Although final biomass and activity of the yoghurt starter cultures are important, short fermentation times are industrially advantageous (Barrette et al. Reference Barrette, Champagne and Goulet2001). Acceleration of the yoghurt fermentation process by supplementation of nutrients for the yoghurt cultures should result in a greater amount of product being produced in the same time span, while requiring no additional infrastructure.
Bacterial growth can be improved in milk by the addition of substances of undefined composition to the growth medium, such as YE. This is most probably due to YE being a reliable source of peptides, amino acids, trace minerals and B-vitamins (Chae et al. Reference Chae, Joo and In2001).
Since YE stimulates growth of various bacteria, including the starters for yoghurt fermentation Streptococcus thermophilus and Lactobacillus bulgaricus, in media other than milk, (Amrane & Prigent, Reference Amrane and Prigent1998; Ghaly et al. Reference Ghaly, Tango and Adams2003) it is of interest whether it would accelerate the yoghurt fermentation process.
Many efforts have been dedicated to screen YE and to identify active components which support cell growth (Yoo, Reference Yoo2009). However, YE powder is a complex mixture and identification of the constituents responsible for cell growth promotion has not yet been achieved (Yoo, Reference Yoo2009). The YE used in the current study is produced by proteolysis of the yeast (Saccharomyces cereviceae) by its own naturally occurring enzymes and consist of the water soluble components of the yeast cell (Champagne et al. Reference Champagne, Gaudreau and Conway2003; Yoo, Reference Yoo2009).
The off-flavours associated with YE or peptones render these substances less suitable for industrial production of commercial dairy products (Elli et al. Reference Elli, Zink, Reniero and Morelli1999; de Lima et al. Reference de Lima, Coelho and Contiero2010). By isolating the accelerant present in YE, the off-flavours would certainly be reduced.
The aim of this study was to investigate whether YE would accelerate yoghurt fermentation time. The aim was furthermore to characterise the compound(s) present in the YE which are responsible for this decrease in yoghurt fermentation time. This data would be useful in formulating the accelerant by combining various pure chemicals. Such a synthetic accelerant might be cheaper to produce, would be of consistent quality, and would not contain the yeast-like taste and flavour.
Materials and methods
YE consists of minerals, vitamins, amino acids and peptides. One of these, or a combination of them, might accelerate growth of starter bacteria. The approach followed here was to determine whether total YE accelerated yoghurt fermentation by a reduction in the time to reach pH 4·6. YE was also fractionated and the accelerating fraction was characterised. In order to avoid fluctuation in fermentation time the investigation was carried out with reconstituted milk powder as substrate.
Reconstitution of milk powder
Skim milk powder (Nestlé, Bryanston, 2021, South Africa) was reconstituted with distilled water (100 g/l) by heating to 50 °C for 60 min.
Starter bacteria
A thermophilic yoghurt culture, FD-DVS YF-L812 Yo-Flex, (Chr. Hansen, Regal Fruits, 1620, South Africa) consisting of Strep. thermophilus and Lb. bulgaricus was used throughout all experiments. A final starter culture inoculum load of 0·104 g/l milk was used.
Yoghurt manufacture
Yoghurt was prepared as described by Tamime & Robinson (Reference Tamime and Robinson1996) and Arkbage (Reference Arkbage2003). No stabilisers were added. Yoghurt prepared in this way served as a control in all yoghurt experiments. The percentage increase or decrease in fermentation times reported in this study refers to the difference in times to reach pH 4·6 in comparison with the control. All fermentation batches were carried out in independent triplicate experiments.
Yoghurt supplemented with YE
YE (Oxoid, Quantum Biotechnologies Ltd, Ferndale, 2160, South Africa) was dissolved in different milk batches in the following concentrations: 0·4, 1, 2, 3, 4, 6, 8, 10 and 15 g/l. A positive control containing 10 g YE/l was used in all other experiments.
Ultrafiltration of YE
YE (10 g/l) was fractionated using an Amicon ultrafiltration cell (model 8200, Amicon Inc., Massachusetts, 01915, USA). The prepared stock solution was fractionated into 9 fractions using 30, 10, 3 and 1 kDa molecular weight cut off (MWCO) membranes successively (Fig. 1).
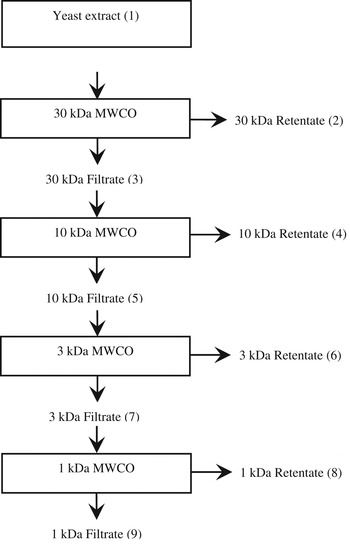
Fig. 1. The 9 fractions obtained after ultra filtration of yeast extract using 4 different molecular size cut off membranes.
From each of the 9 fractions obtained during ultrafiltration, 0·5 ml was added to respective batches of 15 ml milk prior to pasteurisation and their effects on yoghurt fermentation time was determined.
Size exclusion chromatography of YE
Chromatography was carried out on a column (15 mm×1750 mm) of Sephadex G-25-80 (Sigma Aldrich, St. Louis, 63131, USA) with particles size 20–80 μM and a fractionation range of 1–70 kDa, at an elution rate of 30 ml/h. Two millilitres of a 10 g/l YE stock solution was loaded onto the column. The effect of each pooled chromatography fraction on the yoghurt fermentation was determined. The four chromatography fractions were also combined in order to confirm that the reconstituted extract had the same effect on yoghurt fermentation time as unfractionated YE.
Preparation of vitamin, mineral and amino acid solutions
To establish the nature of the accelerant compound, solutions containing minerals, vitamins and amino acids, respectively, were prepared.
A mineral solution generally used in fermentations (du Preez & van der Walt, Reference du Preez and van der Walt1983) was prepared and its effect on the yoghurt fermentation process was established.
The prepared solution contained D-biotin (0·025 mg/l), calcium panthotenate (0·5 mg/l), nicotinic acid (0·5 mg/l), aminobenzoic acid (0·1 mg/l), pyridoxine (0·5 mg/l), thiamine (0·5 mg/l), folic acid (0·004 mg/l), niacin (0·6 mg/l), riboflavin (0·4 mg/l) and inositol (12·5 mg/l). All vitamins were supplied by Sigma Aldrich, St. Louis, 63131, USA.
The amino acid solution contained 50 mg/l of each of the following (all from Merck): L-alanine, L-argininine monohydrochloride, L-asparagine monohydrate, L-aspartic acid sodium salt monohydrate, L-cysteine hydrochloride monohydrate, L-glutamic acid hydrochloride, L-glutamine, glycine, L-histidine, L-isoleucine, L-leucine, L-lysine monohydrochloride, L-methionine, L-phenylalanine, L-proline, L-serine, L-threonine, L-tryptophan, L-tyrosine, uracil and L-valine. A stock solution of all components was prepared.
The concentrations of all three solutions (mineral, vitamin and amino acid) discussed above were referred to as the 1× concentration of each. The respective effects of 1, 5 and 10× of each solution on yoghurt fermentation time was evaluated. A solution which contained a combination of 1× of each of the mineral, vitamin and amino acid solutions was prepared to evaluate their combined effect on the yoghurt fermentation process.
One millilitre of each of the three solutions was individually fractionated by size exclusion chromatography (SEC) to establish the elution time.
Amount of free amino acid groups present in YE
The amount of total free amino acid groups in the four pooled fractions obtained from SEC was determined in triplicate by the Cd-ninhydrin method according to Folkertsma & Fox (Reference Folkertsma and Fox1992).
Effect of amino acids on yoghurt fermentation time
The influence of the individual amino acids present in YE (according to the Oxoid Product Information, 2009) on individual yoghurt fermentations was evaluated. The concentration of each amino acid is reported in Table 1.
Table 1. Concentrations of evaluated amino acids expressed as % w/w of yeast extract (based on Oxoid Product Information on yeast extract LP0021)

The influence of 10 different amino acid combinations on the yoghurt fermentation process was also evaluated. Combinations 1–6 were prepared because these were shown by other researchers to affect growth of bacteria (Table 2). Because L-leucine, L-iso-leucine or L-cysteine were found to increase the fermentation time, four amino acid combinations were compiled that did not contain them. Combinations 7–10 are respectively Combinations 3–6, with these three amino acids excluded.
Table 2. Amino acid combinations reported in literature to stimulate Streptococcus thermophilus growth

Analysis of accelerating chromatography fraction by mass spectrometry
The chromatography fraction containing the accelerant (Fraction 4) was analysed by MS. The peptide sequence information obtained from MS/MS analysis was processed by an in-house Mascot server using the latest Swissprot database (Mascot Version 2.1, Matrix Sciences, London, UK).
Statistical analysis
Statistical analysis was carried out using Excel (Microsoft, Redmond, WA, USA). All results presented are means of triplicates.
Results and discussions
Yoghurt supplemented with YE
The effects of different YE concentrations on yoghurt fermentation time were determined. Results indicated that YE concentrations ranging from 1 to 10 g/l had the same effect and accelerated yoghurt fermentation up to 45 min, or 21%, in respective yoghurt fermentation batches in comparison with the control. The lowest evaluated concentration, 0·4 g/l, decreased fermentation time by only 15 min or 7%. A 43% increase in fermentation time by 15 g/l YE could most likely be ascribed to the increase in initial pH, which consequently influenced starter bacteria growth and subsequent lactic acid production rate.
Effect of ultrafiltration fractions on yoghurt fermentation
The 9 retentate and filtrate fractions obtained from ultrafiltration of YE were used as supplements to respective yoghurt fermentation batches. All 9 the fractions, as well as the positive control, decreased yoghurt fermentation time by 45 min, or 21%, in comparison with the control.
All YE fractions obtained from ultrafiltration decreased yoghurt fermentation time equally. It could therefore be assumed that the accelerating component was present in all fractions and, because it was present in the filtrate of the smallest MWCO membrane, is smaller than 1 kDa. The above conclusions are in accordance with studies by Smith et al. (Reference Smith, Hillier and Lees1975) and Yoo (Reference Yoo2009) indicating that YE contains low molecular weight peptides which have potential as growth enhancers.
Size exclusion chromatography of YE
The separation of proteins in YE is shown in the elution profile (Fig. 2). The eluted peaks observed in Fig. 2 were divided into 4 fractions based on the elution times: Fraction 1: 10–70 min, Fraction 2: 80–180 min, Fraction 3: 190–260 min, Fraction 4: 270–320 min.

Fig. 2. Size exclusion chromatograms of the minerals-, vitamins- and yeast extract solutions. Arrows at 10 and 330 min represent the elution times of blue dextran and mercaptoethanol respectively.
Size exclusion chromatography of vitamin, mineral and amino acid solutions
Each of the three solutions was subjected to SEC (Figs 2 and 3). When the elution time of the Fraction 4 present in YE (Fig. 2) was compared with that of the vitamin, mineral as well as amino acid solutions (Figs 2 and 3), they were very similar.

Fig. 3. Size exclusion chromatograms of yeast extract, the amino acid solution and the free amino groups present in yeast extract.
Free amino groups in YE
The free amino groups coincide with elution of free amino acids (Fig. 3), which confirms that free amino acids are present in the YE, and therefore also in chromatography fraction 4. Although it was evident from Fig. 3 that the bulk of free amino acids (260–320 min) eluted earlier than the bulk of the peptides (280–320 min), it was not possible to separate them completely with this separation method.
Effect of chromatography fractions on yoghurt fermentation
Individual yoghurt fermentations were prepared with each of the four fractions obtained after SEC of YE (Fig. 4) and it was evident that all YE fractions, except Fraction 1, decreased yoghurt fermentation time in comparison with the control. Fraction 1 did not have any effect on the yoghurt fermentation process Fractions 2 and 3 both decreased the fermentation time by 7% in comparison with the control. Fraction 4 however displayed the most significant acceleration, and decreased the time to reach pH 4·6 by 60 min, or 28·6%, which was an even more significant acceleration than that of the positive control, the unfractionated YE, which decreased fermentation time by 45 min, or 21%.

Fig. 4. Yoghurt fermentations with 4 individually pooled size exclusion fractions of yeast extract.
In order to establish whether the combination of the 4 individual fractions had the same effect on the yoghurt fermentation process as the positive control, aliquots of each fraction were combined and the effect was evaluated. Results indicated (Fig. 4) that the fermentation profile of the positive control and the combined fractions were very similar.
Yoghurt production using vitamin, mineral and amino acid solutions
In order to identify the accelerating component, the respective effects of the vitamin-, mineral- and amino acid solutions on yoghurt fermentation time were evaluated.
During preparation of yoghurt batches using the respective solutions, it was observed that all the evaluated mineral and vitamin solution concentrations either reached pH 4·6 in the same time as the control, or increased the fermentation time (Fig. 5). The overall tendencies of the fermentation curves of all mineral and vitamin concentrations evaluated corresponded to that of the control. The accelerant present in the accelerating fraction was therefore not likely to be of mineral or vitamin origin. The yoghurt supplemented with the amino acid solution concentrations as well as the combined solution all followed the same yoghurt fermentation profile as the positive control. This indicated that the amino acids present in the combined solution were responsible for the tendency.
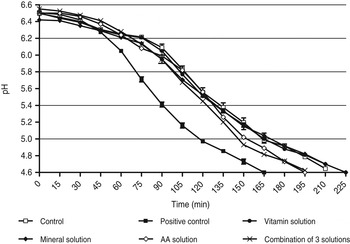
Fig. 5. Yoghurt fermentations using vitamin, mineral and amino acid solutions.
Influence of free amino acids present in YE on yoghurt fermentation evaluated individually and in various combinations
The effect of all the amino acids present in the accelerating fraction on yoghurt fermentation time was firstly evaluated individually. None of the amino acids decreased yoghurt fermentation time in comparison with the control. The supplementation of yoghurt with L-valine, L-threonine, L-alanine, L-glycine and L-serine increased the time to reach pH 4·6 by 7% compared with the control. The effects of L-valine, L-threonine as representatives are shown in Fig. 6. L-leucine, L-iso-leucine and L-cysteine all increased the initial lag phase of the yoghurt fermentation process and increased yoghurt time with at least 50%. The effects of L-leucine as representative are shown in Fig. 6. All the other evaluated amino acids namely L-aspartic acid, L-phenylalanine, L-tryptophan, L-tyrosine, L-methionine, L-glutamic acid, L-arginine, L-proline and L-lysine reached pH 4·6 after 210 min as was the case for the control and had no influence on the fermentation process.
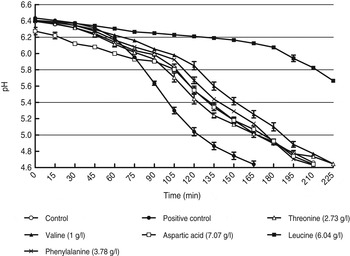
Fig. 6. The effect of individual amino acids on the yoghurt fermentation process.
Due to none of the single amino acids decreasing fermentation time, it was decided to use the amino acids present in the amino acid cocktail in various combinations. None of the 10 evaluated combinations listed in Table 2 reduced the yoghurt fermentation time in comparison with the control.
It was therefore clear that the decrease in fermentation time observed when supplementing yoghurt with YE was not due to a single amino acid or the effect of several free amino acids combined. The reduced fermentation time could therefore only be ascribed to the presence of a peptide. This conclusion was substantiated by Barrette et al. (Reference Barrette, Champagne and Goulet2001) who observed that Lactococci can assimilate peptides and do not require a complete hydrolysis of the proteins into free amino acids (Yoo, Reference Yoo2009).
Although free amino acids are utilised by the starter bacteria, the proteolytic action of the bacterial proteases are responsible for the production of additional amounts of amino acids (Shihata, Reference Shihata2004).
Mass Spectrometry analysis of accelerating chromatography Fraction 4
MS analysis was done on Fraction 4 in order to determine whether specific peptides could be identified from known sources. Results were compared with the database of Saccharomyces cerevisiae due to this yeast being used to prepare YE. During the MS analysis using a threshold of 0·05, 4 enzymes present in Saccharomyces cerevisiae were identified as possible sources.
A further 909 peptides which could not be matched with protein hits were identified which ranged in length from 5–26 amino acids. A possible explanation for the presence of all these unmatched peptides can be that these short peptides can be homologous to a large number of proteins. Another reason for their presence can be that the plastein reaction, a synthesis reaction by proteolytic enzymes, may possibly occur during YE production. The term ‘plastein reaction’ is commonly used to refer to the protease-catalysed process involved in the formation of a plastein from a protein hydrolysate or an oligopeptide mixture, resulting in non-sense sequences which cannot be identified as being derived from specific proteins (Watanabe & Arai, Reference Watanabe, Arai and Hudson1992).
This study provided proof that the accelerant present in YE responsible for the decrease in fermentation time was not of mineral, vitamin or free amino acid origin, but certainly of peptide origin, although synergistic effects between peptides and amino acids cannot be excluded.










