Introduction
Potassium is an essential nutrient in crop production that supports photosynthesis, protein synthesis enzyme activity and water regulation. The effect of K on plant growth is well documented in terms of shoot growth, not least with respect to barley (Høgh-Jensen and Pedersen, Reference Høgh-Jensen and Pedersen2003) and maize (Jordan-Meille and Pellerin, Reference Jordan-Meille and Pellerin2008). Potassium also can alleviate the effects of water stress on crop production by improving crop water retention and disease resistance (Salimi et al., Reference Salimi, Moradi and Banipur2012; Zain and Ismail, Reference Zain and Ismail2016).
Crop demand for K can be considerable, particularly from high yielding forage crops. Salimi et al. (Reference Salimi, Moradi and Banipur2012) showed that forage maize can remove 4.4 kg of K for every tonne of fresh crop, suggesting that a forage maize yield of 50 t/ha will remove around 220 kg K/ha. Ahmed (Reference Ahmed2014) reported that rapidly growing maize crops can remove as much as 6.6 kg K/ha/day. To avoid depleting soils of this macronutrient, the K removed through crop offtake is typically replaced through the application of fertilizers. The most common K fertilizer is potassium chloride (KCl), also known as muriate of potash (MOP).
Global consumption of K is approximately 29 million tonnes per annum, and this is forecast to grow to 31.5 million tonnes by 2022 (Rawashdeh et al., Reference Rawashdeh, Xavier-Oliveira and Maxwell2016). This demand is met by multiple forms of K, originating from different evaporite minerals and manufacturing processes, including MOP, potassium magnesium chloride (KMgCl3) and potassium sulphate (K2SO4, also known as sulphate of potash or SOP). These forms of potash are favoured as they have a higher K content compared with some other K minerals (Zientek et al., Reference Zientek, Hammarstrom and Johnson2010). However, the supply of these high-K, evaporite minerals is finite, therefore new sources are required to meet predicted demand and support food production for the increasing global population. This is a global issue because of the challenges of identifying and extracting new K deposits (Cocker et al., Reference Cocker, Orris and Wynn2016).
Sulphur is a macro element that supports the formation of plant proteins, amino acids, vitamins and enzymes and although not required in the same quantities as the other major elements, it is still essential to maximize crop yield. The need for S is increasing as atmospheric deposition has decreased with the political impetus to reduce S emissions in power generation (Ceccoti, Reference Ceccoti and Rodriguez-Barrueco1996; Webb et al., Reference Webb, Jephcot, Fraser, Wiltshire, Aston, Rose, Vincent and Roth2016). Sulphur deficiency is increasingly common because of decreases in S deposition and increases in S offtake (Dick et al., Reference Dick, Kost, Chen and Jez2008). In barley, Zhao et al. (Reference Zhao, Fortune, Barbosa, McGrath, Stobart, Bilsborrow, Booth, Brown and Robson2006) reported significant yield responses to S with increases of 0.2–1.2 t/ha with an associated improvement in malting quality, while in maize, S deficiency has been reported in parts of the USA (Camberato and Casteel, Reference Camberato and Casteel2017).
Polyhalite is an evaporite mineral; a hydrated K, Ca, magnesium sulphate (MgSO4) salt with the chemical formula: K2SO4.MgSO4.2CaSO4.2H2O. It was first described by Stromeyer (Reference Stromeyer1818) but the discovery of large, more easily-mined deposits of other types of K mineral in Canada meant polyhalite was not commercially exploited (Cocker et al., Reference Cocker, Orris and Wynn2016). Subsequently, there was little interest in polyhalite. However, the discovery of large polyhalite deposits in North Yorkshire, UK, has renewed interest in the material as a multi-nutrient fertilizer. An assessment by Kemp et al. (Reference Kemp, Smith, Wagner, Mounteney, Bell, Milne, Gowing and Pottas2016) established a mineral inventory of 2660 Mt polyhalite in Northeast England, UK. The size and good grade of this deposit are sufficient to allow for mining with no chemical processing required.
Polyhalite contains 113 g/kg K, 110 g/kg Ca, 38 g/kg Mg and 185 g/kg S, and has lower salt index and solubility than for MOP and SOP, respectively (Barbier et al., Reference Barbier, Li, Liu, He, Mylavarapu and Zhang2017). The low solubility of polyhalite means that it might be a useful, slow-release fertilizer (Barbarick, Reference Barbarick1991). The multi-nutrient nature of polyhalite combined with these characteristics suggests that it might offer advantages and synergies in comparison with more conventional sources of either K or S.
Knowledge of how polyhalite performs as a fertilizer is limited although some evidence is available. Fraps and Schmidt (Reference Fraps and H1932) examined its use as a fertilizer on maize and sorghum and reported that availability to crops of K in polyhalite was 96% of the availability of K in MOP and SOP. Lepeshkov and Shaposhnikova (Reference Lepeshkov and Shaposhnikova1958) reported that, for potato crops, polyhalite was as effective as SOP; their results were confirmed by Panitkin (Reference Panitkin1967). Boguszewski et al. (Reference Boguszewski, Drzas and Drzas1968) stated that the fertilizer value of polyhalite was equivalent to SOP plus MgSO4. More recently Terelak (Reference Terelak1975) reported that polyhalite was as effective as MOP plus MgSO4 for a range of crops. Mercik (Reference Mercik1981) reported that, for spring barley, polyhalite out-performed SOP and that it provided an increase in plant Ca and Mg concentrations. Barbarick (Reference Barbarick1991) evaluated polyhalite in glasshouse trials on sorghum-sudangrass and reported its performance to be at least as effective if not superior to SOP. They also conducted column leaching studies and found it to be less soluble than SOP and suggested that it behaved somewhat like a slow-release fertilizer.
Recent experimental work, prompted by the upsurge in interest in polyhalite, has evaluated the performance of the material in supplying K and S to a range of crops. In India, Tiwari et al. (Reference Tiwari, Pandey and Katiyar2015) found polyhalite to be an effective source of S that improved the yield of mustard and sesame in comparison with a control. Polyhalite was also found to be a good source of K in peanut production in Vietnam (Hoang et al., Reference Hoang, Duong, Truong, Ho and Pham2016). Interest in more staple arable crops was partly addressed by Pavuluri et al. (Reference Pavuluri, Malley, Mzimbiri, Lewis and Meakin2017) working on maize in Tanzania; their results highlighted the importance of S nutrition and that polyhalite was a good source of both K and S. How cereal and forage maize crops in temperate conditions respond to polyhalite is unknown although the available evidence suggests that its performance will be similar to other sources of K and S. Addressing this question forms the basis of the work reported here. Potassium and S nutrition are the main focus as these are more often limiting nutrients in field production than Mg and Ca (Clarkson and Hanson, Reference Clarkson and Hanson1980). In commercial agriculture, Ca and Mg seldom limit the yield of field-grown crops and their effects were not studied in the work reported here.
The aim of this study was to examine the performance of polyhalite as a multi-nutrient fertilizer and to compare that performance with commercially available alternatives. This was achieved by reporting the results of four field trials which examined the response of winter barley and forage maize to different rates of polyhalite and commercial alternatives.
Materials and methods
This study draws together the results from four different field trials that shared a number of common features that included location, low-K status soil, and inclusion of polyhalite, MOP and SOP fertilizer treatments.
Site description
Field trials were conducted at the University of Warwick's Crop Centre located in Wellesbourne, Warwickshire, UK (52°11′N, 1°35′W). The trials started in October 2013 and concluded in July 2016. The trial site is situated 45 m above sea level. The soil is a free-draining coarse sandy loam of the Wick series containing 74% sand, 12% silt, 14% clay and 2% organic matter (Whitfield, Reference Whitfield1973).
All the trials were conducted in the low-K status field known as Wharf Ground. This field had been deliberately and systematically mined for K through the use of high biomass crops over a number of years to provide a low-K environment in which response to K fertilizers could be accurately assessed. The field had not been cropped for the previous 5 years. Soil samples were taken pre- and post-drilling and plant samples were taken at harvest. Chemical analysis for the macro and micro nutrients was undertaken by NRM Laboratories, Bracknell, UK. Analysis for extractable P was by Olsen's method (Olsen et al., Reference Olsen, Cole, Watanabe and Dean1954). For K, Mg and Ca, the ammonium nitrate extraction method was used (Ministry of Agriculture, Fisheries and Food, 1981). Available sulphate was extracted from the soil under controlled conditions, using a phosphate buffer extracting solution (Rowell, Reference Rowell1995); a filtered extract of the sample was analysed by Inductively Coupled Plasma Emission Spectroscopy. More detailed information of the analyses in the commercial laboratory, e.g. the wavelengths used, is unavailable. The soil pH was measured potentiometrically using a suspension obtained by stirring soil with water, with a ratio (soil to water) of 1:2.5 (Ministry of Agriculture, Fisheries and Food, 1981). The results of the pre-drilling soil analyses are provided in Table 1. Three out of the four trials showed K values below 80 mg/l which suggested that a positive response to K fertilizer would be expected. The exception was the 2014 maize (trial 3) where K soil concentrations were unexpectedly high at 157 mg/l suggesting that little or no response to K fertilizer would be expected.
Table 1. Available soil nutrient concentrations and soil pH pre-drilling

Existing ground cover was predominantly poor grass which was sprayed with glyphosate approximately 4 weeks prior to drilling. Vegetation was allowed to die back, it was then mowed and ploughed. Seedbeds were prepared using a power harrow. Treatment fertilizers were incorporated pre-seedbed preparation in trials 2 and 3 but were surface applied post-drilling in trials 1 and 4. Details on all fertilizer applications are provided in Tables 2–4.
Table 2. Fertilizer treatments and time of application, for each of the four field trials

Table 3. Treatments, trials 1 and 2 (winter barley): sources of applied K and S, quantities of K and S applied, and treatment names.

C, control; PH, polyhalite; SOP, sulphate of potash (potassium sulphate); MOP, muriate of potash (potassium chloride); MOPG, MOP plus gypsum; *no treatment.
Table 4. Treatments, trials 3 and 4 (forage maize): sources of applied K and S, quantities of K and S applied, and treatment names.
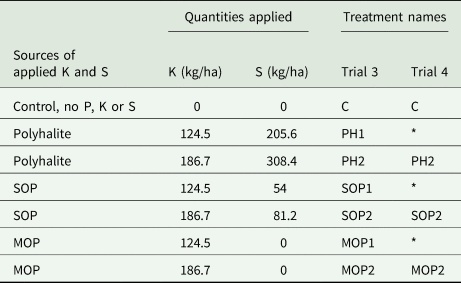
C, control; PH, polyhalite; SOP, sulphate of potash (K2SO4); MOP, muriate of potash (KCl); *no treatment.
Experimental design and management
All four trials used a randomized blocked design, with each block containing every treatment. Details of crop species, varieties, treatment replication, plot size, planting dates and harvest dates are provided in Table 5.
Table 5. Crop species, variety, planting and harvest dates, for each of the four field trials

Polyhalite was supplied by Sirius Minerals PLC (Scarborough, UK) as granules with a size range of 2–4 mm and with an equivalent nutrient content of 116 g/kg K, 121 g/kg Ca, 36 g/kg Mg and 192 g/kg S. The halite content was 3%. All other fertilizers were obtained from commercial sources.
The series of four trials were designed to examine different aspects of the performance of polyhalite as a fertilizer. Each trial contained a zero-K control which provided a baseline against which the fertilizer treatments could be compared. The fertilizer treatments comprised polyhalite, MOP, SOP and CaSO4 (gypsum) at different application rates. These treatments were designed to allow both crop response to individual fertilizers and comparative analysis across the different fertilizers to be examined. Details of the treatments are provided in Tables 3 and 4. Within each trial, all the experimental plots received the same amount of N to ensure that the crop response (to K and S) was not restricted by a lack of N. The N rate was derived from the UK Fertilizer Manual RB209 (Defra, 2010); details of N and non-treatment fertilizer applications are provided in Table 2. In summary, the trials were designed as follows:
• Trial 1: Winter barley. This trial was designed to provide a direct comparison of three types of K fertilizers: polyhalite, SOP and MOP. All plots received 100 kg N/ha as ammonium nitrate.
• Trial 2: Winter barley. This trial was designed to compare the supply of K and S from polyhalite and MOP plus gypsum (MOPG). All plots received 140 kg N/ha as ammonium nitrate in spring.
• Trial 3: Forage maize. This trial was designed to provide a direct comparison of three types of K fertilizers: polyhalite, SOP and MOP. All plots received 43.7 kg P/ha and 50 kg N/ha (as mono-ammonium phosphate + ammonium nitrate) as a basal dressing and 50 kg N/ha (as ammonium nitrate) as a top-dressing.
• Trial 4: Forage maize. This trial was designed to provide a direct comparison of three types of K fertilizers: polyhalite, SOP and MOP. All plots received 43.7 kg P/ha and 50 kg N/ha (as mono-ammonium phosphate + ammonium nitrate) as a top-dressing immediately after drilling and another 50 kg N/ha (as ammonium nitrate) as a top-dressing.
Treatments applied are detailed in Table 3 (trials 1 and 2) and Table 4 (trials 3 and 4). The trials provide an examination of fertilizer efficacy (polyhalite v. MOP v. SOP), of crop (winter barley and forage maize) and of year (both crops were grown over two years).
Crop management followed typical commercial practice to control weeds, pests and diseases. Winter barley (trials 1 and 2) received both pre- and post-emergence herbicides and a three-stage fungicide programme. No insecticides were used. Forage maize (trials 3 and 4) also received a pre- and post-emergence herbicide but no fungicides or insecticides. All crops remained free of major pest or disease problems although some hand-weeding was undertaken to ensure that competition from weeds did not influence the results. Forage maize (trial 4) was irrigated twice in June 2015 to provide 40 mm water to ensure that nutrient uptake was not restricted by a lack of soil moisture. The crops were harvested at commercial maturity, the development stage at which commercial harvest typically takes place for these crops: barley was harvested using a plot combine harvester and the maize was harvested by hand. Details of harvest dates and harvested areas can be found in Table 5.
Calculations and statistical analysis
Harvest assessment for all crops included fresh and dry weight of above-ground biomass, and in addition, grain yield and moisture content for barley. Sub-samples were analysed for N, K and S to allow for the calculation of nutrient offtake and percentage nutrient recovery. Nutrient offtake was estimated by multiplying the dry weight by nutrient concentration. Treatment means, treatment differences and Tukey multiple comparisons were calculated using analysis of variance (ANOVA) with the statistical software Genstat 13.2 (VSN International, 2010). Apparent nutrient recovery (ANR) was calculated using the approach:
ANR = ((nutrient uptake fertilized–nutrient uptake unfertilized)/nutrient applied) × 100
Results
The response of winter barley to fertilizer treatments (trials 1 and 2)
Trial 1 winter barley grain yields and whole crop dry weights at harvest had ranges of 2.33–7.59 and 4.5–11.4 t/ha, respectively (Table 6). Fertilizer treatment differences were significant for both of these yield variables (P < 0.001), with all treatments, except those based on MOP, giving significantly higher yields in comparison to the control (no K or S). Harvest indices varied less between fertilizer treatments, but again treatment differences were significant (P = 0.039) with higher values in all treatments except those based on MOP, in comparison to the control. The lack of a yield response to K highlighted the influence of S, since the yield of the polyhalite and SOP treatments was approximately double that of the control and MOP treatments. The performance of polyhalite and SOP was similar.
Table 6. Trial 1 (barley 2013/14): whole crop yield and offtake of K and S.

C, control [no K or S]; PH1, polyhalite [42 kg/ha K, 68 kg/ha S]; PH2, polyhalite [83 kg/ha K, 137 kg/ha S]; SOP1, sulphate of potash (potassium sulphate) [42 kg/ha K, 18 kg/ha S]; SOP2, sulphate of potash (potassium sulphate) [83 kg/ha K, 36 kg/ha S]; MOP1, muriate of potash (potassium chloride) [42 kg/ha K, no S]; MOP2, muriate of potash (potassium chloride) [83 kg/ha K, no S].
*Different letters indicate statistical difference at a 95% confidence interval.
Whole crop nutrient concentration and offtake values show a considerable difference between the fertilizer treatments in terms of their ability to provide K and S (Table 6). The highest K concentrations were provided by MOP and were approximately 37% higher than either polyhalite or SOP, however, the higher concentrations did not translate into either higher offtake values (as these were 28% lower than polyhalite and SOP), or higher yield, which was half that of the polyhalite and SOP treatments.
The influence of fertilizer type on the concentration and offtake of S was mixed. Polyhalite was the most effective source of S and produced the highest offtake amounts but interestingly, there was little difference between MOP and SOP in their S concentration, although S offtake by SOP was double that of MOP. In overall terms, polyhalite performed a little better than SOP and they both significantly outperformed MOP and the control.
In contrast, the trial 2 results were not as informative since the crop over-wintered poorly and there were no significant differences between any of the treatments for grain yield, whole crop dry weight or harvest index at harvest (Table 7). However, nutrient concentration and uptake did vary. In all fertilizer treatments, except MOPG1, K concentrations were significantly higher than the control and the same pattern was true for K offtake. Where additional S was applied, in the polyhalite and MOPG treatments, S concentrations were significantly higher than either the control or MOP2 although the higher concentrations did not always translate into higher S offtake. In overall terms, no robust conclusions on the effect of fertilizer type can be drawn from the results of trial 2.
Table 7. Trial 2 (barley 2015/16): whole crop yield and offtake of N, K and S.

C, control [no K or S]; PH1, polyhalite [42 kg/ha K, 68 kg/ha S]; PH2, polyhalite [83 kg/ha K, 137 kg/ha S]; MOPG1, muriate of potash (potassium sulphate) plus gypsum [42 kg/ha K, 68 kg/ha S]; MOPG2, sulphate of potash (potassium sulphate) plus gypsum [83 kg/ha K, 96 kg/ha S]; MOP2, muriate of potash (potassium chloride) [83 kg/ha K, no S].
*Different letters indicate statistical difference at a 95% confidence interval.
The response of forage maize to fertilizer treatments (trials 3 and 4)
Overall, 2014 was a good year for the production of forage maize and crop growth responded positively to all the fertilizer treatments. Dry weight yields for the fertilizer treatments were significantly higher than the control but there was no significant difference in yield between polyhalite, SOP and MOP (Table 8). The lack of significant yield differences in response to varied K applications suggests that in general the crop was adequately supplied with K. Potassium offtake results were also not correlated to the applied rate of K. There was a relatively high K value at planting (157 mg/l, index 2; Table 1).
Table 8. Trial 3 (forage maize 2014): whole crop yield and offtake of N, K and S.

C, control [no K or S]; PH1, polyhalite [125 kg/ha K, 206 kg/ha S]; PH2, polyhalite [187 kg/ha K, 308 kg/ha S]; SOP1, muriate of potash (potassium sulphate) [125 kg/ha K, 54 kg/ha S]; SOP2, sulphate of potash (potassium sulphate) [187 kg/ha K, 81 kg/ha S]; MOP1, muriate of potash (potassium chloride) [125 kg/ha K, no S]; MOP2, muriate of potash (potassium chloride) [187 kg/ha K, no S].
*Different letters indicate statistical difference at a 95% confidence interval.
The crop did respond positively to added S, with S concentrations being greater at the higher application rates. Sulphur offtake followed the same pattern but was not correlated well with dry weight yield suggesting luxury uptake rather than a requirement. In overall terms, there was little difference between polyhalite and SOP and they both had a slight advantage over MOP but all three outperformed the control.
In contrast, 2015 was a poor year for forage maize as unfavourable weather limited biomass accumulation and yields were significantly lower in comparison to 2014 (Table 9). At harvest, there was little difference in whole crop dry weight yield between any of the treatments, including the control, although there were differences in nutrient concentration and offtake. In comparison to the no-K control, the application of polyhalite, SOP and MOP contributed to significantly higher K concentration and offtake. Polyhalite and SOP increased crop S concentration but this was not translated into extra biomass as S offtake was similar for all treatments.
Table 9. Trial 4 (forage maize 2015): whole crop yield and offtake of N, K and S.

C, control [no K or S]; PH2, polyhalite [187 kg/ha K, 308 kg/ha S]; SOP2, sulphate of potash (potassium sulphate) [187 kg/ha K, 81 kg/ha S]; MOP2, muriate of potash (potassium chloride) [187 kg/ha K, no S].
*Different letters indicate statistical difference at a 95% confidence interval.
Comparison between fertilizer treatments
The four trials revealed season-to-season differences across all three key parameters. In winter barley, the control, whole-crop yield in 2013/14 was double that of 2015/16 with corresponding different nutrient uptake amounts for both K and S. In 2013/14, the crop responded to added nutrients, but this was not the case in 2015/16. Winter barley grain yield also responded to added nutrients in 2013/14. In forage maize, the control yields in 2014 and 2015 were similar but whereas the crop responded to added nutrients in 2014, it did not in 2015.
The four individual trials provided an examination of the different fertilizers and showed that polyhalite, SOP and MOP are all effective sources of nutrients. The relative performance of the treatments over different crops and growing conditions is shown in Figs 1–3. Values for total dry crop yield, K offtake and S offtake are presented relative to the results for polyhalite.

Fig. 1. Relative analysis of total dry weight yield for each trial. The reference fertilizer is polyhalite which is set at 100% with all other values relative to that. Control had no fertilizer applied; polyhalite is represented by PH2, sulphate of potash by SOP2 or MOPG2, and muriate of potash by MOP2. Bars indicate SED (9 d.f.).

Fig. 2. Relative analysis of K offtake (kg/ha) for each trial. The reference fertilizer is polyhalite which is set at 100% with all other values relative to that. Control had no fertilizer applied; polyhalite is represented by PH2, sulphate of potash by SOP2 or MOPG2, and muriate of potash by MOP2. Bars indicate SED (9 d.f.).

Fig. 3. Relative analysis of S offtake for each trial. The reference fertilizer is polyhalite which is set at 100% with all other values relative to that. Control had no fertilizer applied; polyhalite is represented by PH2, sulphate of potash by SOP2 or MOPG2, and muriate of potash by MOP2. Bars indicate SED (9 d.f.).
The three fertilizer treatments outperformed the control in three out of the four trials. This meets the expectation based on the low nutrient status of the experimental soils and suggests that the results are robust. The exception was trial 4 in which forage maize underperformed in a poor growing year.
In terms of total dry crop yield, the performance of polyhalite was similar to MOP in three out of the four trials and better in one (Fig. 1). Polyhalite treatments were similar to SOP in two out of four trials, performed better in one and worst in one. The growing season had a considerable influence on fertilizer performance and crop biomass accumulation. Where growing conditions were more favourable, as in trials 1 and 3, the advantage of providing both K and S resulted in higher dry weight yield; SOP in particular performed very well. However, in contrast, in poorer growing years, as in trials 2 and 4, the differences in yields were smaller and the application of S provided little or no advantage and the performance of polyhalite, SOP and MOP treatments was broadly similar.
All three fertilizers contained K and the pattern of K offtake was similar to total crop dry weight (Fig. 2). Trials 2 and 4 provided similar responses to fertilizer treatments in conditions of relatively low nutrient accumulation, while trials 1 and 3 showed a greater response, and significant differences, in higher nutrient accumulation conditions. In trial 1, where S may have been deficient and limited crop yield, polyhalite and SOP both performed well. In trial 2, where similar conditions were present, SOP outperformed polyhalite.
The recovery of K-fertilizers by the crops was variable. In trial 1, the crop recovered an average of 48% and 40% of the K contained within polyhalite and SOP, respectively; but only 9% of the K in MOP (Table 6). Recovery fell in trial 2 to less than 10% for all three fertilizers making interpretation difficult (Table 7). Trial 3 provided more robust results and showed, with the exception of SOP2 treatment, that recovery of K from all three fertilizer types was broadly similar, being between 24% and 57% (Table 8). Recovery of K in trial 4 was generally low although polyhalite and SOP slightly out-performed MOP (Table 9).
Only polyhalite and SOP contained S and this was reflected in both the overall yield and S uptake (Fig. 3). With the exception of trial 4, where the response to added nutrients was low, polyhalite and SOP enabled greater crop uptake of S in comparison with MOP and control treatments. Values for S-ANR are presented in Tables 6–9 where relevant, but the quantities supplied by the different fertilizers are not comparable and therefore make evaluation difficult.
Discussion
The aim of this research was to examine the agronomic value of polyhalite and to reassess its contribution to crop nutrition. Polyhalite contains four plant nutrients (K, S, Ca and Mg) but the focus here is K and S. Naturally occurring multi-nutrient fertilizers are uncommon, so two aspects of its performance are discussed: firstly, the performance of the fertilizer as an effective source of crop nutrients, and secondly, the performance of the individual nutrients to judge their value when supplied from alternative products.
Polyhalite as fertilizer
The first question was addressed by comparison of crop yield response to different fertilizer types. In trial 1, where crop yield did not respond to K, polyhalite provided crop-available S and performed slightly better than the nutrient-equivalent SOP. Where the crop responded to K (trial 3), polyhalite performed as well as both SOP and MOP as a source of plant-available K and S. Where a crop did not respond to either K or S (trials 2 and 4), the performance of polyhalite was not different to SOP, MOP or MOPG, demonstrating that there was no negative impact of its use. These results support the early findings of Fraps and Schmidt (Reference Fraps and H1932) and Lepeshkov and Shaposhnikova (Reference Lepeshkov and Shaposhnikova1958) and confirm that polyhalite is an effective multi-nutrient fertilizer. More recent work on potato found that polyhalite performed as well as MOP and SOP as a source of K (Mello et al., Reference Mello, Pierce, Tonhati, Almeida, Netto and Pavuluri2018), was similar or superior to MOP in corn (Dal Molin et al., Reference Dal Molin, Nascimento, Teixeira and Benites2020), and was a more efficient fertilizer for supplying K, Ca, Mg and S relative to equivalent soluble salts in wheat (Yermiyahu et al., Reference Yermiyahu, Zipori, Faingold, Yusopov, Faust and Bar-Tal2017). This evidence suggests that polyhalite is indeed an effective source of plant nutrients.
Polyhalite is a natural mineral and as such, its nutrient balance is fixed so while it may be a good source of nutrients, it does not automatically mean that it is an effective fertilizer. The molecular ratio of K : Ca : Mg : S is approximately 3 : 3 : 1 : 5 which suggests an excess of Ca and S should the application be tailored to K requirements. Yermiyahu et al. (Reference Yermiyahu, Zipori, Faingold, Yusopov, Faust and Bar-Tal2017) recommended that the application rate should be adjusted to provide sufficient Ca and Mg and that additional fertilizers be used as a source of K. This study took a different approach and tailored the K application without regard for the other nutrients but found no detrimental results as a consequence of higher Ca and S amounts. Our research did not address the agronomic effects of Ca and Mg in polyhalite, but the earlier work of Mercik (Reference Mercik1981) reported increases in plant Ca and Mg concentrations in response to polyhalite application. Under some conditions, it is expected that these nutrients will benefit crop production.
Nutrients within polyhalite
The second question was addressed by examination of individual nutrient concentrations and offtake values. On its own, nutrient offtake can be misleading as it is heavily influenced by biomass yield but considered together with concentration it can provide an assessment of fertilizer performance. Multi-nutrient fertilizers present a challenge because of potential interactions between nutrient effects. Since fertilizer application for supply of K and S is typically more important than fertilizer application for supply of Ca and Mg (because K and S are more often limiting compared with Ca and Mg), our approach has been to concentrate on the former.
Potassium performance
The inverse relationship between K concentration and K uptake in trial 1 is interesting: the highest concentrations and lowest offtake values belonged to MOP but the opposite was true for polyhalite, which also returned the highest biomass yield. This suggests that MOP was a better source of K than either polyhalite or SOP but that K concentration was not a good indicator of biomass yield; we assume that it was the availability of S that drove biomass accumulation, since biomass yield was positively and significantly correlated to S offtake. At low K rates, Dal Molin et al. (Reference Dal Molin, Nascimento, Teixeira and Benites2020) reported that polyhalite was less effective than MOP but other studies have suggested that their performances are comparable (Pavuluri et al., Reference Pavuluri, Malley, Mzimbiri, Lewis and Meakin2017; Bernardi et al., Reference Bernardi, Souza and Vale2018; Mello et al., Reference Mello, Pierce, Tonhati, Almeida, Netto and Pavuluri2018). One possible explanation is that the lower solubility of polyhalite in comparison to other fertilizers might restrict K availability at lower application rates (Yermiyahu et al., Reference Yermiyahu, Zipori, Faingold, Yusopov, Faust and Bar-Tal2017). In this series of trials, polyhalite was a better source of K than SOP but it was the availability of S that most influenced biomass accumulation.
Levels of ANR can provide further evidence of the availability of nutrients from fertilizers, although the results from these four trials are mixed in terms of their usefulness. The most robust data came from trials 1 and 3, while the lack of responses in trials 2 and 4 render them less useful. Recovery of K-fertilizer within trial 1 was good from polyhalite and SOP, but poor from MOP. Polyhalite and SOP were broadly similar suggesting that material breakdown and nutrient availability were similar; their advantage over MOP is likely to be their sulphur content rather than better availability of K. The addition of S is known to facilitate N uptake (Withers et al., Reference Withers, Tytherleigh and ODonnell1995) and trials 1 and 4 provide some evidence of this. Recovery of K-fertilizer in trial 3 was similar for polyhalite, SOP and MOP suggesting that overall, all three fertilizers performed equally well despite some evidence that polyhalite might be slower to dissolve in soil water.
In trial 4, despite the lack of yield effects, the application of polyhalite, SOP and MOP contributed to significantly higher K concentration and offtake in comparison to the control. This, and the higher K offtake values, demonstrates that the fertilizer treatments were able to supply additional K but that the crop was unable to use it to increase biomass production.
Sulphur performance
The K and S contents of polyhalite and SOP are quite different: polyhalite contains the equivalent of 11.6% K and 19.2% S while SOP contains 41.5% K and 18% S. To provide the same K application rate requires more polyhalite, which consequently provides much more S. The 186.7 kg K applications in trial 3, using SOP and polyhalite, delivered 81.2 and 308.4 kg S, respectively. Both these amounts exceed plant requirements. Although the polyhalite treatments returned the highest S concentrations in all trials except trial 4, the difference between polyhalite and SOP was rarely significant; the exception to this was in trial 1. These results suggest that, although polyhalite contains a large amount of S, not all of it was captured by the crop. There was no evidence for a toxic effect, but sulphate is readily leached out of soil into ground water, potentially affecting soil mineral content and water quality (Ren et al., Reference Ren, Pan, Zeng and Jiao2017). Soils with low acid buffering capacity and that are Ca-poor may be vulnerable, therefore, to loss of Ca and Mg anions when sulphate cations are in excess and leaching. Further work would be beneficial to examine the acid buffering capacity of receiving soils (Bouwman et al., Reference Bouwman, Van Vuuren, Derwent and Posch2002).
It was observed that there was little difference between MOP and SOP in their S concentration, although S offtake by SOP was double that of MOP. The assumption is that the more readily available source of S in SOP supported better and earlier crop development, and that the MOP treated crop was able to scavenge sufficient soil S by harvest to return similar concentration values but not to attain similar yield.
In-field performance
Nutrient recovery, and subsequently plant nutrient concentration, is influenced by the availability of nutrients within the soil matrix and while these trials were not designed to examine the breakdown of the fertilizer materials, some observations can be made. Fertilizers were either incorporated into soils prior to drilling (trials 2 and 3) or surface applied post-emergence (trials 1 and 4). Of the fertilizers used, polyhalite was the most resistant to breakdown when surface applied, and some residual material was still visible on the soil surface for a few weeks after the other materials had disappeared. These observations support the work of Barbarick (Reference Barbarick1991), who reported that polyhalite behaved like a slow release fertilizer, and Barbier et al. (Reference Barbier, Li, Liu, He, Mylavarapu and Zhang2017) who reported the solubility of polyhalite to be lower than MOP and SOP. Reduced dissolution rate can be advantageous in longer season crops since it ensures a supply of nutrients over a longer period. For polyhalite, it appears that there are no short-term disadvantages as yields were similar or slightly better compared to SOP and MOP in these trials.
In conclusion, the results show that polyhalite was an effective fertilizer, supplying nutrients as effectively as alternative materials. The research focused on K and S but there is evidence to suggest some positive synergy due to the presence of Ca and Mg. At this early stage in the revaluation of polyhalite, any potential synergy provided by multiple nutrients should be valued even though there is little understanding of the mechanism or quantification of the effect. Polyhalite may be useful as a slow release multi-nutrient fertilizer as it dissolves more slowly than some alternative materials (MOP and SOP) and this characteristic may allow greater flexibility in terms of application timing.
The commercial usefulness of polyhalite as a fertilizer for mainstream crop production will depend on the relative costs of alternatives, together with consideration of any advantages due to its multi-nutrient status.
Acknowledgements
We would like to thank the technical staff at the University of Warwick's Crop Centre, in particular Susannah Chapman and Harry Tricklebank, who managed the field trials, and Timothy Lewis at Sirius Minerals PLC for providing the polyhalite.
Financial support
This work was funded by Sirius Minerals PLC (now Anglo American PLC)
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
Not applicable.















