INTRODUCTION
Production of crop-derived feed protein is low in Europe and therefore, European animal production is highly dependent on imported soybean (Glycine max (L.) Merr.). In the northernmost European regions, such as Finland, alternatives for producing crop-based protein are limited (Peltonen-Sainio & Niemi personal communication). Rapeseed (both turnip rape, Brassica rapa L. and oilseed rape, Brassica napus L.), field peas (Pisum sativum L.) and faba bean (Vicia faba L.) represent the major alternatives for crop-based protein sources (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Hyövelä and Nissilä2011a; Peltonen-Sainio & Niemi personal communication). However, the cereals that are currently grown on c. 1·1 million ha in Finland may, along with intensified rapeseed production and expanded cultivation of grain legumes, contribute to targeted improvements in national protein production capacity (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen and Nissilä2012). This potential, however, needs to be supported through investments in industries that use non-protein crop components, such as bio-ethanol production, which produces protein-enriched distiller feed (Härmälä Reference Härmälä2010).
Investigating the potential for increasing seed crop-based protein production in many regions of the world is critical because global changes, such as population growth, urbanization and more meat-intensive diets, require an increase in global protein crop production during the same period that climate change could substantially challenge crop production volumes and certainties (Parry et al. Reference Parry, Canziani, Palutikof, van der Linden and Hanson2007). However, the capacity to replace imported soybean is dependent on the availability of alternative protein sources and differences between domestic animal species in their responses to protein source. For example, use of alternative protein sources for monogastric livestock, poultry and pigs is challenging and may be limited due to risks of negative effects on growth and productivity (Etienne Reference Etienne1977; Perttilä et al. Reference Perttilä, Valaja, Partanen, Jalava and Venäläinen2002; Crépon et al. Reference Crépon, Marget, Peyronnet, Carrouée, Arese and Duc2010; Jezierny et al. Reference Jezierny, Mosenthin and Bauer2010). In contrast, feed for cattle in Finland could be based fully on domestic protein sources, such as protein-enriched silage, legumes in grass mixtures, rapeseed meal and to a lesser extent distiller feed, malting residues, peas and faba bean (Vanhatalo et al. Reference Vanhatalo, Ahvenjärvi and Jaakkola2004; Huuskonen et al. Reference Huuskonen, Khalili and Joki-Tokola2007).
Full protein self-sufficiency is not a realistic target for a country such as Finland, but the present low self-sufficiency in crop-based protein can be improved (Peltonen-Sainio & Niemi personal communication). This has been set as a target in recent national food security and feed strategies. Expanding the production of protein crops is not solely a question of reducing dependency on imported feed constituents per se, it is also an approach to diversify northern cropping systems and benefit as much as possible from the versatile ecological services that more extensive introduction of legumes and rapeseed could provide to agroecosystems (Stoddard et al. Reference Stoddard, Hovinen, Kontturi, Lindström and Nykänen2009; Köpke & Nemecek Reference Köpke and Nemecek2010; Gan et al. Reference Gan, Liang, Wang and McConkey2011; Peltonen-Sainio & Niemi personal communication). The key questions related to expansion of protein crop production that the present paper addresses are: (i) on which crops and to what degree should the increase in self-sufficiency be based, (ii) how can production of rapeseed and grain legumes be arranged regionally, taking into account their present regional status, climatic, edaphic and other regionally varying constraints that may cause uncertainty in production, (iii) what is the upper limit for sustainably expanded production and thereby, self-sufficiency in protein crops in the northernmost European areas and (iv) how will this upper limit change over time due to an extended growing season induced by climate warming? One of the northernmost European countries, Finland, represents an interesting, even extreme, case regarding the means of enhancing protein crop production (Peltonen-Sainio & Niemi personal communication).
FEATURES OF CURRENT PROTEIN CROP PRODUCTION IN FINLAND
The proportion of protein crop area compared with that of cereals is currently low in Europe (Fig. 1). It is highest (c. 0·20) in central European countries such as the Czech Republic, France, Germany and the UK, and lowest (⩽0·10) in northern and southern Europe. Consequently, protein self-sufficiency, the proportion of plant protein produced compared with that used, is low, averaging c. 0·30 in Europe. In Finland, it is generally expected to be 0·25 at its highest, but can be as low as 0·15 during some years. The dependency on imported soybean meal is currently high for monogastric animals. However, in commercial feed mixtures for cattle up to 0·90 of protein is derived from rapeseed meal, and the remainder that comes from soybean meal is quite easy to replace with other protein sources. In contrast, >0·90 of protein in commercial feed mixtures for poultry and some 0·50 in those for pigs originates from soybean meal.
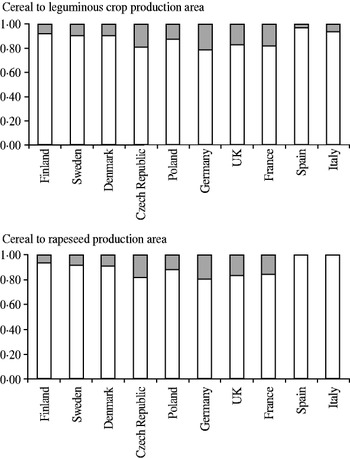
Fig. 1. Examples of relative differences in total production of cereals and protein seed crops in Czech Republic, Denmark, Finland, France, Germany, Italy, Poland, Spain, Sweden and United Kingdom in 2004–8. The shaded sections represent the proportion of (a) legumes or (b) rapeseed in total with cereals. Data from FAO (2012).
Spring turnip rape (B. rapa) is the major protein crop grown in Finland (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Hyövelä and Nissilä2011a). However, it is estimated that due to climate warming and the subsequent extension of the growing season (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Hakala and Ojanen2009a), spring sown oilseed rape (B. napus) could replace turnip rape in current major production regions within several decades (Peltonen-Sainio et al. Reference Peltonen-Sainio, Hakala, Jauhiainen and Ruosteenoja2009b). In such scenarios, turnip rape remains important as a crop introduced into novel regions, where the shorter growing season requirement of turnip rape, compared with that of oilseed rape, is appreciated when managing production uncertainties. Therefore, it is essential to maintain active turnip rape breeding programmes to sustain the transition to higher national production areas, and thereby to avoid serious production uncertainties.
Peas are an exceedingly neglected crop in Finland, while faba bean production has expanded rapidly, reaching 10000 ha within recent years. Alternative protein crop production might become possible with climate warming, including lupins (Lupinus sp.) and sunflower (Helianthus annuus L.) (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Hakala and Ojanen2009a). It is, however, likely that their production capacities would not be high enough to replace rapeseed, pea and faba bean production in the near future. Novel crops could, however, represent an interesting opportunity for further diversification of northern agroecosystems. Despite this, lupins and sunflower are expected to have only minor potential as protein crops in Finland as there has been little experience of their management, lack of breeding programmes, scattered and/or minor research activities and no experience in their use in industrial processes. Furthermore, in many regions across Europe, rapeseed out-yields sunflower, as do peas, lupins (Fig. 2) and even soybean, the latter again being a competitive yield producer in Central to South Central Europe.

Fig. 2. Examples of relative yield differences between different protein seed crops when grown in Czech Republic, France, Germany, Hungary, Italy, Poland and/or Spain. Data from FAO (2012).
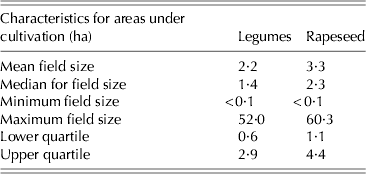
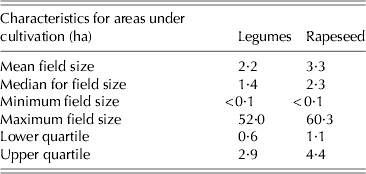
Production intensity, but also increased risks of crop failures, may affect the typical aspects of rapeseed and legume production, especially characteristics of fields where they are cultivated. Typically legumes are grown in smaller individual fields than rapeseed in Finland (Table 1), with means of 2·2 and 3·3 ha and medians of 1·4 and 2·3 ha, respectively. In early 2000s, faba beans made up a relatively minor contribution to total legume area compared with pure stands of peas or mixtures including legumes. However, within recent years the total area under faba bean has been increased considerably, which has also stimulated interest in its use in commercial feed mixtures. Other leguminous crops such as common beans (Phaseolus vulgaris L.) and vetch (Vicia sativa L. and Vicia villosa Roth.) are only grown in small field patches.
Table 1. Field size of leguminous and rapeseed crops in Finland according to dataset for 2000–10 from Tike (2012)
Regional differences and limitations for protein crop production today
Production of grain legumes is heavily concentrated in two southern regions of Finland, Varsinais-Suomi and Uusimaa, with total areas averaging over 1000 ha in each, but varying considerably over 20 years (Fig. 3). Several hundred hectares are grown in other southern and coastal regions, while elsewhere production of legumes is negligible. Pure stands of leguminous crops characterize the southern regions, while these become marginal northwards, where again mixtures with legumes dominate and where vetch is grown as a ley (Table 2).
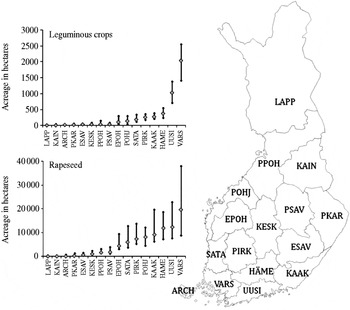
Fig. 3. Variation in annual production area (ha) of leguminous crops (and crop mixtures) and rapeseed in different regions of Finland during 2000–10. Mean area over years is shown with a grey square and the range across years with lines. ARCH, archipelago; UUSI, Uusimaa; HÄME, Häme; VARS, Varsinais-Suomi; SATA, Satakunta; PIRK, Pirkanmaan; KAAK, Kaakkois-Suomi; ESAV, Etelä-Savo; PSAV, Pohjois-Savo; PKAR, Pohjois-Karjala; KESK, Keski-Suomi; EPOH, Etelä-Pohjanmaa; POHJ, Pohjanmaa; PPOH, Pohjois-Pohjanmaa; KAIN, Kainuu and LAPP, Lapland. Data from Tike (2012).
Table 2. Regional characteristics as proportion of fields having different leguminous crops and crop mixtures in 1997–2001. The geographical locations of different regions are shown in Fig. 3. Data from Tike (2012)

* Area under faba bean has increased drastically near to 2010, when it was 9430 ha compared with some 150 ha in the above years.
† Grasslands including clover or other leguminous species in mixture are not included.
‡ Includes both Phaseolus vulgaris var. nanus and var. vulgaris.
The production regions that are either typical or non-typical for legumes are similar to those for rapeseed. However, the total regional areas are vastly greater for rapeseed than for leguminous crops, the differences being up to four times greater (Fig. 3). Regarding regional differences in legume field sizes, 5–10 ha fields are most common in the southern regions, while 1–3 ha fields dominate elsewhere (Fig. 4). In contrast, rapeseed fields are typically 5–10 ha throughout the country, even in the northernmost production areas where there are high production risks and the crops make a very marginal contribution to total national production (Fig. 5). Hence, rapeseed is likely to be cultivated in larger than average fields.

Fig. 4. Regional differences in frequency of different field sizes (from <0·05 ha up to >20 ha) in which leguminous crops and crop mixtures were grown in Finland during 2000–10. Data from Tike (2012).
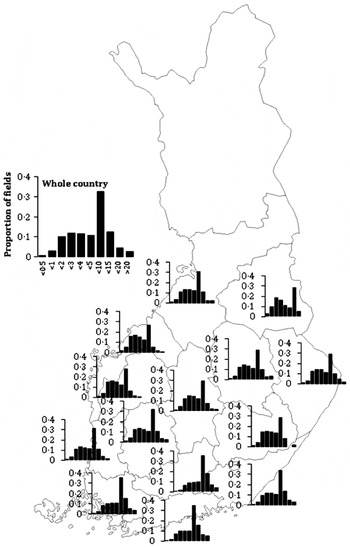
Fig. 5. Regional differences in frequency of different field sizes (from <0·05 ha up to >20 ha) in which rapeseed was grown in Finland during 2000–10. Data from Tike (2012).
Legumes in pure stands or mixtures?
Pure stands of legumes are more common in terms of the number of fields and their total area when compared with crop mixtures that include legumes. However, regional differences are marked, as referred to above (Table 2). Traditionally, pure stands of faba bean have been scarce, but current expanded production is largely based on pure stands. Peas are grown in pure stands as well as in mixtures with cereals (Stoddard et al. Reference Stoddard, Hovinen, Kontturi, Lindström and Nykänen2009; Kontturi et al. Reference Kontturi, Laine, Niskanen, Hurme, Hyövelä and Peltonen-Sainio2011) and both cropping systems have advantages and disadvantages.
One of the evident advantages of growing pea–cereal mixtures is that yield production is enhanced and/or more stable (Hauggaard-Nielsen & Jensen Reference Hauggaard-Nielsen and Jensen2001; Hauggaard-Nielsen et al. Reference Hauggaard-Nielsen, Andersen, Jørnsgaard and Jensen2006). Often, the impacts of abiotic and biotic stresses are buffered when mixtures are used and thereby yield losses or, in extreme cases, total crop failures are less likely (Trenbath Reference Trenbath1993; Altieri Reference Altieri1999). This is especially important in the northernmost European growing areas with high risks of recurrent crop failure (Mukula & Rantanen Reference Mukula and Rantanen1987; Peltonen-Sainio & Niemi personal communication). Furthermore, legumes intercropped with cereals decrease the risk of plant disease infection (Bulson et al. Reference Bulson, Snaydon and Stopes1997; Hauggaard-Nielsen et al. Reference Hauggaard-Nielsen, Jønsgård, Kinane and Jensen2008; Schoeny et al. Reference Schoeny, Jumel, Rouault, Lemarchand and Tivoli2010; Fernandez-Aparicio et al. Reference Fernandez-Aparicio, Shtaya, Emeran, Allagui, Kharrat and Rubiales2011). Also, in mixtures, insect pest attack is often less severe than in sole crops because associated non-host plant species in the mixtures make it more difficult for the insect herbivores to find their host plant (Finch & Collier Reference Finch and Collier2000). For example, aphid infestations were reduced when faba bean was intercropped with oats (Avena sativa L.) or barley (Hordeum vulgare L.) compared with a sole faba bean crop (Patriquin et al. Reference Patriquin, Baines, Lewis and Macdougall1988). Annual intercrops can also enhance weed suppression (Szumigalski & Van Acker Reference Szumigalski and Van Acker2005). Another benefit is that legumes provide nitrogen to the companion crop (Hauggaard-Nielsen et al. Reference Hauggaard-Nielsen, Gooding, Ambus, Corre-Hellou, Crozat, Dahlmann, Dibet, Von Fragstein, Pristeri, Monti and Jensen2009; Kontturi et al. Reference Kontturi, Laine, Niskanen, Hurme, Hyövelä and Peltonen-Sainio2011) and also to the following crop in the rotation, thereby reducing the need for chemical fertilizers and improving the energy efficiency and carbon footprint of the cropping system (Tosti & Guiducci Reference Tosti and Guiducci2010; Gan et al. Reference Gan, Liang, Wang and McConkey2011). However, the yields of mixture components may vary substantially (Kontturi et al. Reference Kontturi, Laine, Niskanen, Hurme, Hyövelä and Peltonen-Sainio2011), which often challenges their use as animal feed.
Table 3 contains data from pea–oat mixtures grown for 3 years in three locations in Finland (see also Kontturi et al. Reference Kontturi, Laine, Niskanen, Hurme, Hyövelä and Peltonen-Sainio2011 for details), the main conclusion being that irrespective of pea cultivar, year or location, oats always benefited from intercropping at the expense of the pea crop, which was the secondary component in the mixture. The reduction in the proportion of pea yield in a pea–oat mixture (compared to original proportion in sown seed) ranged from 0·06 to 0·73. This means that the pea component probably provided important ecological services, such as nitrogen fixation, which aided the oat crop, but that pea was not successful in competition with oats (Kontturi et al. Reference Kontturi, Laine, Niskanen, Hurme, Hyövelä and Peltonen-Sainio2011). The protein content of the companion oat crop was 0·2–1·6% higher compared with oats grown in a pure stand, depending on pea cultivar and the proportion of peas in the mixture. This was also the case when peas were intercropped with other cereals (Hauggaard-Nielsen et al. Reference Hauggaard-Nielsen, Andersen, Jørnsgaard and Jensen2006). The protein content of peas changed from +0·9 to −0·8% when grown in mixture compared with the pure stand. Also total protein yields/ha produced by mixtures were often somewhat higher than those in pure oat stands. These findings emphasize the role of mixtures with legumes as a sustainable alternative for enhancing protein crop production with reduced production uncertainties. Their future roles are also worth emphasizing, particularly in the northern regions where they are currently grown (though only on very small areas), but also elsewhere, when larger legume cropping areas are targeted. By using mixtures, especially during the transition phase, both abiotic and biotic constraints and induced production risks and uncertainties are likely to be better managed. Success in avoiding crop failures maintains farmer interest and commitment to cultivation of protein crops, whereas annual fluctuations in total cropping areas are likely to result from poor initial experiences.
Table 3. Reduction of pea yield in pea–oat mixtures when compared with the seed mixtures (w/w) sown with 0·93 and 0·85 of pea (standard deviation (s.d.) in parentheses) according to 3 years of experiments organized in Varsinais–Suomi (VARS), Häme and Etelä–Pohjanmaa (EPOH) regions of Finland. Also ranges for yield of pea–oat mixtures are shown. See also Kontturi et al. (Reference Kontturi, Laine, Niskanen, Hurme, Hyövelä and Peltonen-Sainio2011)

NEED, POTENTIAL AND MEANS FOR EXPANSION OF PROTEIN CROP PRODUCTION
Total volumes of imported protein, as rapeseed, soybean and their meals, approximately reflect the need to expand domestic protein crop production. In the 2000s, the imports of rapeseed, rapeseed meal and soybean meal increased steadily. In contrast, the role of soybean seed as a protein source in feed mixtures dropped as the imports of unprocessed soybean decreased (Table 4). Assuming an extraction rate of 0·60, the import of rape meal is estimated to correspond to c. 200 Gg (200000 t) rapeseed, and the total domestic rapeseed production to correspond to c. 72 Gg rapeseed meal. This means that in general there is a potential need to increase national rapeseed production by three times the current level, or alternatively, replace part of the demand for rapeseed with home-produced grain legumes. Furthermore, in total c. 150 Gg of soybean meal were imported into Finland in recent years, with a peak of c. 170 Gg in 2008 (i.e. the last recorded year in Table 4). Some of the soya-based protein in feeds of monogastric animals can be substituted by other sources of protein. Hence, soybean dependency could be eased by successful expansion of national rapeseed and legume production.
Table 4. Total quantities and value of the most imported crop-based protein commodities, rapeseed, rapeseed meal, soybean and soybean meal into Finland in the 2000s, according to FAO (2012)

The extent of the potential to substitute imported protein sources by nationally enhanced production is dependent on regional differences in: (a) production risks and constraints, (b) limitations to expansion potential resulting from rotation requirements that are more demanding for legumes and rapeseed when compared with other crops grown in Finland on a large scale and (c) availability of suitable land for protein crop production. In the present paper, production risks were considered to be too great in regions with a low likelihood for growing seasons that frequently exceed 1000 °Cd (temperature sum, base +5 °C, calculated from sowing to mid-September according to 30-year climate datasets; see Peltonen-Sainio & Niemi personal communication for further details). These areas were defined as high-risk areas. This was also in line with the present, regional prevalence of areas under legumes and rapeseed (except rapeseed for Etelä-Pohjanmaa (EPOH) and Pohjanmaa (POHJ) regions with large areas despite substantial climatic risks). Therefore, it was considered unlikely that the area under protein crops would be expanded in regions with high risk of short growing seasons and thereby, recurrent problems with maturation and harvest. However, the current (though negligible) areas for rapeseed and legumes were retained in estimates for those risky sites.
Rotation requirement caused by biotic risks
Rotation requirements were taken into account in the process of estimating regional expansion potential: only 0·20 of suitable land could be devoted to rapeseed or to legumes in order to have the same total cropping areas from one year to another (i.e. to avoid high annual fluctuations in total cropping areas and production volumes). However, rapeseed and legumes were included in the same rotation because they do not share the same pathogens or insect pests. In contrast, the proportion of grassland with clover (Trifolium sp.) was estimated according to statistics from Evira (Finnish Food Safety Authority 2012a,b). These data included annual quantities of both certified and imported clover seed. Thereby, the estimated total of 115000 ha were not included in the potential legume expansion area, in order to avoid legumes occurring too often in a rotation.
The crop rotation requirement is critical when sustainable increases in cropping areas are targeted. Rotation of crops in the same field generally reduces plant disease problems (Trenbath Reference Trenbath1993; Krupinsky et al. Reference Krupinsky, Bailey, McMullen, Gossen and Turkington2002). Minimum intervals between cropping of a particular crop in the same field depend on the longevity of survival structures of their major pathogens. Other possible host plants of the pathogen also affect the duration of the rotation period (Zegada-Lizarazu & Monti Reference Zegada-Lizarazu and Monti2011) and crops sharing major pests should not be grown together in the same rotation. Inclusion of legumes and rapeseed in the same rotations, as was done in the present approach, is justified because they do not share the same major pathogens. White mould (Sclerotinia sclerotiorum) can cause stem rot on legumes and rapeseed (Willets & Wong Reference Willets and Wong1980). The disease increased in rapeseed in Finland in the 1980s when the area of rapeseed rapidly increased (Hannukkala Reference Hannukkala1988). The initial inoculum can originate from fields surrounding the current crop and therefore crop rotation has not offered a means for effective control of the disease (Wahmhoff et al. Reference Wahmhoff, Hedke, Von Tiedemann, Nitzsche and Ulber1999). Another potential disease complex to be considered is damping-off and root rots caused by Rhizoctonia solani. The pathogen can attack both rapeseed and legumes. The disease on rapeseed is mainly caused by anastomosis group 2 of the pathogen (Verma Reference Verma, Sneh, Jabaji-Hare, Neate and Dijst1996), while legumes are mainly affected by other anastomosis groups (Hwang et al. Reference Hwang, Howard, Chang, Sneh, Jabaji-Hare, Neate and Dijst1996). There is no published evidence on the potential threat of the Rhizoctonia complex for rapeseed and legumes.
For peas, a 5–6-year interval is traditionally recommended to reduce the effects of the root rot pathogen Aphanomyces euteiches (Persson et al. Reference Persson, Bødker and Larsson-Wikström1997; Gaulin et al. Reference Gaulin, Jacquet, Bottin and Dumas2007). The oospores of the pathogen can survive in soil for over 10 years (Gaulin et al. Reference Gaulin, Jacquet, Bottin and Dumas2007). The accumulation of the infectious oospores in soil to a destructive level can take several rounds of short intervals in pea production, but thereafter the soil, once heavily infested, is almost impossible to clean again during any reasonable crop interval (Oyarzun et al. Reference Oyarzun, Gerlagh and Hoogland1993; Persson et al. Reference Persson, Bødker and Larsson-Wikström1997), and yield losses of up to 0·80 have been reported in soils infested with A. euteiches (Gaulin et al. Reference Gaulin, Jacquet, Bottin and Dumas2007). The pathogen is relatively widespread in Scandinavia (Persson et al. Reference Persson, Bødker and Larsson-Wikström1997). No systematic survey has been carried out on its occurrence in Finland, but in the studies of Engqvist & Ahvenniemi (Reference Engqvist and Ahvenniemi1997) the pathogen was found at 10 pea cultivar test sites, with average crop losses of 0·35. The pathogen is restricted to leguminous plants and is reported to be most destructive for peas and lucerne (Medicago sativa L.) (Oyarzun et al. Reference Oyarzun, Gerlagh and Hoogland1993; Gaulin et al. Reference Gaulin, Jacquet, Bottin and Dumas2007). A. euteiches strains infecting peas are not, however, pathogenic to faba bean (Levenfors et al. Reference Levenfors, Wikström, Persson and Gerhardson2003), but some strains may attack Vicia sp. (Gaulin et al. Reference Gaulin, Jacquet, Bottin and Dumas2007). Therefore, a 5-year interval was used in the present study between peas and faba bean, which is also sufficient to control stubble-borne root, stem and leaf diseases such as Mycosphaerella pinodes and Phoma medicaginis var. pinodella (Oyarzun et al. Reference Oyarzun, Gerlagh and Hoogland1993; Davidson & Ramsey Reference Davidson and Ramsey2000).
For rapeseed, the traditionally recommended interval is very similar to those of legumes, mainly due to risks represented by club-root (Plasmodiophora brassicae) (Colhoun Reference Colhoun1958). P. brassicae is a soil-borne pathogen belonging to the Kingdom Rhizaria (Cercozoa) (Bass & Cavalier-Smith Reference Bass and Cavalier-Smith2009). The pathogen is highly specialized on cruciferous plants and consequently legumes are not infected (Karling Reference Karling1968). The resting spores of P. brassicae can remain infectious for 20 years (Dixon Reference Dixon2009): in a work by Wallenhammar (Reference Wallenhammar1996), the half-life of the spores in soil without cruciferous plants averaged 3·6 years and full eradication of the pathogen took 17 years. Thus, crop rotation is important in controlling the disease, but complete eradication is difficult to achieve in practice. Also, many common volunteer host plants, including cruciferous weeds, can maintain viable inoculum in soil (Dixon Reference Dixon2009). Rapeseed also has stubble-borne leaf spot and stem rot diseases caused by Alternaria spp. (Humpherson-Jones Reference Humpherson-Jones1989) and Leptosphaeria maculans (Phoma lingam) (Naseri et al. Reference Naseri, Davidson and Scott2008) that can survive in plant debris for a few years. These diseases do not infect legumes and can be managed by introducing a cropping interval for club-root control (Zegada-Lizarazu & Monti Reference Zegada-Lizarazu and Monti2011). Although rotation of crops in the same field reduces the risk of plant diseases, it is not necessarily sufficient in the case of insect pests because of the better dispersal ability of insects from neighbouring fields or the surrounding landscape. Therefore, with insect pests ‘area-wide pest management’ is more important than traditional crop rotation on a small scale (Sexson & Wyman Reference Sexson and Wyman2005; Huusela-Veistola & Jauhiainen Reference Huusela-Veistola and Jauhiainen2006). Only weakly dispersing pests such as pea midge (Contarinia pisi) in legumes and pod midge (Dasineura brassicae) in rapeseed, can be kept under control by crop rotation (Coaker Reference Coaker, Burn, Coaker and Jepson1987). Furthermore, many pests of rapeseed and legumes are polyphagous and their control using crop rotation is limited.
Challenges caused by weed infestations may also limit frequency of grain legumes and rapeseed in crop rotations. Finnish farmers often argue that herbicides with broad efficacy against weeds without, e.g. causing retarded growth on legume and rapeseed crops are no longer sufficiently available. However, the survey of Salonen et al. (Reference Salonen, Hyvönen and Jalli2005) carried out on farmers’ pea fields showed that herbicides provided relatively good control, though the costs were high, especially when compared to the costs caused by weed management in cereals. According to a Canadian study, weedy plots often caused significant yield losses in canola and peas when compared with weed-free plots (Harker Reference Harker2001; Beckie et al. Reference Beckie, Johnson, Blackshaw and Gan2008). Weeds are especially challenging in organic farming where intercropping is a means of improving competition against weeds (Hauggaard-Nielsen et al. Reference Hauggaard-Nielsen, Ambus and Jensen2001; Salonen et al. Reference Salonen, Hyvönen and Jalli2005). The abundantly available nitrogen provided by a pea crop during early growth stages boosted subsequent growth of weeds (Hauggaard-Nielsen et al. Reference Hauggaard-Nielsen, Ambus and Jensen2001), but intercrops again provided high capacity for weed suppression (Corre-Hellou et al. Reference Corre-Hellou, Dibet, Hauggaard-Nielsen, Crozat, Gooding, Ambus, Dahlmann, Von Fragstein, Pristeri, Monti and Jensen2011). The extent of weed interference is also dependent on growing conditions and crop management. In general, advanced and even plant stand establishment favoured competitiveness of rapeseed (Bullied et al. Reference Bullied, Van Acker, Marginet and Kenkel2006), as did a rise in the density of pea crop stands (Hauggaard-Nielsen et al. Reference Hauggaard-Nielsen, Andersen, Jørnsgaard and Jensen2006; Lemerle et al. Reference Lemerle, Verbeek and Diffey2006). However, genetically modified crops such as herbicide-resistant rapeseed do not provide a means of managing weeds in rapeseed in Finland, due to strong public opposition to genetically modified crops.
Other constraints for expansion
In addition, regional differences in suitability of fields were taken into account by discarding fields that were <0·5 ha. These small fields are often impractically located, e.g. they may be next to shadowing forests, or far away from the farm centre, which makes their management even more costly and energy inefficient (Myyrä & Pietola Reference Myyrä and Pietola2002); furthermore, it is more difficult to monitor infestation of pests and diseases regularly (preferably on a daily basis). Therefore, management of pests and diseases is also likely to be less efficient in these fields. As a whole, these small fields contributed only negligible amounts (0·01 for rapeseed and 0·02 for legumes) to total area under production, despite their high number. Therefore, taking small fields into protein crop cultivation should be avoided when higher total production capacity is the goal.
An average of 0·15 of the total area was discarded due to probable risk of compacted, poor condition soils (according to national survey, see more details in Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Laitinen, Salopelto, Saastamoinen and Hannukkala2011b). One could argue that tap-rooted rapeseed would have beneficial, ‘rehabilitating’ effects on compacted soils, but that would occur at the expense of rapeseed yields. Furthermore, only fields with soil types reasonably favourable for production of rapeseed and legumes (e.g. Arenosol-Podzols, excluding tilly soils, Regosols, Cambisol-Gleysols and Histosols) were considered to have potential for expansion of protein crop production, while fields with less favourable soils were discarded. The data used for regional assessment originated from the Finnish Soil Database (for more details see Lilja et al. Reference Lilja, Uusitalo, Yli-Halla, Nevalainen, Väänänen and Tamminen2009).
Theoretical maximum and realistic potential for expansion
The estimations of current expansion potential indicated a theoretical maximum for increase in production area (when only regional production risks and rotation requirements were considered) for the present, heavily neglected legume production, of c. 240000 ha, and for rapeseed c. 170000 ha (Table 5). The mean figures for cultivated area in the 2000s were 4500 and 85000 ha, respectively. Therefore, in total, the theoretical maximum potential for increase in area is c. 400000 ha and the present future maximum potential area c. 500000 ha. When taking all of the limiting factors for expansion into account, a realistic potential for current protein crop production is obviously much less, c. 200000 ha in total (Table 6). This means that the current area under legumes and rapeseed could be doubled. Doubling the total area under protein crops, however, means a relatively higher increase in total production (Gg), as national seed yields of heavily neglected peas are higher (2200 kg/ha) than those for rapeseed (1400 kg/ha), which has declined in yield since the late 1990s (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen and Hannukkala2007). Therefore, the relatively higher potential increase in pea area is attributable to a higher increase in the total national production estimate relative to change in total area.
Table 5. Mean cultivation area, regional total cultivated land (in 2011) and currently existing theoretical, maximum potential for increase in cropping area for leguminous crops and crop mixtures, as well as for rapeseed. Current potential is estimated only for areas that have manageable production risks (see Peltonen-Sainio & Niemi personal communication). Future potential, induced by a prolonged growing season attributable to projected climate change, estimated for 30-year period centred on 2025 and 2055 according to 19 climatic models (see Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Hakala and Ojanen2009a). Dot indicates region lacking potential. The geographical locations of different regions are given in Fig. 3. Data from Tike (2012)

Table 6. Potential cropping areas for leguminous crops and crop mixtures as well as for rapeseed today and in 2025 and 2055, when estimated as a theoretical maximum potential, potential excluding small, often scattered fields and a realistic potential further discarding unsuitable soil types, compacted soils and grasslands with leguminous species (omitted only from area of leguminous crop). Estimate for total production expects yield levels to be comparable to those recorded for 2000–10. The geographical locations of different regions are shown in Fig. 3. Data from Finnish Soil Database (see Lilja et al. Reference Lilja, Uusitalo, Yli-Halla, Nevalainen, Väänänen and Tamminen2009) and Tike (2012)

* Discard is based on the proportion of unsuitable soils that takes into account soil type (Lilja et al. Reference Lilja, Uusitalo, Yli-Halla, Nevalainen, Väänänen and Tamminen2009) as well as fields that have high risk for soil compaction/poor soil conditions (averages 0·15, see Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Laitinen, Salopelto, Saastamoinen and Hannukkala2011b), but also grasslands, including clover (Trifolium sp.) are omitted from realistic potential for leguminous crop due to the rotation requirement.
† Low realistic potential for ESAV results from high proportion of Arenosol–Podzol/tilly soils and grassland with leguminous species.
–: regions with no potential.
‡ Total may not exactly match the sum of each column due to rounding.
Even in the case of no expected rise in yield levels, using mean regional yields from the 2000s, a large increase in total production (Gg) should result from a prolonged growing season due to climate warming and expansion of cultivation to novel regions currently with only negligible contributions to production capacity (Table 6). However, availability of suitable land restricts expansion more in the northern, novel production regions than in the southern regions, which have less potential for further expansion. Therefore, it was estimated that in the future (2025 and 2055) the potential area under protein crops could be more than triple the present area (Table 6).
CAN WE ASPIRE FOR HIGHER PER HECTARE YIELDS DURING THE TRANSITION TOWARDS EXPANDED CROPPING AREAS?
When estimating future total production potential (Gg), yield levels were not expected to rise, despite the fact that potential yields have steadily increased, especially by plant breeding, during recent decades (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen and Hannukkala2007, Reference Peltonen-Sainio, Jauhiainen and Laurila2009c) and are generally expected to increase further due to prolonged growing seasons and the introduction of slower maturing cultivars (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Hakala and Ojanen2009a). Stagnated mean yields were used in the present approach because national mean yields have levelled off or even declined since the late 1990s, depending on the crop, despite achievements in increasing genetic yield potentials. Furthermore, even in the case of a prolonged growing season in the future, climatic and biotic constraints will probably limit the realization of genetic yield potentials (Hakala et al. Reference Hakala, Hannukkala, Huusela-Veistola, Jalli and Peltonen-Sainio2011; Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen and Hakala2011c; Trnka et al. Reference Trnka, Olesen, Kersebaum, Skjelvåg, Eitzinger, Seguin, Peltonen-Sainio, Rötter, Iglesias, Orlandini, Dubrovský, Hlavinka, Balek, Eckersten, Cloppet, Calanca, Gobin, Vucetic, Nejedlik, Kumar, Lalic, Mestre, Rossi, Kozyra, Alexandrov, Semerádová and Zalud2011). One can also expect that limits to the expansion of cultivated area and having new farmers who are not necessarily experienced with special crop production may create some problems, which again could contribute to stagnation of national rapeseed and grain legume yields in the future.
The effects of expanded production on biotic stresses could be even more critical. Host-specific herbivores and diseases are dependent on spatial and temporal distribution of their host plants. Therefore, pest problems are likely to increase when a particular crop becomes more widely grown (Altieri & Letourneau Reference Altieri and Letourneau1982). Larger cropping areas improve host discovery and pest colonization. Dispersion of crops, including size and degree of isolation of host–plant patches, affects infestation levels. Pest risks tend to be higher when the scale and frequency of cropping of the particular crop increases. Therefore, the expansion of protein crops may increase the risk of infestation by their crop-specific pests and diseases. An example is the pea moth (Cydia nigricana), where infestation levels have increased along with the expansion of pea production (Huusela-Veistola & Jauhiainen Reference Huusela-Veistola and Jauhiainen2006). As protein crop areas increase, pest problems and the need for insecticides will probably be enhanced further by climate change and parallel cropping of spring and winter rapeseed (Hakala et al. Reference Hakala, Hannukkala, Huusela-Veistola, Jalli and Peltonen-Sainio2011). This is problematic because even at the present time, excessive use of pyrethroid insecticides increases the risk of pyrethroid-resistant pest populations developing.
In Australia, expansion of pea area from 40000 ha in 1983 to 155000 ha in 1994 resulted in an average yield reduction of 0·30. This was attributed mainly to increases in complexes of various foliar diseases, including Mycosphaerella pinodes and P. medicaginis var. pinodella (Davidson & Ramsey Reference Davidson and Ramsey2000). In Europe, these pathogens cause a range of foot and root rots, which increase in short rotations with peas. The most detrimental increase in root rots is caused by soil-borne Aphanomyces euteiches in cropping systems where overall frequency of legumes is too high (Oyarzun et al. Reference Oyarzun, Gerlagh and Hoogland1993). An increase in the incidence of, and crop losses caused by, club root is the most obvious risk in expansion of cruciferous protein crops. In Finland, club root was previously relatively common, but limited to cruciferous vegetable production (Linnasalmi & Toiviainen Reference Linnasalmi and Toiviainen1991). The rapid increase in rapeseed production during the 1980s probably increased the proportion of club root-infested fields and symptomatic plants were found in up to 0·20 of inspected fields in a survey carried out in 1982–88 (Hannukkala Reference Hannukkala1988). In 2007–9 the proportion of club root-infected fields in Finland again increased to 0·30, though the level of infestation was reasonably low in most fields (Rastas et al. Reference Rastas, Hannukkala and Latvalaforthcoming). A similar increase in club root incidence coincided with an increase in rapeseed production elsewhere in Europe (Zegada-Lizarazu & Monti Reference Zegada-Lizarazu and Monti2011) and Canada (Donald & Porter Reference Donald and Porter2009; Howard et al. Reference Howard, Strelkov and Harding2010).
All the above-mentioned potential constraints to unit area yield increase suggest that it will be difficult to intensify protein crop production sustainably at the same time as cultivation areas are expanded. The intensification effect could be consequential in the sense that ecological services provided by protein crops enhance yields of other crops, such as the cereals that are often grown in monocultures. For example, the beneficial effects of rapeseed on yields of the subsequent crop mainly result from its capacity to break the cereal monocultures (Kirkegaard et al. Reference Kirkegaard, Simpfendorfer, Holland, Bambach, Moore and Rebetzke2004; Smith et al. Reference Smith, Kirkegaard and Howe2004), because cereals and rapeseed lack common pests and diseases. Furthermore, including tap-rooted rapeseed in rotations may improve soil physical conditions where soils are not yet too heavily compacted (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Laitinen, Salopelto, Saastamoinen and Hannukkala2011b). In fact, it is possible or even likely that during the transition to larger areas, simply maintaining the current yield levels of protein crops per unit area will represent a challenge. However, the declines in rapeseed yield recorded since the late 1990s were not attributable to the large increases in rapeseed production in northern growing areas such as EPOH (Fig. 3) during recent decades (Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen and Hannukkala2007). Therefore, for farmers to commit to national food security and feed strategies in the long term, incentives, insurance or other mechanisms are needed to protect against crop failures and marked yield variability, especially during the early phase of transition. In addition to the average yield, it is important that the risks associated with cultivation are kept under control. In 1995–2010, the coefficient of variation for between-year national average pea yield/ha was 0·17, whereas that for average rapeseed yield was below 0·13. These figures suggest a higher yield risk incurred by growing legumes and rapeseed than barley with its coefficient of variation at 0·11. However, this does not necessarily imply a higher income risk associated with legumes than with grains, as in some cases the gross margin may be higher than that for grains.
In order for farmers to commit to national food security and feed strategies in the long term, stronger economic incentives for cultivation are needed. Public policies could create options for higher revenues (e.g. area payments or marketing support to increase sale prices of protein crops) or aiming at lowering production costs per unit yield. Production costs of legumes could be reduced effectively by supporting the production of high-quality seed (because seed can account for c. 0·70 of the variable costs of grain legume production; Juntti et al. Reference Juntti, Pihamaa and Heikkilä2005), and by promoting the use of more efficient production technology (including land improvements and plant breeding investments). Fertilizer and crop protection are important items for cost reduction in rapeseed production. Measures to lower per unit production costs rather than output value are more viable because an increase in the yield revenue also increases feed prices and can thus reduce the competitiveness of livestock production (e.g. Niemi Reference Niemi2006). Feed is the primary use of leguminous protein crops. As crop rotation is an important aspect in protein crop production, promoting efficient crop rotation and collaboration among farms (such as specialized feed production to gain economies of scale and temporary exchange of land parcels between farms to facilitate efficient crop rotation) can be an important policy tool, particularly in the short term.
Another important policy option is to support and enhance the knowledge of farmers by providing information (e.g. education programmes, research and development). Due to higher yield risk, risk management mechanisms are also needed to protect against crop losses. Crop farmers, like others, are typically risk-averse (Pratt Reference Pratt1964; Arrow Reference Arrow1965; Koundouri et al. Reference Koundouri, Laukkanen, Myyrä and Nauges2009). This implies that risk represents a cost. Policies could, for instance, support the use of risk-reducing inputs or provide a safety net in the event of crop loss. Currently a safety net against severe crop damage is organized through the national crop damage compensation scheme. Public policies could also co-finance yield insurance or a fund, should it be available, to reduce costs associated with annual yield variation. However, policy measures must take into account international agreements and public support.
HOW DO ESTIMATED INCREASES IN TOTAL PRODUCTION MATCH WITH PROTEIN IMPORTS?
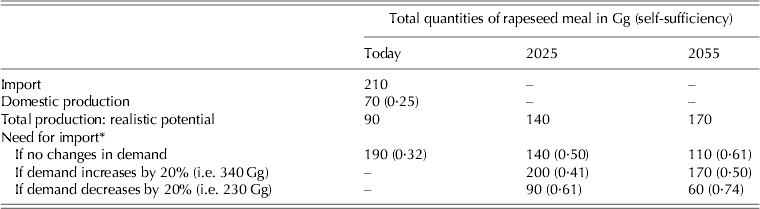
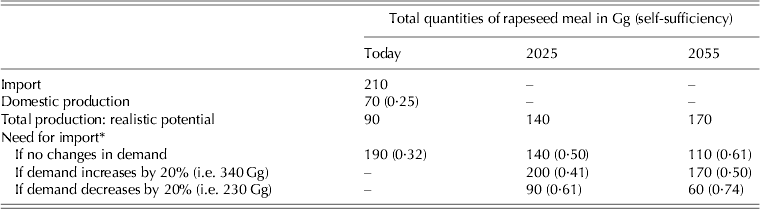
Rapeseed and rapeseed meal together are imported into Finland in larger quantities than soybean meal (Table 4). When estimating the potential for increasing self-sufficiency in rapeseed meal production, imported quantities together with present domestic production set the reference value. When the estimations were based on realistic expansion potential and the assumption that yields are not going to increase due to reasons given above, no case or scenario could be characterized according to which domestic rapeseed production could completely substitute for present import levels. By successfully and fully expanding the rapeseed production area to correspond with the realistic potential area for Finland (Table 6), self-sufficiency in rapeseed meal production could increase from some 0·25 up to 0·32 (Table 7).
Table 7. Import and domestic production of rapeseed meal (Gg) and estimates for total production capacities according to realistic potential area for today, 2025 and 2055 (centred mean over 30 years) in the case that mean yields/ha do not change compared with those of the 2000s. Three scenarios were used: animal production and need for rapeseed based protein remain the same ones as during recent years, or need for rapeseed meal increases or decreases by 20%. Estimate for rapeseed meal self-sufficiency is shown in parentheses for all scenarios. Rapeseed is transformed to correspond to rapeseed meal by multiplying the seed quantities by 0·60
* According to realistic potential for expanded area without changes in yield levels.
– : no relevance.
Due to possible climate change-induced increases in the (northern) cropping area, rapeseed meal self-sufficiency could further increase to 0·50 by 2025 and 0·61 by 2055 if the present demand for rapeseed meal reflects that of the future. However, in the case that the future need for rapeseed meal increases by 20%, estimated self-sufficiencies would decline to 0·41 and 0·50, respectively. However, an equal (20%) decline in demand would increase rapeseed meal self-sufficiency to 0·61 by 2025 and even 0·74 by 2055. This would mean that a 40–50% reduction in the future demand for rapeseed meal would correspond with the estimated future domestic production potential, i.e. to gain full self-sufficiency in rapeseed production. By 2025 and 2055, spring rapeseed will probably dominate, though in the middle of the century winter types may also contribute to total rapeseed production since winters are projected to get milder due to climate warming (Jylhä et al. Reference Jylhä, Fronzek, Tuomenvirta, Carter and Ruosteenoja2008; Peltonen-Sainio et al. Reference Peltonen-Sainio, Jauhiainen, Hakala and Ojanen2009a).
The above estimates suggest that if enhanced domestic rapeseed production is used to substitute for imported rapeseed and rapeseed meal, imported soybean meal cannot be substituted by rapeseed. If substitution of soybean meal by rapeseed is reasonable, for example due to the negative attitude towards genetically modified soybean that currently characterizes global markets, rapeseed imports would not decline drastically despite the considerable estimated increases in domestic production. In fact, rapeseed is already the protein source that largely substitutes for soybean in industrial feed mixtures (especially so for cattle) due to its optimal protein quality in grass silage-based diets (Shingfield et al. Reference Shingfield, Vanhatalo and Huhtanen2003; Huuskonen et al. Reference Huuskonen, Khalili and Joki-Tokola2007).
FAPRI (2011) estimated that the price of both soybean and rapeseed will decline by 2020. However, the projected decrease in the price of rapeseed meal will be much more rapid than the decrease in the price of soybean meal. In fact, soybean meal prices are projected to decrease quite modestly within the next 10–15 years. By contrast, USDA (2011) projects the price of soybean meal to decrease more rapidly than estimated by FAPRI (2011). OECD-FAO (2011) has also projected a downward overall trend in the prices of protein meals. In each case, the decrease is associated with increased production of bioenergy, which also increases the production of distiller's by-products. These estimates suggest that there may be greater economic incentives to use domestic rapeseed production to reduce soybean meal imports rather than to reduce rapeseed meal imports into Finland. This view can be supported also by the fact that there may be surplus of European rapeseed meal available.
The price of feed is a key to the competitiveness of livestock production. For instance, Niemi et al. (Reference Niemi, Sevon-Aimonen, Pietola and Stalder2010) estimated that a 10% increase in the price of soybean meal would reduce returns on the area occupied by one pig by almost €60. This is quite a significant amount because the cost of building a pig unit is typically c. €500–600 per capita (updated from MAF 2006). Further simulations using a similar approach suggest that there are economic incentives, especially to substitute pea seed for soybean meal. The benefit of pea is its traditionally rather low price.
When comparing with current imports of soybean meal (c. 180 Gg/year), it seems that the estimated realistic potentials for enhanced, domestic grain legume production could, in principle, replace soybean. The total production volumes for grain legumes, according to the realistic potential for expansion at current yield levels, were estimated to be 180, 340 and 400 Gg for 2011, 2025 and 2055, respectively. With typical protein contents these production quantities correspond to c. 70 Gg protein (c. 0·38 protein content) in soybean meal, and 40–50 Gg protein in current domestic grain legumes, 75–100 Gg for 2025 and 90–115 Gg for 2055 (depending on balance between production of peas with c. 0·22 and faba bean with c. 0·29 of seed protein). If the protein contents differ from the means shown above that instantly affects replacement capacity.
In current industrial feed mixtures, soybean meal contributes only 0·10 of used protein sources for cattle feed, 0·50 for pig feed and 0·40 for poultry feed, with total national production of 600, 350 and 300 Gg of feed mixtures, respectively (Tike: Information Centre of the Ministry of Agriculture and Forestry 2012). Soybean meal is replaced mainly by rapeseed meal. Peas and faba bean would also be possible substitutes if their production were successfully expanded. With regard to maximum substitution proportions, protein content and quality (amino acid composition) are essential traits.
Despite the lower crude protein content of peas, but only slightly lower protein digestibility compared with soybean meal, peas are considered an excellent energy and protein source for fattening pigs and gestating sows and for poultry layers and broilers. For fattening pigs and gestating sows, it is possible to replace all soybean meal with peas as the main protein source, if the diets are balanced for the needs of ileal digestible amino acids, mainly methionine, the content of which is rather low in peas (Gatel et al. Reference Gatel, Grosjean and Leuillet1988, Reference Gatel, Grosjean and Castaing1989a; Grosjean et al. Reference Grosjean, Bastianelli, Bourdillon, Cerneau, Jondreville and Peyronnet1998; Partanen et al. Reference Partanen, Valaja, Jalava and Siljander-Rasi2001; Szabó et al. Reference Szabó, Jansman, Babinszky, Kanis and Verstegen2001). For layers and broilers, 0·60–0·70 of soybean meal could be replaced by peas (Valkonen Reference Valkonen2005; Venäläinen Reference Venäläinen2005). Fortification of diets with methionine and tryptophan are needed for poultry when large amounts of peas are used. For piglets, pea protein concentrate seems to lower the digestibility of energy and protein, so it cannot replace soybean meal successfully (Valencia et al. Reference Valencia, Serrano, Centeno, Lázaro and Mateos2008). However, peas could be used up to 300 g/kg in feed without adverse effects if some soybean meal or other easily digestible protein sources were included in the feed (Gatel et al. Reference Gatel, Fekete and Grosjean1989b; Partanen et al. Reference Partanen, Siljander-Rasi and Alaviuhkola2006). For lactating sows, the maximum inclusion of peas in the diet would be c. 240 g/kg (Gatel et al. Reference Gatel, Grosjean and Leuillet1988), which corresponds to a replacement of about 0·70 of the soybean meal.
Faba beans with rapeseed meal can replace soybean meal when fattening pigs if digestible amino acids are balanced in the diet. During the growing period, however (up to c. 55 kg live weight), inclusion of more than 200 g/kg will reduce the growth performance of the pigs. For older fattening pigs, up to 260 g/kg faba bean in the diet, replacing all soybean meal, does not adversely affect growth performance (Partanen et al. Reference Partanen, Alaviuhkola, Siljander-Rasi and Suomi2003). The digestibility of energy and protein components of faba bean is reduced because of the presence of tannins (Jezierny et al. Reference Jezierny, Mosenthin and Bauer2010). Tannins also reduce the palatability of faba bean, which is considered one reason why its inclusion should be limited to 120 g/kg in piglet diets (Partanen et al. Reference Partanen, Siljander-Rasi and Alaviuhkola2006). According to Buron & Gatel (Reference Buron and Gatel1992) and Etienne et al. (Reference Etienne, Duee and Pastuszewska1975), faba bean would be suitable for gestating and lactating sows at proportions of 0·15 and 0·21 in feed, respectively. However, Etienne (Reference Etienne1977) suggested that faba bean in the sow diet would cause higher embryonic mortality than soybean meal. Updated information on sow feeding with faba bean is evidently needed. The use of faba beans in the diets of poultry is restricted due to the anti-nutritional factors vicine and convicine. For laying hens, only 0·05 faba beans could be used (Crépon et al. Reference Crépon, Marget, Peyronnet, Carrouée, Arese and Duc2010; Koivunen et al. Reference Koivunen, Valaja, Tuunainen, Valkonen, Ceylan, Ciftci and Adabi2011). There is, however, wide genetic variation between faba bean cultivars in vicine and convicine contents (Crépon et al. Reference Crépon, Marget, Peyronnet, Carrouée, Arese and Duc2010). Therefore, a potential increase in feed use of faba beans for poultry is possible.
In conclusion, many factors currently restrict expansion of rapeseed and legume production in Finland, which has >2 M ha of agricultural land, some 200000 ha of which could be devoted to protein crop production. This would mean a doubling of current production, mainly through expanded cultivation of highly neglected legumes, peas in particular. Rapeseed protein self-sufficiency could increase from the present 0·25 up to 0·32, and by 2025 and 2055 up to 0·50 and 0·60, respectively. In the case of legume production, expansion of domestically grown legumes according to present potential could replace 0·50–0·60 of imported soybean meal at the present time, and replace it completely by 2025. Replacing soybean meal is easily managed for cattle, to some extent for pigs, but represents a challenge for poultry.
The work was financed by the Finnish Ministry of Agriculture and Forestry as a part of an ongoing consortium project entitled ‘Strengthening domestic protein self-sufficiency under the pressure of global changes (OMAVARA)’. The authors are also grateful to the numerous projects (RYPSINOSTE, KOVAHERNE, MoniPalko, ILMASOPU and LIHAKETJU) that provided information and understanding used in estimations of protein crop production potentials. Harri Lilja is acknowledged for his help in using the national soil database.