, using CM as substrate we found the largest difference in adhesion, ten times between the less and the most adherent strains, but no significant differences were observed. Bacterial adhesion to the plate without substrate was lower than 30 CFU/ml.

Fig. 2. Adhesion of Lb. kefiri to porcin (a) stomach mucin, (b) small intestine mucus extract and (c) colon mucus extract. Experiments were performed in 96 wells plates, which were coated with the corresponding substrate and incubated with 108 CFU ml. All experiments were performed at least three times. None of the data showed significant differences (P>0·05).
On the other hand, the adhesion assays of surface protein extracts from the seven strains of Lb. kefiri revealed with anti S-layer specific antibodies indicate that S-layer proteins from all bacteria were able to bind to MUCIN, SIM or CM, and no differences were observed among them (data not shown). In agreement with these results, the adhesion of surface proteins (mainly S-layer proteins) to MUCIN was confirmed by SDS-PAGE of bound proteins for all the strains studied here (Fig. 3).

Fig. 3. Adhesion of S-layers from Lb. kefiri strains to MUCIN. SDS-PAGE 12·5% was revealed using silver staining. Lanes 1: molecular weight marker, 2 and 9: MUCIN without any S-layer, 3: Lb. kefiri CIDCA 8321, 4: CIDCA 8345, 5: CIDCA 8348, 6: CIDCA 83111, 7: CIDCA 83113, 8: CIDCA 83115, 10: JCM 5818.
Taking into account all these results, we selected two aggregating strains (CIDCA 8348 and CIDCA 83115) and one non-aggregating strain (JCM 5818) of Lb. kefiri to perform complementary studies.
Effect of removal of surface components on bacterial adhesion
The extraction of surface components using LiCl or NaOH significantly reduced the adhesion of Lb. kefiri to MUCIN, SIM or CM for the three strains tested relative to the untreated controls (Fig. 4a–c). However, the SDS-PAGE analysis of LiCl and NaOH extracts showed that a significant proportion (up to 50%) of surface proteins (S-layer proteins) were not completely eliminated from bacterial surface after these treatments (Fig. 4d). On the other hand, a slight reduction of bacterial adhesion was observed for Lb. kefiri CIDCA 8348 and 83115 after treatment with proteinase K, although no significant differences were registered (Fig. 4). The viability of bacterial cells, assessed by plate counting, was not affected by these treatments.
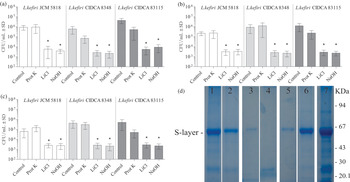
Fig. 4. Adhesion of Lb. kefiri CIDCA 8348, CIDCA 83115 and JCM 5818 to (a) MUCIN, (b) SIM and (c) CM after treatment with 0·1 g/l proteinase K 30 min at 37 °C, 5 m LiCl or 0·01 m NaOH during 30 min at 4 °C. All experiments were performed at least three times. *Significant difference relative to controls (P<0·05) (d) SDS-PAGE (colloidal blue staining) of proteins from Lb. kefiri CIDCA 8348 cells after treatment with LiCl or NaOH, or without any treatment. SN: proteins in supernatants after dialysis. Lane 1: bacteria after treatment with 5 m LiCl, lane 2: SN dialysis 5M LiCl, lane 3: bacteria after treatment with 0·1 g/l proteinase K, lane 4: SN dialysis 0·1 g/l proteinase K, lane 5: bacteria after treatment with 0·01 m NaOH, lane 6: SN dialysis 0·01 m NaOH, lane 7: bacteria without any treatment.
Effect of surface proteins addition on bacterial adhesion
Adhesion assays employing strains Lb. kefiri CIDCA 8348, CIDCA 83115 and JCM 5818 were performed in order to evaluate the influence of surface proteins (surface protein extracts) addition on bacterial interaction with gastric mucin or intestinal mucus components. The addition of their own surface protein extract (mainly composed by its S-layer protein) significantly increased adhesion of aggregating strains Lb. kefiri CIDCA 8348 and 83115 to MUCIN and SIM (P<0·05), meanwhile no changes were observed for adhesion of non-aggregating strain Lb. kefiri JCM 5818 to these substrates (Fig. 5). Regarding the interaction with CM components, addition of S-layer proteins only increased the adhesion of Lb. kefiri CIDCA 83115 (Fig. 5).

Fig. 5. Adhesion of whole Lb. kefiri CIDCA 8348, CIDCA 83115 and JCM 5818 to MUCIN, SIM or CM with addition of own S-layer protein extract (15 μg/ml). Experiments were performed in 96 wells plates coated with the corresponding substrate and incubated with 108 CFU/ml. All experiments were performed at least three times. *Significant difference compared to each control (P<0·05).
Effect of sugar addition on bacterial interaction with mucin and mucus
It was previously described that a lectin-like activity attributed to the presence of S-layer proteins in the surface, mediates the interaction of Lb. kefiri cells with other microorganisms (Golowczyc et al. Reference Golowczyc, Mobili, Garrote, Serradell, Abraham and De Antoni2009). In order to test the influence of this kind of interactions in mucus-adhesion ability, a series of assays in presence of different sugars was performed. None of the seven sugars tested had the capablity to inhibit the adhesion of whole bacteria to MUCIN, SIM or CM at assessed conditions (data not shown). Noteworthy, the increase in the adherence of bacteria to MUCIN, SIM or CM produced by the addition of their own surface protein extracts is hampered by the presence of certain sugars (P<0·05). Figure 6 shows the results obtained in these experiments for Lb. kefiri CIDCA 8348. The addition of mannose, fructose, rhamnose or galactose significantly reduced bacterial adhesion in presence of its surface proteins extract either to MUCIN or to SIM; meanwhile the addition of lactose, glucose or sucrose did not modify bacterial adhesion. On the other hand, sugar effect on adhesion to CM showed a similar pattern, although differences were observed regarding the effect of fructose or glucose addition and the strong inhibition observed in presence of mannose (100 times lower). Similar effects were observed for Lb. kefiri CIDCA 83115 with the same sugars, with the exception of mannose, which did not show inhibition for this strain (data not shown).
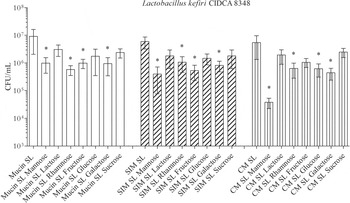
Fig. 6. Effect of sugars in mucus-adhesion. Adhesion of Lb. kefiri CIDCA 8348 with addition of own S-layer protein extract (15 μg/ml) to MUCIN, SIM or CM in presence of sugars (0·08 m). All experiments were performed at least three times; *Significant difference relative to SL Lk CIDCA 8348 (P<0·05).
DISCUSSION
The adhesion to mucus layer that coats the intestinal tract is the first step required for commensal and probiotic organisms to interact with host cells and elicit any particular response (Van Tassell & Miller, Reference Van Tassell and Miller2011). In this work, we reported for the first time the ability of Lb. kefiri to adhere to commercial porcine gastric mucin as well as to mucus components extracted from piglet small intestine and colon. In our experimental approaches, no differences were observed among adhesion of aggregating and non-aggregating Lb. kefiri strains to MUCIN or SIM. However, regarding each particular strain, some differences were observed in CM adhesion. We observed a tendency to a lower adhesion to CM than to MUCIN or SIM, indicating that mucus composition of distinct intestinal compartments offers differential adhesion characteristics to Lb. kefiri strains. The degree of adhesion for all the strains seems to be not very tight (percentage of bacterial adhesion ranged from 0·08 to 5·0%) compared with data reported for other probiotic lactobacilli such as L. rhamnosus GG (Ouwehand et al. Reference Ouwehand, Kirjavainen, Grönlund, Isolauri and Salminen1999), L. jonhsonii LA1 (Ouwehand et al. Reference Ouwehand, Tölkko, Kulmala, Salminen and Salminen2000) or L. plantarum 299v (Tallon et al. Reference Tallon, Arias, Bressollier and Urdaci2007). In agreement with our results, a considerable variation in mucus adhesion ability has been reported for different probiotic bacteria (Tuomola et al. Reference Tuomola, Ouwehand and Salminen2000; MacKenzie et al. Reference MacKenzie, Jeffers, Parker, Vibert-Vallet, Bongaerts, Roos, Walter and Juge2010).
Many researchers have reported that functionality of surface components can be responsible for probiotic properties of several different microorganisms (Lebeer et al. Reference Lebeer, Vanderleyden and De Keersmaecker2008; Wang et al. Reference Wang, Wei, Yuan, Li, Li, Li and Li2008; Sánchez et al. Reference Sánchez, Arias, Chaignepain, Denayrolles, Schmitter, Bressollier and Urdaci2009; Kleerebezem et al. Reference Kleerebezem, Hols, Bernard, Rolain, Zhou, Siezen and Bron2010; Mastromarino et al. Reference Mastromarino, Cacciotti, Masci and Mosca2011). We studied the effect of extraction of surface components, mainly S-layer proteins, in that interaction. Our results show that bacterial treatment with LiCl or NaOH significantly reduced the ability of Lb. kefiri cells to adhere to gastric mucin and intestinal mucus, although surface proteins were not completely eliminated. We previously reported that these bacteria are highly hydrophobic (Londero et al. Reference Londero, Abraham, Gomez Zavaglia, Illanes Frontaura, León Pelaez, Serna Cataño, Quintero Yepes, Kakisu, Tymczyszyn, De Antoni, Garrote, Serradell, Golowczyc, Gerbino, Mobili, Perez and Carasi2009) and we observed that the performed treatments dramatically reduced this condition. It must be noted that the use of chemical methods to extract the S-layers from the surfaces might result in the simultaneous extraction of other surface components which might be involved in adhesion (Antikainen et al. Reference Antikainen, Antón, Sillanpaa and Coronen2002; Jakava-Viljanen & Palva, Reference Jakava-Viljanen and Palva2007). However, proteinase K-treated bacteria showed only a slight reduction in mucus adhesion. These findings suggest that besides S-layer extraction or changes in hydrophobicity, each particular treatment causes other sets of modifications on bacterial surfaces which affect the ability of Lb. kefiri to interact with mucins and mucus components. In consequence, other experiments were carried out to evaluate the role of surface proteins from Lb. kefiri in bacterial adhesion to mucus components.
We observed that the addition of surface protein extract significantly increases the degree of adhesion of Lb. kefiri aggregating strains to gastric mucin and intestinal mucus, suggesting that surface proteins (mainly S-layer proteins) with some physicochemical features could mediate bacterial adhesion to these substrates (Mobili et al. Reference Mobili, Serradell, Trejo, Avilés-Puigvert, Abraham and De Antoni2009). Besides, taking into account that S-layer-carrying bacteria could secrete these proteins into surrounded media, it is possible to hypothesise that the presence of these proteins in the GI tract could enhance the bacterial adhesion to mucus layer, improving the interaction of these bacteria with the epithelium. To our knowledge, although several authors have proposed that S-layers mediate adhesion to epithelial cells (Schneitz et al. Reference Schneitz, Nuotio and Lounatma1993; Hynönen et al. Reference Hynönen, Westerlund-Wikström, Palva and Korhonen2002; Avall-Jääskeläinen et al. Reference Avall-Jääskeläinen, Lindholm and Palva2003; Frece et al. Reference Frece, Kos, Svetec, Zgaga, Mrsa and Susković2005), none of them have directed their experimental approaches toward the study of the contribution that extracellular S-layer proteins components could be involved in this phenomenon. On the other hand, several lactobacilli mucus adhesins were identified in Lb. reuteri (Rojas et al. Reference Rojas, Ascencio and Conway2002; Roos & Jonsson, Reference Roos and Jonsson2002; Wang et al. Reference Wang, Wei, Yuan, Li, Li, Li and Li2008), Lb. plantarum WCFS1 (Pretzer et al. Reference Pretzer, Snel, Molenaar, Wiersma, Bron, Lambert, de Vos, van der Meer, Smits and Kleerebezem2005) and Lb. acidophilus NCFM (Buck et al. Reference Buck, Altermann, Svingerud and Klaenhammer2005).
Additionally, since it was previously described by our workgroup that a lectin-like activity (mainly attributed to bacterial S-layer proteins) mediates the co-aggregation of Lb. kefiri with yeast cells (Golowczyc et al. Reference Golowczyc, Mobili, Garrote, Serradell, Abraham and De Antoni2009), we performed a series of experiments to evaluate the influence of the presence of different sugars on bacterial adhesion to mucus. Our results support the idea that sugar residues are not mediating the direct adhesion of bacterial cells. However, the assays performed with Lb. kefiri CIDCA 8348 allowed us to consider the possibility that sugars could be involved in the interaction of surface proteins and mucus components. Since it has been described that most of S-layer proteins present a rather smooth outer surface and a more corrugated inner surface which can be exposed in proteins recrystallised from solution conformational structure in solution (Schuster & Sleytr, Reference Schuster and Sleytr2009), we hypothesise that the structure of the S-layer molecules (the major component of the surface protein extracts) in solution could differ from that observed on the surface of the bacteria thus leading to different interactions with sugars. Further studies will be needed in order to gain inside into the degree of participation of specific sugar moieties in the mucus-adhesion mechanism. On the other hand, the use of isogenic mutants of Lb. kefiri deficient in genes encoding S-layer proteins would allow confirmation of the role of these surface proteins in mucus-adhesion process in the future.
In conclusion, these food-isolated bacteria adhere to gastrointestinal mucus and the extraction of surface components, mainly S-layer proteins, abrogates this phenomenon. This probiotic characteristic could influence their interactions with the host and with gut microbiota. On the other hand, it is an important property to understand the contribution of Lb. kefiri strains to the health-promoting properties traditionally associated to kefir-consumption and becomes an essential issue regarding the biotechnological potentiality of Lb. kefiri for food industry.
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (PICT 00479/06), CONICET, Universidad Nacional de La Plata (Project 11/X548), and Bordeaux Science Agro, Ministère de l'Agriculture Français. P Carasi is a fellow of CONICET; G De Antoni is a researcher of the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC-PBA); M Serradell is a member of the Carrera de Investigador Científico y Tecnológico of CONICET. MC Urdaci is researcher of Bordeaux Science Agro, Université de Bordeaux. P Carasi was supported by Boehringer Ingelheim Fonds (travel grants programme).








