Introduction
Alfalfa (Medicago sativa L.) has one of the highest feeding values of leguminous forages (Zhang et al., Reference Zhang, Grimi, Jaffrin, Ding and Tang2017), and is an excellent feed ingredient preserved as hay, haylage or silage for lactating dairy cows (Schmidt et al., Reference Schmidt, Hu, Mills and Kung2009). In comparison with grasses, it has a higher content of crude protein (CP), greater concentration of organic acids and minerals; however, it is difficult to ensile due to its high buffering capacity for acidic conditions, in combination with the low water-soluble carbohydrates (WSC) concentrations (McDonald et al., Reference McDonald, Henderson and Heron1991; Ogunade et al., Reference Ogunade, Kim, Jiang, Weinberg, Jeong and Adesogan2016).
The utilization of microbial additives for the purpose of achieving a proper fermentation and improving digestibility has generated great interest, which is evidenced by the fact that inoculants have been developed as silage additives for over 40 years (McDonald et al., Reference McDonald, Henderson and Heron1991; Dunière et al., Reference Dunière, Sindou, Chaucheyras-Durand, Chevallier and Thévenot-Sergentet2013). These products included strains of homofermentative lactic acid bacteria (HoLAB) or heterofermentative lactic acid bacteria (HeLAB) (Muck, Reference Muck2010). Administration of HoLAB often boosts silage fermentation by transforming available sugars into lactic acid, thus accelerating the rate of decrease in pH (Contreras-Govea et al., Reference Contreras-Govea, Muck, Mertens and Weimer2011). It should be pointed out that HoLAB inoculants are generally preferred for legume silages as they minimize dry matter (DM) losses through a higher lactic acid production. On the other hand, HeLAB species produce lactic acid and carbon dioxide, as well as traces of ethanol or acetic acid as by-products (Borreani et al., Reference Borreani, Tabacco, Schmidt, Holmes and Muck2018). This leads to DM losses associated with gas production and reduces the feeding value of silage (Ni et al., Reference Ni, Wang, Cai and Pang2015; Borreani et al., Reference Borreani, Tabacco, Schmidt, Holmes and Muck2018). However, HeLAB inoculants are valuable in enhancing aerobic stability since moderate acetic acid production has the potential to inhibit yeasts and moulds, responsible for initiating spoilage upon exposure to air (Muck et al., Reference Muck, Nadeau, McAllister, Contreras-Govea, Santos and Kung2018; Ferrero et al., Reference Ferrero, Piano, Tabacco and Borreani2019). For this reason, modest DM losses from HeLAB treatment should be compensated with improvements in aerobic stability and reduced losses at feed out (Borreani et al., Reference Borreani, Tabacco, Schmidt, Holmes and Muck2018). As both LAB types take different approaches to direct fermentation in the silo, their combination could have potential advantages and complementary effects (Zhang et al., Reference Zhang, Li, Wang, Zeng, Hu and Cui2009).
Even though many authors have reported several benefits of LAB inoculation on fermentation patterns, there are still unanswered questions and challenges about the extent of variability in the effects of inoculants on the preservation of silage and the impact of the interactions between inoculants and other covariates (for instance, LAB species, LAB application rate, concomitant use of enzymes, study duration and silo scale). Some studies have shown that inoculants enhance the attributes of silages (Zielińska et al., Reference Zielińska, Fabiszewska and Stefańska2015; Liu et al., Reference Liu, Lai, Lu, Guo and Luo2016; Li et al., Reference Li, Wang, Zhang, Lin and Yang2018), but in others experiments, LAB did not consistently decrease ammonia nitrogen production (NH3-N) (Kozelov et al., Reference Kozelov, Iliev, Hristov, Zaman and McAllister2008), did not preserve DM and CP content (Chilson et al., Reference Chilson, Rezam, Drewnoski, Price and Hunt2016), failed to improve feed efficiency (Rabelo et al., Reference Rabelo, Lara, Basso, Härter and Reis2018) and inhibit undesirable microorganisms (Twarużek et al., Reference Twarużek, Dorszewski, Grabowicz, Szterk, Grajewski and Kaszkowiak2016). Disagreements in responses to LAB addition are multifactorial, and can possibly be attributed to essential factors such as forage maturity, harvesting conditions, moisture content, silage density, mode and application rate of LAB, epiphytic LAB population, sugar availability, plant DM concentration, ensiling duration, efficacy of the inoculant strains and interactions between microbial species in the inoculant and chemical components within the forage (Santos and Kung, Reference Santos and Kung2016; Ozduven and Celebicam, Reference Ozduven and Celebicam2017). Additional factors that may explain the reported variability include using experiments with insufficient statistical power and inappropriate experimental designs (Arriola et al., Reference Arriola, Oliveira, Ma, Lean, Giurcanu and Adesogan2017).
A meta-analysis is a highly valuable statistical tool whose objective is to summarize, integrate and contrast the results of a large number of primary studies that investigate the same topic (Shelby and Vaske, Reference Shelby and Vaske2008). As a result, the meta-analysis generates a more accurate estimate of the effect size of a particular event with greater statistical power than if only one single study was considered (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009). With this perspective in mind, the objective of this work has been to quantitatively summarize published research studies so as to evaluate the magnitude of effects of HoLAB and HeLAB on fermentation parameters, nutritive value, microbiological composition, as well as the outcomes on aerobic stability of alfalfa silage.
Materials and methods
Search strategy
PubMed, ScienceDirect and Scopus databases were screened for articles restricted by language (English, Spanish and Portuguese). The studies included in this meta-analysis were selected only if they were randomized and controlled trials using alfalfa silage, and results were published in peer-reviewed journals between 1980 and 19 April 2018. To evaluate the effects of applying LAB inoculants on fermentation parameters, nutritive value, microbiological composition and aerobic stability of alfalfa silage, peer-reviewed manuscripts were retrieved using the terms ‘silage’, ‘alfalfa’, ‘lucerne’ and ‘inoculant’. Studies must have examined uninoculated and inoculated treatment groups, held treatments comprising only LAB and reported response variables with the measures of variance (standard deviation, standard error or variation coefficient). Reviews, duplicate reports, experiments that used different forage species and a number of studies that evaluated other additives were excluded. The term ‘study’ refers to a scientific article, which can involve one or more experiments. Preliminary screening of titles and abstracts was carried out for eligibility to this study according to the inclusion and exclusion criteria.
Outcomes and definitions
Supplementation with LAB was analysed as a tool which may improve fermentation parameters, nutritive value, microbiological composition and aerobic stability of alfalfa silage. In all studies, the same method was used to measure aerobic stability, which was defined as the number of hours that silage remained stable before increasing more than 2°C above the ambient temperature (Kung and Ranjit, Reference Kung and Ranjit2001). When the study included more than one inoculant, or when different doses of the same inoculant were used, each inoculated group was compared with the uninoculated group separately.
Data extraction
Information on study design, the number of replicates, means and variances was extracted from each research report. Data for pH, DM concentration (g/kg), neutral detergent fibre (NDF), acid detergent fibre (ADF), NH3-N (g/kg total N), CP, WSC, ash, ethanol, lactate, acetate, propionate and butyrate, in vitro DM digestibility-48 h (IVDMD-48 h), counts of LAB, yeasts, moulds (log10 cfu/g) and aerobic stability (h) were used to estimate outcomes. Certain response variables (DM recovery, lignin, acid detergent-insoluble nitrogen, counts of clostridia and mycotoxins) were retained in the analysis as there were relatively few comparisons that met our selection criteria. For each study, the methodology employed to achieve the results was assessed in detail. However, no scores were used to exclude studies (Lean et al., Reference Lean, Rabiee, Duffield and Dohoo2009).
Statistical analysis
The statistical analysis was conducted in Comprehensive Meta Analysis version 2.2 (2011). Due to continuous variables being analysed, results were evaluated by examining the raw mean differences between the inoculant treatment and controls with 95% confidence intervals using a random-effects model. In this model, the true effect may vary from experiment to experiment; we have included between-experiment variability (true heterogeneity) as well as sampling error (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009). To account for variation in precision across studies, the inverse of the squared standard error of each treatment mean was used as a factor in the weight statement of the model.
Heterogeneity, meta-regression and publication bias
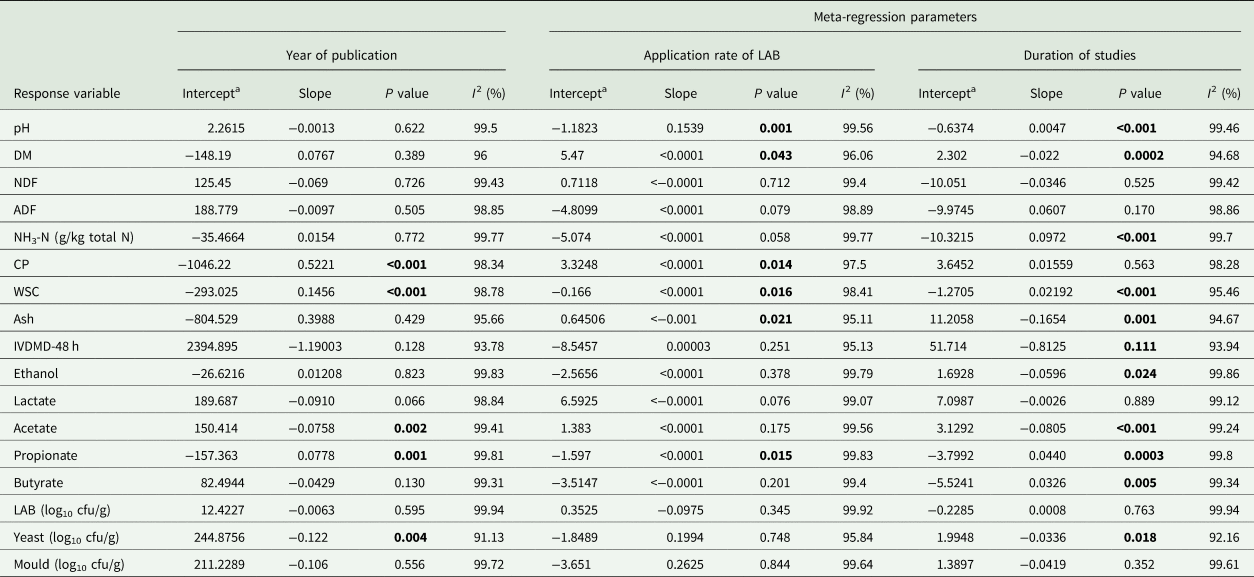
A meta-regression analysis was performed to examine heterogeneity sources in the treatment effects. Meta-regression allowed assessing the relationship between year of publication, application rate of LAB inoculant (which ranged from 4 to 7 log10 cfu/g) and duration of the studies as covariates, and silage attributes as outcome variables.
A priori sub-group analyses were planned depending on factors that could potentially influence the magnitude of the treatment: (1) for the purpose of grouping the newest articles, we used the last 10 years (before 2009 v. after 2009) as a pre-specified cut-off; (2) type of inoculum (mono-strain v. multi-strain); (3) among mono-strain inoculum, type of LAB (HoLAB v. HeLAB); (4) LAB species used (with Lactobacillus buchneri, with L. plantarum, with Pediococcus acidilactici and with Enterococcus faecium); (5) enzymatic additives addition (with fibrolytic enzymes v. without fibrolytic enzymes); (6) study duration (from 30 to 60 days v. more than 60 days); and (7) silo type (laboratory or farm scale).
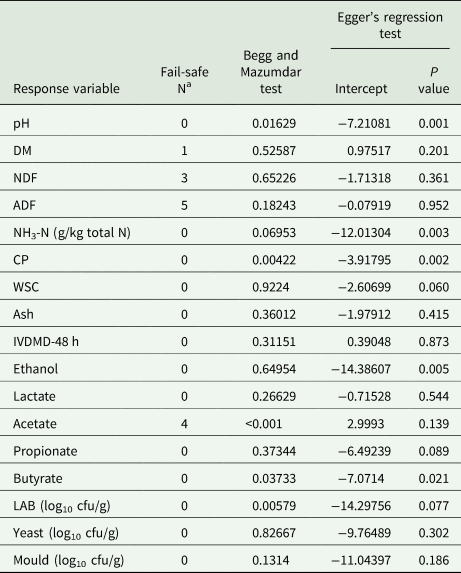
Heterogeneity among studies was assessed using the DerSimonian and the Laird test (Q-statistic). The degree of heterogeneity was quantified with the Inconsistency index (I 2-statistic; Higgins and Thompson, Reference Higgins and Thompson2002). An adjusted rank correlation test using the Egger method (Egger et al., Reference Egger, Davey Smith, Schneider and Minder1997) and the Begg test (Begg and Mazumdar, Reference Begg and Mazumdar1994) was used to assess publication bias. It was considered that there was bias if both statistical methods were significant (P < 0.01). When there was any evidence of publication bias, the ‘trim’ and ‘fill’ method (Duval and Tweedie, Reference Duval and Tweedie2000) was applied to estimate the quantity and magnitude of missing studies and resultant unbiased effect size. Significance was declared at P ⩽ 0.05 and tendencies at 0.05 > P < 0.1.
Results
Excluded studies
The literature search yielded 2173 scientific articles on alfalfa silage inoculants. Of the studies identified at the beginning of the meta-analysis, 1976 were excluded on the basis of publication type: articles involving other forage species or mixed crops (n = 429), other additives (n = 88), or both of them (n = 265), reviews (n = 89), duplicate reports (n = 593), wrong topics (n = 505) and studies using inoculants for other purposes (n = 7) were rejected. In addition, experiments which were eligible for quantitative review were exempted due to lack of statistical information for conducting a meta-analysis (n = 25), studies conducted to assess the impact of certain pathogens like Escherichia coli (n = 2), manuscripts involving hay or haylage (n = 12), papers using simulation models (n = 3), no full-text articles (n = 12), no English, Spanish or Portuguese language full articles (n = 3), studies that analysed the efficacy of symbiotics (n = 1), yeasts or propionic acid bacteria (n = 3), books or book chapters (n = 46) and summaries (n = 42) (Fig. 1).

Fig. 1. Flow diagram of studies selected for meta-analysis in this work.
Overview of included studies
At the end of the literature review, 48 studies (131 experiments) were included in this meta-analysis to estimate the role of HoLAB and HeLAB for alfalfa silage. Most of the research papers reviewed did not assess the LAB inoculants' effect over all the parameters under study. Consequently, the number of studies included in the meta-analysis differed in each variable considered. Of the screened experiments, 58 were published before 2009 and the remaining 73 after 2009. Fifty-nine experiments included mono-strain inoculum and 71 included multi-strain LAB. Among mono-strain inoculum, 50 experiments were carried out using HoLAB, whereas nine utilized HeLAB. A total of 18 studies used L. buchneri (nine alone and nine in combination with other LAB), 82 used L. plantarum (31 alone and 51 in combination with other LAB), 27 used P. acidilactici (two alone and 25 in combination with other LAB) and 26 used E. faecium (one alone and 24 in combination with other LAB). Inoculants were incorporated with enzymes (20) or without enzymes (111). Studies were conducted for ⩽60 days (76), or for >60 days (52). In most of the experiments (118), the inoculant was employed in laboratory-scale silos, while 13 studies were executed in farm-scale silos.
Alfalfa silage conservation
The effects of LAB inoculation on silage quality across studies are depicted in Table 1. In the pooled estimate, inoculation with LAB decreased silage pH, NDF, ADF and NH3-N, whereas DM and CP were increased compared to controls. In contrast, there were no statistical differences in WSC, IVDMD-48 h and ash. Additionally, LAB inoculation reduced acetate, propionate, ethanol and butyrate concentrations, whereas increased lactate. Moreover, LAB inoculation reduced the counts of yeasts and moulds, but did not alter LAB counts. Finally, LAB inoculation improved aerobic stability (Table 1).
Table 1. Effects of LAB inoculants on fermentation parameters, nutritive value, microbiological composition and aerobic stability of alfalfa silage (g/kg DM, unless otherwise stated)
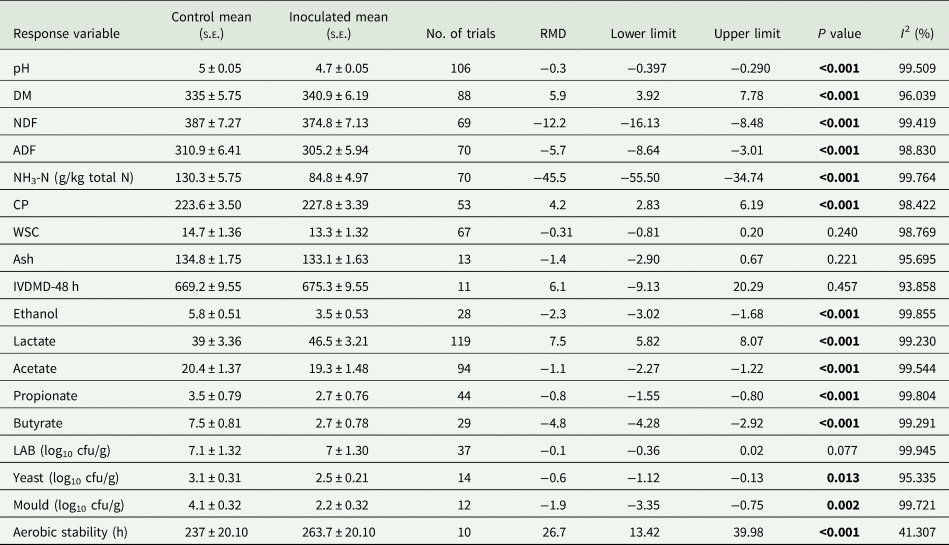
RMD, raw mean difference between inoculated and uninoculated treatments; DM, dry matter; NDF, neutral detergent fibre; ADF, acid detergent fibre; NH3-N, ammoniacal nitrogen; CP, crude protein; WSC, water-soluble carbohydrates; IVDMD-48 h, in vitro DM digestibility at 48 h; LAB, lactic acid bacteria.
Results were expressed as the arithmetic mean ± standard error of the mean (S.E.M). Bold values denote statistical significance at the P < 0.05 level.
Significant heterogeneity (I 2 statistic >50%) was observed across all silage quality response variables, except for aerobic stability (I 2 = 41.3%). Hence, sub-groups were evaluated in order to identify the sources of variability. In accordance with the sub-group analysis, and only considering significant variables in the pool estimate, inoculation decreased pH in all conditions (P < 0.001), except when enzymes were applied (P = 0.957) (Tables 2 and 3).
Table 2. Sub-group analysis comparing the effects of silage inoculation on nutrient composition and microbiological profile of alfalfa silage according to the LAB species
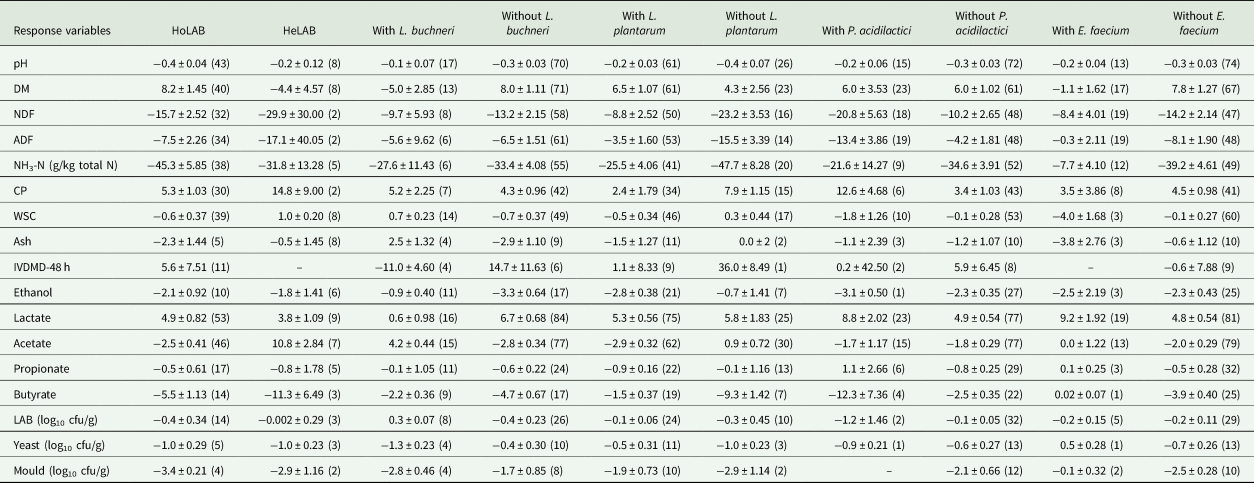
RMD, raw mean difference between inoculated and uninoculated treatments; HoLAB, homofermentative lactic acid bacteria; HeLAB, heterofermentative lactic acid bacteria; DM, dry matter; NDF, neutral detergent fibre; ADF, acid detergent fibre; NH3-N, ammoniacal nitrogen; CP, crude protein; WSC, water-soluble carbohydrates; IVDMD-48 h, in vitro DM digestibility at 48 h; LAB, lactic acid bacteria.
Results were expressed as the RMD ± S.E.M and values in parentheses indicate the number of studies. There was no sub-group analysis for aerobic stability.
Table 3. Sub-group analysis comparing the effects of silage inoculation on nutrient composition and microbiological profile of alfalfa silage considering factors that could potentially influence the magnitude of the treatment

RMD, raw mean difference between inoculated and uninoculated treatments; DM, dry matter; NDF, neutral detergent fibre; ADF, acid detergent fibre; NH3-N, ammoniacal nitrogen; CP, crude protein; WSC, water-soluble carbohydrates; IVDMD-48 h, in vitro DM digestibility at 48 h; LAB, lactic acid bacteria.
Results were expressed as the RMD ± S.E.M and values in parentheses indicate the number of studies. There was no sub-group analysis for aerobic stability.
Dry matter increased significantly with inoculation when studies were shorter than 60 days (P < 0.001), in those experiments in which inoculants were applied to mini silos (P < 0.001) and by HoLAB when mono-strain inoculums were used (P < 0.001). Silage inoculation with LAB significantly decreased NDF (P < 0.001). Nevertheless, inoculants had no effects when HeLAB were used (P = 0.320). Moreover, inoculation decreased ADF (P < 0.001). This effect was observed in studies that used mono-strain inoculants (P < 0.001) and HoLAB (P < 0.001), and in mini silos (P < 0.001) (Tables 2 and 3).
Inoculants improved silage protein preservation (P < 0.001) in the experiments carried out after 2009 (P < 0.001), in the absence of enzymes (P < 0.001), by mono-strain LAB (P < 0.001) and by HoLAB when single-strain inoculums were used (P < 0.001). LAB reduced (P < 0.001) NH3-N concentrations in the pool estimate and in the sub-group analysis, with the exception of studies that reported concomitant use of enzymes (P = 0.286) (Tables 2 and 3).
With respect to organic acids, ethanol concentrations considerably decreased in all conditions (P < 0.001), except when HeLAB (P = 0.202) or enzymes (P = 0.294) were used. In contrast, lactate significantly increased (P < 0.001) when LAB were inoculated, and effects were independent of the sub-group considered. Inoculation with LAB decreased acetate concentrations (P < 0.001), but HeLAB significantly increased this organic acid (P < 0.001). Propionate decreased in studies conducted before 2009 (P < 0.001) and in the absence of enzymes (P < 0.001), when LAB were inoculated in laboratory-scale silos (P < 0.001), with a multi-strain inoculum (P < 0.001), and in experiments shorter than 60 days (P < 0.001). The positive effect on butyrate was observed when the experiments were performed by mono-strain LAB (P < 0.001) and HoLAB (P < 0.001), in the absence of enzymes (P < 0.001) and when studies were executed for more than 60 days (P < 0.001) (Tables 2 and 3).
Regarding microbiological composition, the number of yeast counts was reduced (P < 0.001) when inoculants were used. Conversely, they increased in studies conducted before 2009 (P < 0.001) and in those carried out for less than 60 days (P < 0.001). No effects were observed on yeast counts with multi-strain inoculum (P = 0.735) and with the use of enzymes (P = 0.094). The inoculation of LAB showed a positive impact on moulds counts (P = 0.002), but the reduction was not significant with the use of enzymes (P = 0.105) (Tables 2 and 3).
There were no significant differences regarding the use of HeLAB, simultaneous use of enzymes and long storage periods, which can be attributed to the small number of comparisons found. The low number of studies that incorporate these covariates limited our ability to detect significant effects.
Finally, regarding aerobic stability, this parameter was higher in inoculated alfalfa silage (P < 0.001) (Table 1). Nevertheless, it was not possible to conduct a sub-group analysis because all the studies were conducted after 2009, with multi-strain inoculum, without the administration of enzymes, for less than 60 days and in mini silos.
Inoculation with HoLAB (which included L. plantarum, P. acidilactici and E. faecium among others, with the prevalence of the first one) or HeLAB (with L. buchneri as the predominant species) showed opposite behaviour in the following response variables: DM (P = 0.015), WSC (P < 0.001) and acetate concentrations (P < 0.001). While HoLAB increased DM and decreased the concentrations of WSC and acetate, HeLAB reduced DM and increased WSC and acetate concentrations (Table 2).
Considering the LAB species included, the absence of L. buchneri decreased pH, NDF, ADF, acetate and propionate concentrations (P < 0.05), while increased DM and lactate (P < 0.001). Inoculation with L. buchneri was effective in decreasing the number of yeasts (P < 0.001). CP increased (P < 0.05), while NH3-N, ethanol, butyrate and moulds counts (P < 0.05) were reduced both in the presence and in the absence of L. buchneri in the inoculated group (Table 2).
Treating alfalfa silage with L. plantarum increased DM concentration (P < 0.001). This microorganism was also able to induce a reduction in ethanol (P < 0.001), propionate (P < 0.001) and acetate concentrations (P < 0.001). The absence of L. plantarum produced an increase in CP (P < 0.001). In addition, a reduction of yeast counts was observed in the inoculated group in the absence of this species (P < 0.001). Other measured parameters such as pH, NDF, ADF, NH3-N, butyrate and moulds counts decreased (P < 0.05), while lactate increased both in the presence and in the absence of L. plantarum in the inoculated group (P < 0.001) (Table 2).
A significant increase in DM and a reduction in NH3-N and propionate (P < 0.05) were observed in the inoculated group in the absence of P. acidilactici. Silage inoculation with P. acidilactici tended to increase DM content (P = 0.092). Meanwhile, P. acidilactici inoculation reduced pH, NDF, ADF, butyrate and yeast counts. CP and lactate concentrations increased, whereas ethanol decreased (P < 0.05) both in the presence and in the absence of P. acidilactici in the inoculated group (Table 2).
Inoculation with E. faecium significantly reduced WSC (P = 0.017), whereas its absence decreased ADF, acetate, butyrate, yeasts, moulds (P < 0.05) and tended to decrease propionate concentrations (P = 0.061). On the contrary, DM and CP increased, while ethanol decreased in the absence of this microorganism (P < 0.001). pH, NDF, NH3-N were reduced (P < 0.05), while lactate was increased (P < 0.001) both in the presence and in the absence of E. faecium in the inoculated group (Table 2).
Based on the results from the meta-regression analysis, interactions were observed between the year of publication and CP (P < 0.001), WSC (P = 0.001), acetate (P = 0.002), propionate (P = 0.001) and yeasts (P = 0.004). Moreover, the application rate of LAB was associated with pH (P = 0.001), DM (P = 0.043), CP (P = 0.014), WSC (P = 0.016), ash (P = 0.021) and propionate (P = 0.015). Finally, the duration of studies was correlated with pH (P < 0.001), DM (P = 0.0002), NH3-N (P < 0.001), WSC (P < 0.001), ash (P = 0.001), ethanol (P = 0.024), acetate (P < 0.001), propionate (P = 0.0003), butyrate (P = 0.005) and yeast counts (P = 0.018) in the meta-regression (Table 4). Yet, the coefficient was under 50%, except for the duration of studies in WSC (adjusted R 2 = 0.72). Therefore, the year, rate of inoculation and length of studies had a reduced impact in the remaining response variables.
Table 4. Summary of random weighted meta-regression analysis for independent variables (year of publication, application rate of LAB and duration of studies) that influenced the effects between inoculated and uninoculated treatments for alfalfa silage quality parameters (g/kg DM, unless otherwise stated)

LAB, lactic acid bacteria; DM, dry matter; NDF, neutral detergent fibre; ADF, acid detergent fibre; NH3-N, ammoniacal nitrogen; CP, crude protein; WSC, water-soluble carbohydrates; IVDMD-48 h, in vitro DM digestibility at 48 h.
Bold values denote statistical significance at the P < 0.05 level.
a Intercept: constant in the model.
As part of this study, beside Egger's regression test, Begg and Mazumdar rank correlation test, Duval and Tweedie's trim and fill method were used to detect publication bias in the studies included for each of the response variables analysed. The results are shown in Table 5. There was a general tendency in having few publication biases for pH, CP and butyrate; however, the large number of scientific articles included in this meta-analysis provides valid results beyond the potential bias (Table 5).
Table 5. Publication bias detection (g/kg DM, unless otherwise stated)

DM, dry matter; NDF, neutral detergent fibre; ADF, acid detergent fibre; NH3-N, ammoniacal nitrogen; CP, crude protein; WSC, water-soluble carbohydrates; IVDMD-48 h, in vitro DM digestibility at 48 h; LAB, lactic acid bacteria.
a Number of studies required to reverse the effects are calculated on the condition of P = 0.05.
Discussion
This quantitative meta-analysis of data from several randomized controlled experiments showed that the use of LAB inoculants decreased pH in the pooled estimate. Results also suggested that the nutrients in alfalfa silage were well preserved by inoculation with LAB, as indicated by lower NDF and ADF concentrations, and higher DM and CP in the pooled estimate as compared with the control silage. The NDF and ADF of silages are important quality parameters and are expected to be lower in inoculated alfalfa. Certain inoculants contain bacteria that could secrete specific enzymes, mainly cellulases and xylanases, that may contribute to degrade these structures and increased fibre digestion (Adesogan et al., Reference Adesogan, Arriola, Jiang, Oyebade, Paula, Pech-Cervantes, Romero, Ferraretto and Vyas2019). The decreased NDF and ADF concentration could be also related to better WSC preservation following inoculation, which could reduce NDF and ADF contents by a ‘dilution effect’. The reduction of NDF and ADF in treated silage compared with the control evidenced favourable anaerobic conditions for the fermentation process, degradation of cell walls providing soluble carbohydrates to fermentative microorganisms and hydrolysis of the most available forage structural carbohydrates (Kozelov et al., Reference Kozelov, Iliev, Hristov, Zaman and McAllister2008; Rabelo et al., Reference Rabelo, Mari, Reis, Silva and Santos2016; Wang et al., Reference Wang, Guo, Li, Chen, Dong and Shao2019). Bearing in mind the sub-group analysis, although some categories did not show significant differences, in all cases, there was a reduction in both parameters.
Independently of the factors that could influence the treatment magnitude, DM content increased, except when experiments lasted for more than 60 days and when HeLAB were applied. The lack of effect with prolonged storage time can be related to the degradation of some nutrients and cell walls by bacterial enzymes activity and acidic conditions in silage during fermentation (Sariçiçek et al., Reference Sariçiçek, Yildirim, Kocabaş and Özgümüş Demir2016). On the other hand, since obligate HeLAB are characterized by CO2 production in the conversion of lactic acid and carbohydrates to acetic and propionic acids, they would decrease DM content (Weinberg et al., Reference Weinberg, Khanal, Yildiz, Chen and Arieli2010; Randby et al., Reference Randby, Weisbjerg, Nørgaard and Heringstad2012). The higher CP and the lower content of NH3-N indicated limited proteolysis. In this meta-analysis, LAB inoculants exhibited their potential to protect feed proteins in alfalfa silage. The positive effects of LAB inoculants on nitrogen fractions can be accounted for the rapid acidification of the forage below the optimal pH for plant protease activity (Wang et al., Reference Wang, Wang, Zhou and Feng2009). During ensiling, plant enzymes such as carboxypeptidase (optimum pH 5.2) and acid proteinase (optimum pH 4.5) may play major roles in protein degradation, while aminopeptidase loses most of its activity in the initial phase of fermentation since its optimal pH is close to 7.0 (McKersie and Buchanan-Smith, Reference McKersie and Buchanan-Smith1982). Accordingly, if the pH of forage is reduced as rapidly as possible, it will contribute to proteolysis inhibition (Wang et al., Reference Wang, Wang, Zhou and Feng2009).
With reference to organic acids, in treated silages, lactic acid concentrations were largely increased, whereas the levels of ethanol, acetic, propionic and butyric acids were clearly lower than in uninoculated silages. LAB have a positive effect on the extent and rate of lactic acid production in the silage, hence stimulating a rapid drop in pH and suppressing the growth of clostridia and other undesired anaerobic microorganisms (Oude Elferink et al., Reference Oude Elferink, Krooneman, Gottschal, Spoelstra, Faber and Driehuis2001). Although inoculation with LAB reduced acetate concentrations, overall HeLAB significantly increased it. While inoculation with HoLAB led to silages with high lactic acid contents, inoculation with HeLAB resulted in higher levels of acetic acid (Chen et al., Reference Chen, Dong, Li and Shao2018). It is well documented (Heinl and Grabherr, Reference Heinl and Grabherr2017) that this could be due to the capacity of HeLAB to degrade lactic acid to acetic acid under anoxic conditions. Acetic acid is one of the most effective substances for inhibition of spoilage microorganisms by decreasing their maximum growth rate (Danner et al., Reference Danner, Holzer, Mayrhuber and Braun2003). A level of acetic acid of 1.5–3.0% in the DM could inhibit yeast growth in silages exposed to air in the feed out phase (Acosta Aragón et al., Reference Acosta Aragón, Jatkauskas and Vrotniakienė2012). In the same way, propionic acid levels were lower in inoculated silages. Although a certain amount of propionic acid is desirable in order to minimize possible growth of yeasts and improve aerobic stability, it could affect the voluntary intake and utilization of silage-based diets (Nishino et al., Reference Nishino, Yoshida, Shiota and Sakaguchi2003). In conserved forage, butyric acid and ethanol are equally undesirable. In the present work, lower amounts of ethanol appeared in inoculated alfalfa silages. Ethanol, which is a yeast end product, has little preservative effect in silage, and it causes extremely high losses in DM and energy (Kung et al., Reference Kung, Shaver, Grant and Schmidt2018). With respect to butyrate, this organic acid confers poor palatability, reducing the voluntary feed intake in animals (Kung, Reference Kung2010). Frequently, uninoculated silages have relatively high contents of butyrate, related to the activity of clostridia derived from soil or slurry contamination (Danner et al., Reference Danner, Holzer, Mayrhuber and Braun2003; Liu et al., Reference Liu, Lai, Lu, Guo and Luo2016).
Taking into account the microbiological composition, all treated silages had lower counts of yeasts and moulds than the control ones in the pool estimate. On the contrary, the inclusion of inoculants did not significantly raise LAB counts. However, it should be noted that LAB reached a concentration of at least 106 cfu/g in either group after ensiling. It was also worth noting that, regardless of the number of final LAB, when an inoculant fails to produce an adequate amount of lactic acid in the process of silage fermentation to reduce pH and suppress the growth of harmful microorganisms, the resulting silage will be of poor quality (Ni et al., Reference Ni, Wang, Cai and Pang2015). In the studies summarized in this analysis, the counts of LAB were similar, but it was possible to detect significant differences in the aforementioned variables in favour of the treated group. Moreover, it was possible that differences in the numbers of LAB were only seen in the early stages of fermentation. The fast increase in LAB counts frequently observed in inoculated silages in early fermentation indicates that LAB strains are competitive among the epiphytic communities. Moreover, the reduction in the LAB population after this initial peak is expected because low pH and lack of fermentable substrates result in bacterial death (Xu et al., Reference Xu, He, Zhang and Kong2017; Nascimento Agarussi et al., Reference Nascimento Agarussi, Gomes Pereira, de Paula, da Silva, Santos Roseira and Fonseca e Silva2019).
Regarding yeasts, these microorganisms are the main initiators of aerobic spoilage by metabolizing valuable sugars and lactic acid, thus raising the pH and allowing an increase of silage inner temperature (Pahlow et al., Reference Pahlow, Muck, Driehuis, Oude Elferink, Spoelstra, Buxton, Muck and Harrison2003). Finally, moulds complete the deterioration of silages (Dolci et al., Reference Dolci, Tabacco, Cocolin and Borreani2011). For well-preserved silage, the concentration of moulds and yeasts should not exceed 3–4 log10 cfu/g (McEniry et al., Reference McEniry, O'Kiely, Clipson, Forristal and Doyle2006). In the present study, inoculating alfalfa at the time of ensiling altered the resulting fermentation by reducing the level of yeasts and moulds in 1–2 log10 cfu/g compared with untreated silage. On the other hand, yeast counts were increased in studies conducted for less than 60 days. Apparently, the slow development of HeLAB, which are the main antifungal agents, explains why the effects of these microorganisms are manifest only during the late storage phase of ensiling (Schmidt et al., Reference Schmidt, Hu, Mills and Kung2009).
The results observed in the pool estimate have led us to hypothesize that alfalfa silage inoculants provide a stable acidic pH with a suitable proportion of organic acids after opening the fermented plant material. Thereby, growth of yeasts and moulds in the presence of oxygen is inhibited and also heating of the silage is prevented. Classical microbial inoculants containing only HoLAB were shown to have no significant influence on aerobic stability, primarily because lactic acid by itself is not an effective antimycotic agent (Filya and Sucu, Reference Filya and Sucu2007). A more promising approach seems to be related to the use of HeLAB. Addition of these microorganisms improves aerobic stability through the production of acetic and propionic acids with strong antifungal properties (Zielińska et al., Reference Zielińska, Fabiszewska and Stefańska2015). However, our meta-analysis summarized limited reports on the ability of microbial inoculants to improve the aerobic stability of alfalfa silage, and it was not possible to perform a sub-group analysis to investigate its influence on the response. Hence, future research should be conducted to further examine the effects of HoLAB, HeLAB and their combinations during aerobic exposure. Although the inclusion of HeLAB on forages with a low DM content does not appear to be appropriate due to the excessive fermentation (Jatkauskas et al., Reference Jatkauskas, Vrotniakiene, Ohlsson and Lund2013), the aforementioned inoculants should be utilized so as to avoid the air deterioration that could occur during the feed out phase or due to poor management (Yuan et al., Reference Yuan, Wen, Wang, Desta, Dong and Shao2018). For instance, silage moved from one silo structure to another, silage fed from intermediate feeding piles, and silos with large exposed surfaces are good candidates for treatment with HeLAB. Besides, over-sized silos, with slow feed out rate, poor packing and maintained at 30°C, are more prone to aerobic deterioration, so combinations of several strains with different mechanisms of action should be considered (Ashbell et al., Reference Ashbell, Weinberg, Hen and Filya2002; Kung, Reference Kung2010).
According to the sub-group analyses, the use of enzymes did not offer supplemental effects on silage pH. The result in studies applying enzymes could be attributed to the fact that all the trials involved employed HeLAB, too. It might be hypothesized that the HeLAB L. buchneri was a confounder and had possibly a major influence on this effect, as using this microorganism implies a more heterolactic fermentation, consisting in the conversion of pentoses or hexoses into lactic acid, CO2 and other products, mainly acetic acid, ethanol and propionic acid (McDonald et al., Reference McDonald, Henderson and Heron1991). Furthermore, the absence of enzymes significantly increased CP and IVDMD-48 h, while decreased NH3-N, ethanol, propionate, yeasts and moulds counts. Therefore, this meta-analysis indicates no benefit of the LAB treatment with combined fibrolytic enzymes. Though this is in agreement with the results obtained by Lynch et al. (Reference Lynch, Baah and Beauchemin2015), one plausible explanation could be that the number of experiments that employed enzymes and measured certain variables was relatively small, thus this observation should be interpreted with caution.
Summary of findings included in this meta-analysis denoted that L. buchneri, L. plantarum, P. acidilactici and E. faecium were mostly administered as multi-species inocula due to the synergistic effects when bacteria are applied together (Blajman et al., Reference Blajman, Páez, Vinderola, Lingua and Signorini2018). Therefore, the infrequent use of individual inoculants (except for L. plantarum) may have limited our ability to detect LAB species-related impacts on the measures of silage quality. Still, this meta-analysis evidenced that the main goal of L. plantarum, P. acidilactici and E. faecium administration was the preservation of the nutritional quality of ensiled alfalfa (Oliveira et al., Reference Oliveira, Weinberg, Ogunade, Cervantes, Arriola, Jiang, Kim, Li, Gonçalves, Vyas and Adesogan2017), whereas reduction in harmful microorganisms was the most consistent benefit of L. buchneri (Liu et al., Reference Liu, Dong and Shao2018).
This work produced a synthesis and contrast of results among a large number of primary studies. To our knowledge, this is the first meta-analysis to compare the addition of HoLAB and HeLAB for alfalfa silage. In the pool estimate, positive effects due to the application of microbial silage inoculants were found in most of the evaluated parameters. Regarding the sub-group analysis, inoculation with HoLAB is recommended as it has been shown to contribute to a lesser loss of nutritional value and to improve the chemical parameters of alfalfa silages. Moreover, this meta-analysis provided evidence that using either HoLAB or HeLAB enhanced microbiological composition. In spite of the previous statement, further studies are needed to examine the effects of HoLAB and HeLAB with different biotechnological features and in appropriate proportions on digestibility and animal performance. Additionally, more studies are required to identify the effects of LAB inoculants on silage preserved in farm-scale silos as well as on how LAB combined with enzymes affects silage quality. Lastly, research should be conducted to analyse the ability of bio-inoculants to inhibit clostridia and decontaminate silages of mycotoxins produced by them, so as to finally standardize commercial alfalfa silage inoculants.
Acknowledgements
The authors would like to thank Lecturer Liliana Silber for her English manuscript proofreading service.
Financial support
This research was financially supported by the National Institute of Agricultural Technology (INTA, Argentina) and The National Agency of Scientific and Technological Promotion (FONCyT, Argentina), Projects PICT-2017-0665 and PICT-2016-0256.
Conflict of interest
None.
Ethical standards
Not applicable.