INTRODUCTION
Understanding the temporal loss of nutrients from slow release fertilizers (defined as any material with plant nutritive value that is made available in time through microbial activity or chemical reaction influenced by temperature and moisture status) after application is critical in determining and designing practices to reduce and/or control nutrient losses. Animal manures are a viable slow release source of nutrients for row crop agriculture (Aina & Egolum Reference Aina and Egolum1980; Singh et al. Reference Singh, Rana, Kapur, Sharma and Bhandari1983; Masek et al. Reference Masek, Schepers, Mason and Francis2001; Moss et al. Reference Moss, Reeves, Lin, Torbert, McElhenney, Mask and Kezar2001; Bhadoria et al. Reference Bhadoria, Prakash, Kar and Rakshit2003; Yang et al. Reference Yang, Li, Mahli, Wang, Suo and Wang2004), forage crops (Burns et al. Reference Burns, Westerman, King, Overcash and Cummings1987; Huneycutt et al. Reference Huneycutt, West and Phillips1988; Edwards & Daniel Reference Edwards and Daniel1994a), and pasture lands (McLeod & Hegg Reference McLeod and Hegg1984; Owens et al. Reference Owens, Edwards and Van Keuren1984). Application of animal manures has also been shown to improve and enhance soil physical, biological, and chemical properties (Sweeten & Mathers Reference Sweeten and Mathers1985; Schjonning et al. Reference Schjonning, Elmholt, Munkholm and Debosz2002; Shirani et al. Reference Shirani, Hajabbasi, Afyuni and Hemmat2002; Marschner et al. Reference Marschner, Kandeler and Marschner2003; Plaza et al. Reference Plaza, Hernandez, Garcia-Gil and Polo2004; Wienhold Reference Wienhold2005). However, land-applied manure has been implied and shown to have negative water quality implications (Heathman et al. Reference Heathman, Sharpley, Smith and Robinson1995; McFarland & Hauck Reference McFarland and Hauck1995, Reference McFarland and Hauck1999; Eghball & Gilley Reference Eghball and Gilley1999; Sauer et al. Reference Sauer, Daniel, Nichols, West, Moore and Wheeler2000; Pote et al. Reference Pote, Reed, Daniel, Nichols, Moore, Edwards and Formica2001; Santhi et al. Reference Santhi, Arnold, Williams, Hauck and Dugas2001; Smith et al. Reference Smith, Jackson and Pepper2001; Gilley et al. Reference Gilley, Risse and Eghball2002; Tarkalson & Mikkelson Reference Tarkalson and Mikkelsen2004).
Available literature comparing organic and inorganic forms of fertilizer exists (Edwards & Daniel Reference Edwards and Daniel1994a, Reference Edwards and Danielb; Heathwaite et al. Reference Heathwaite, Griffiths and Parkinson1998; Eghball & Gilley Reference Eghball and Gilley1999; Eghball Reference Eghball2000) but nutrient loss from manure compared to commercially available slow release forms of fertilizers is not well documented. The primary objective of the present study was to compare and contrast the amount of N lost in overland flow from organic and inorganic fertilizers applied to grassland. Specifically, the goal was to determine the amount of ammonium-N and nitrate-N transported in overland flow from composted dairy manure, poultry litter, sulphur-coated urea, and ammonium nitrate applied to grassland and relate those losses to time after application.
MATERIALS AND METHODS
A three-factor block study was designed to test the null hypothesis that the amount and timing of NO3-N and NH4-N losses in plot scale surface runoff from manufactured fertilizers would not be different from like losses measured from natural products. The three factors were block, treatment, and time since application. Each block consisted of four plots. Within each block, one of four treatments was assigned to each plot. The treatments consisted of a one-time fertilizer application in the form of NH4NO3, sulphur-coated urea, composted dairy manure, or poultry litter.
Four run-over troughs (0·6 m wide×3·7 m long×0·3 m deep) were constructed in a greenhouse and filled with an Austin (fine-silty carbonatic, thermic, Typic Haplustolls) clay soil. The troughs were turfed with squares of commercially available dwarf bermudagrass and positioned on a 2·87° slope. A similar plot box approach was used successfully by McDowell & Sharpley (Reference McDowell and Sharpley2002) and Kleinman et al. (Reference Kleinman, Sharpley, Veith, Maguire and Vadas2004) to measure phosphorus losses in surface runoff. Kleinman et al. (Reference Kleinman, Sharpley, Veith, Maguire and Vadas2004) reported a strong correlation between phosphorus losses measured in the plot boxes and field data. Six 6·3 mm openings were positioned along the length of the plots to allow drainage of infiltrated water. The troughs were constructed with extended sidewalls that permitted the introduction and containment of overland flow. The overland flow was channelled to a 379 litre storage tank positioned on a 454 kg continuous recording floor scale. The overland flow events were simulated at 7-day intervals for a period of 10 weeks. Because of space limitations, only one block could be run at a time. The soil and turf were replaced in each trough prior to initiating each block run. With each block, the soil and turf were replaced in a consistent manner. Each trough was filled with soil to a depth of 0·15 m. The soil was levelled and lightly tamped by dropping a wooden board from 0·2 m. The turf was positioned, sprinkled with 14 litres of water, and tamped in an identical manner a second time to assure good contact with the soil surface.
Prior to fertilizer application, the plots were subjected to a 30 min overland flow event to obtain background nutrient concentrations. No significant (P>0·05) differences in background NO3-N or NH4-N concentrations by plot were measured (Table 1); however, background concentrations were considerably greater in blocks 2 and 3 compared to block 1. The turf was purchased from a commercial vendor and differences in background concentrations were attributed to the management of the turf prior to procurement. After the initial flush/run, each plot received a single fertilizer treatment in the form of NH4NO3, sulphur-coated urea, composted dairy manure, or poultry litter. Nutrient concentrations of the composted dairy manure and poultry litter (Table 2) were measured prior to each application to determine the mass of material to be added. The one time application rate was equivalent to 187 kg N/ha. The plots were irrigated once weekly at a rate of 6·3 mm volumetric depth, a volume equivalent to 6·3 mm deep over the entire surface area, or in this case 14·0 litres.
Table 1. Background NO3-N and NH4-N concentrations (mg/l) from each trough prior to treatment application for each block

Table 2. Concentration (g/kg) of NFootnote * in fertilizer used to calculate mass of material for 187 kg/ha application rate for composted dairy manure and poultry litter treatments used in each block
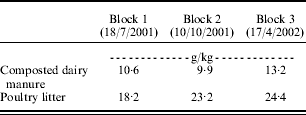
* Nitrogen proportion in NH4NO3 was 33·0 g/kg; sulphur-coated urea was 38·0 g/kg.
Overland flow (125 mm/h) was introduced weekly for a period of 30 min via a water dispersion device located further up the slope. The device was constructed of plastic pipe and designed to evenly distribute the water over the width of the plot. The overland flow source was potable tap water. At the conclusion of each overland flow event, water in the storage tank was agitated and a water sample was collected. The samples were acidified and refrigerated at 4°C until analysis. The samples were analysed colorimetrically for NO3+NO2-N and NH4-N concentrations using a Technicon Autoanalyzer IIC and methods published by Technicon Industrial Systems (1973a, b, 1976). From this point forward, NO3-NO2-N will be notated as NO3-N.
The pollutant concentration and the volume of water collected in the tank were used to calculate a total pollutant loss. Losses were not corrected for amount delivered in source water. Statistical analyses were performed on pollutant loss using a multifactor ANOVA and the statistical software Statgraphics (Manugistics 2000). The dependent variable was pollutant loss while block, treatment, and days since application were identified as factors. Treatment means, within each time period and means within each treatment over time were evaluated using the Tukey HSD statistic and a 0·05 significance level.
RESULTS
No significant differences (P>0·05) were noted in measured discharge volumes by treatment over time. The largest NO3-N loss was observed with the initial overland flow event, especially with respect to the ammonium nitrate treatment. The proportion of NO3-N loss resulting from the first overland flow event compared to the total measured loss was 0·40 for the ammonium nitrate treatment, 0·17 for sulphur-coated urea, 0·25 for composted dairy manure, and 0·26 for poultry litter. The losses were generally reduced with subsequent overland flow events. Time since application, treatment, and the interaction of time and treatment were all significant parameters in NO3-N loss (Fig. 1, Table 3). Thus, an analysis of treatment means within each treatment and time period was conducted.

Fig. 1. Mean (n=3) NO3-N loss by treatment for the 10-week period of study. Treatments include ammonium nitrate (closed circles), sulphur-coated urea (open circles), composted dairy manure (closed triangles), and poultry litter (open triangles). Treatment (P<0·001), s.e.=0·42, days since application (P<0·001), s.e.=0·66, and interaction of treatment and days (P<0·001), s.e.=1·32, were significant.
Table 3. Mean (standard deviation) NO3-N loss by treatment and time following application

NO3-N loss from composted dairy manure and poultry litter treatments was consistently less than the loss from ammonium nitrate and sulphur-coated urea treatments during all sampling points of the 10-week study. Significant differences (P<0·05) in NO3-N losses through time were measured for ammonium nitrate and composted dairy manure treatments (Table 3). Measured NO3-N loss from the ammonium nitrate treatment significantly decreased until 21 days after application, while the initial NO3-N loss from the composted dairy manure treatment was not significantly reduced until 28 days after application. No significant difference (P>0·05) was measured in NO3-N loss over time from the sulphur-coated urea or poultry litter treatments.
Similar to the measured NO3-N losses, the measured NH4-N losses in the first event, expressed as a proportion of the total losses, were 0·34 for ammonium nitrate, 0·22 for sulphur-coated urea, 0·15 for composted dairy manure, and 0·36 for poultry litter. Treatment and time since application were identified as significant (P<0·05) parameters defining NH4-N losses (Fig. 2, Table 4). However, interaction between treatment and time since application was not found to be significant (P>0·05). Significant (P<0·05) differences were measured in NH4-N losses through time for ammonium nitrate, sulphur-coated urea, and poultry litter (Table 4) treatments. These differences were measured at 21 days after application for the manufactured fertilizers, ammonium nitrate and sulphur-coated urea, and 14 days in the poultry litter treatment. No differences were measured in NH4-N loss from composted dairy manure through time. The only measured treatment difference (P<0·05) in NH4-N loss through time occurred 5 weeks after application (Table 4). During that time period, the ammonium loss from sulphur-coated urea was significantly greater than that measured from the composted dairy manure.

Fig. 2. Mean (n=3) NH4-N loss by treatment for the 10-week period of study. Treatments include ammonium nitrate (closed circles), sulphur-coated urea (open circles), composted dairy manure (closed triangles), and poultry litter (open triangles). Treatment (P<0·05), s.e.=0·102, and days since application (P<0·001), s.e.=0·162, were significant. Interaction of treatment and days since application (P<0·55), s.e.=0·325, was not significant.
Table 4. Mean (standard deviation) NH4-N loss by treatment and time following application

Cumulative NO3-N and NH4-N losses for the manufactured fertilizers and livestock manures are highlighted in Figs 3 and 4. A significant difference (P<0·05) in cumulative NO3-N losses for the 10-week period was measured between manufactured and livestock manure fertilizers (Fig. 3). Cumulative NH4-N losses (Fig. 4) were a magnitude less than the cumulative NO3-N losses but no significant differences (P>0·05) in cumulative NH4-N losses were measured. NO3-N recovered in surface runoff totalled 68·2 kg/ha (a proportion equivalent to 0·37 of applied N) from the application of ammonium nitrate. Cumulative NO3-N recovered in surface runoff from sulphur-coated urea application was 46·1 kg/ha (0·25 of applied N). The cumulative NO3-N losses measured from the ammonium nitrate and sulphur-coated urea treatments were significantly greater (P<0·05) than those same losses measured from composted dairy manure and poultry litter treatments. The cumulative NO3-N amount recovered from composted dairy manure was 19·3 kg/ha while the amount recovered from poultry litter treatment was 13·7 kg/ha. Cumulative losses from composted dairy manure and poultry litter treatments were not significantly different (P>0·05).

Fig. 3. Cumulative NO3-N recovered (n=3) in the overland flow for the 10-week study period. Nitrogen application rate was equivalent to 187 kg/ha. s.e.=25·07.

Fig. 4. Cumulative NH4-N recovered (n=3) in the overland flow for the 10-week study period. Nitrogen application rate was equivalent to 187 kg/ha. s.e.=7·73.
DISCUSSION
The proportions of nutrients recovered in the first runoff event are consistent with those reported by Linde & Watschke (Reference Linde and Watschke1997) and Easton & Petrovic (Reference Easton and Petrovic2004) on turf systems. In each of those studies, the greatest loss of applied nutrients was measured in the first runoff event following application. This result suggests that the initial period following application of these fertilizers would be the most vulnerable time for surface runoff losses and highlights the need to make fertilizer applications in accordance with weather forecasts, namely delaying application when rainfall probability is high. Additionally, this finding emphasizes the need to be selective when choosing fertilizer type.
When selecting fertilizer type, a holistic approach that considers both environmental and economic impacts should be used. Of the manufactured products used in the present study, ammonium nitrate is the most economical and rapidly acting. But from a crop production standpoint, ammonium nitrate provides the greatest crop scorch potential. Additionally, if the crop dictates that the fertilizer is applied in one dosage, the risk for surface loss increases. As noted here, the initial and cumulative NO3-N and NH4-N losses were greatest for the ammonium nitrate treatment.
Of the manufactured products, sulphur-coated urea is a slow release alternative source of nitrogen to ammonium nitrate. One of the primary advantages of using a slow release fertilizer is reducing the risk or availability for surface loss. However, by reducing the potential for surface runoff loss, the release of nitrogen available for plant use is also delayed. In the current study, sulphur-coated urea was effective at reducing the initial vulnerability to NO3-N and NH4-N losses but over the length of the study the magnitude of NO3-N and NH4-N losses was not statistically different from measured losses from the ammonium nitrate treatment.
In the case of sulphur-coated urea, the NO3-N losses in time were not statistically different, suggesting a consistent low level of available N. Under warm moist conditions, urea hydrolyses to form ammonium, which is available for plant uptake or transport in runoff. The NH4-N in the runoff from the sulphur-coated urea plot levelled off 14 days after application, suggesting that the sulphur coating may be degraded within the first 7 days. While NH4-N has been shown to be a major component of the inorganic N lost in runoff when rainfall occurs immediately after fertilizer application (Torbert et al. Reference Torbert, Potter and Morrison1996, Reference Torbert, Potter, Hoffman, Gerik and Richardson1999), relatively small amounts of N were lost in the NH4-N form compared to NO3-N in the present study. In the case of the manufactured fertilizers, a large portion of the N applied in the fertilizer would have been in the NH4-N form with all N in the sulphur-coated urea application and half of the N in the ammonium nitrate application initially in NH4 form.
Like sulphur-coated urea, composted dairy manure and poultry litter are considered slow release fertilizers. They have an advantage of providing some micronutrients and also are associated with a low risk of crop scorch. The disadvantages of manures include the relatively high rates that need to be applied to supply sufficient nutrients, the handling requirements, potential odour nuisance and the sometimes slow release of nutrients. This means that nutrient supply is not always consistent with crop needs. Additionally, if manures are regularly applied to the same cropping area on the basis of crop nitrogen requirement, a build-up of soil phosphorus is likely.
NH4-N is the first inorganic N form to be released from the decomposition of organic matter. However, NH4-N in soil will be adsorbed onto the clay particles and the NH4-N in soil solution will quickly be converted to NO3-N. Under the growing conditions of the sward examined in the present study, only small amounts of N were lost in the NH4-N form compared to the NO3-N form, suggesting that losses of the more mobile and available NO3-N may be of greater concern in the management of overland flow in grassland production. Compared to measured NO3-N losses from ammonium nitrate application, losses from composted dairy manure and poultry litter applications were consistently less throughout the study and significantly less until 42 days after application. These findings were consistent with those of Easton & Petrovic (Reference Easton and Petrovic2004). Conversely, while small in magnitude, the initial NH4-N losses from poultry litter were greater than the initial losses of composted dairy manure and sulphur-coated urea. In addition, the initial fraction of NH4-N loss compared to the cumulative total loss was greatest for poultry litter when compared to all other treatments.
The ammonium transported in runoff from the composted dairy manure was somewhat consistent for the 10-week study. The consistent low-level release is one of the distinct advantages of composting. During the composting process, nutrients in manure are stabilized by micro-organisms into organic compounds that decompose more slowly and are therefore released more slowly over time (The Composting Council 1996). The slow release of nutrients from the animal manure products tested here would potentially result in a significantly smaller contribution of N loss in overland flow to the environment compared to manufactured fertilizer products. This is consistent with the findings of Easton & Petrovic (Reference Easton and Petrovic2004), which indicated that manufactured fertilizer sources produced higher NO3-N concentrations and fluxes. Since the same amount of total N was applied with each of the fertilizer treatments, it can be concluded that the application of N through animal manure will be less vulnerable to the initial losses of N to the environment. However, from a crop production viewpoint and depending on the plant nutrient requirements, if the manures were the only form of fertilizer applied, the plant would most likely be stressed due to nitrogen deficiency.
CONCLUSIONS AND RECOMMENDATIONS
The main conclusion from the present work is that, from an environmental perspective, the initial surface runoff loss of NO3-N and NH4-N from application of composted dairy manure and poultry litter pose less of an environmental risk than application of manufactured fertilizer products. Based on the results from the present experiment the null hypothesis was rejected. A significant difference (P<0·05) in cumulative (10-week) NO3-N loss was measured between manufactured (NH4NO3 and sulphur-coated urea) and natural products (composted dairy manure and poultry litter) but no significant difference (P>0·05) in total (10-week) NH4-N loss was measured between treatments; however, NH4-N loss from composted dairy manure was less than half when compared to all other treatments. Additionally, significant differences (P<0·05) in NO3-N loss through time were measured for two of the treatments (ammonium nitrate and composted dairy manure). All differences were measured within 21 days after treatment. Measured NH4-N losses through time were significant (P<0·05) for ammonium nitrate, sulphur-coated urea, and poultry litter. NO3-N recovered in the surface runoff, expressed as a proportion of applied N, was 0·37 for ammonium nitrate, 0·25 for sulphur-coated urea, 0·10 for composted dairy manure, and 0·07 for poultry litter. Cumulative NH4-N losses were an order of magnitude less than NO3-N losses.
Based on the results from the present study the following recommendations can be suggested. First, fertilizer application should be made based on weather forecasts in an effort to reduce or avoid initial losses. Second, a holistic approach, which considers both the environmental and economic implications, should be taken when selecting fertilizers. Selection should be made based on availability, potential for initial and cumulative losses, and crop requirements. Third, an integrated approach that considers the combination of animal manures and manufactured fertilizers should be considered. The potential benefits of such an integrated approach include reduced risk of runoff losses, reduced risk of possible crop yield reductions resulting from nitrogen deficiency when using animal manure as the sole source of nitrogen and improved nutrient balance.
The authors would like to acknowledge and thank Robert Chaison, Georgie Mitchell, and Ron Whitis for their respective analytical, technical, and engineering assistance in this research effort.










