Introduction
Despite ongoing research during the last few years, tail-biting is still an important challenge in both conventional and alternative husbandry systems (Walker and Bilkei, Reference Walker and Bilkei2006; EFSA, 2007; Sonoda et al., Reference Sonoda, Fels, Oczak, Vranken, Ismayilova, Guarino, Viazzi, Bahr, Berckmans and Hartung2013), although its prevalence is higher under conventional husbandry conditions (McGlone et al., Reference McGlone, Sells, Harris and Hurst1990; Cox and Cooper, Reference Cox and Cooper2001; Walker and Bilkei, Reference Walker and Bilkei2006). In the past, most farmers have docked the tails of their piglets within the first days of life to prevent tail-biting, which was only a cure for the symptom but did not solve the cause (Valros et al., Reference Valros, Munsterhjelm, Hänninen, Kauppinen and Heinonen2016). However, routinely executed tail-docking is prohibited by the Council Directive 2008/120/EG and the ‘German Order for the Protection of Production Animals used for Farming Purposes and other Animals kept for the Production of Animal Products’ (German designation: Tierschutz-Nutztierhaltungsverordnung) (TierSchNutztV, 2017). However, tail-docking of single animals is allowed with an exemption to prevent this animal or other animals from damage.
All forms of tail-biting show a multifactorial genesis as described in the literature (Moinard et al., Reference Moinard, Mendl, Nicol and Green2003; Sonoda et al., Reference Sonoda, Fels, Oczak, Vranken, Ismayilova, Guarino, Viazzi, Bahr, Berckmans and Hartung2013; D'Eath et al., Reference D'Eath, Arnott, Turner, Jensen, Lahrmann, Busch, Niemi, Lawrence and Sandøe2014; Valros et al., Reference Valros, Munsterhjelm, Hänninen, Kauppinen and Heinonen2016), and until today no adequate measure against tail-biting has been found. However, the occurrence of stressful situations is an important factor which has been identified to cause tail-biting (Munsterhjelm et al., Reference Munsterhjelm, Simola, Keeling, Valros and Heinonen2013). Stress in weaning pigs is caused by several influences, for instance, management factors such as separation from the sow (Scheffler et al., Reference Scheffler, Stamer, Traulsen and Krieter2014), a change of diet caused by the lack of suckling milk (Hötzel et al., Reference Hötzel, de Souza, Costa and Machado Filho2011), the movement to another pen (Hötzel et al., Reference Hötzel, de Souza, Costa and Machado Filho2011) and contact with new pen mates (Weary et al., Reference Weary, Jasper and Hötzel2008). Furthermore, environmental parameters such as climate (Taylor et al., Reference Taylor, Main, Mendl and Edwards2010) or intrinsic factors such as health status (Schrøder-Petersen and Simonsen, Reference Schrøder-Petersen and Simonsen2001) play major roles in the genesis of tail-biting. One measure to reduce the risk of a tail-biting outbreak prescribed in the Council Directive 2008/120/EG is free access to a sufficient amount of manipulable material, which often consists of a high amount of crude fibre and satisfies the rooting behaviour of the pigs. Conventional piglet rations often contain a comparatively low amount of crude fibre. This is because raising the crude fibre quota reduces the energy density of the feed wherefore farmers worry about a decrease in daily growth performance (Edwards, Reference Edwards2003; Presto Åkerfeldt et al., Reference Presto Åkerfeldt, Nihlstrand, Neil, Lundeheim, Andersson and Wallenbeck2018). However, crude fibre has positive effects on the intestinal tract (Wenk, Reference Wenk2001; Holinger et al., Reference Holinger, Früh, Stoll, Graage, Wirth, Bruckmaier, Prunier, Kreuzer and Hillmann2018), reduces pen mate manipulation (Holinger et al., Reference Holinger, Früh, Stoll, Graage, Wirth, Bruckmaier, Prunier, Kreuzer and Hillmann2018) and therefore could reduce the risk of tail-biting. The feeling of satiety is prolonged due to longer retention time in the stomach and the longer time for digestion (de Leeuw et al., Reference de Leeuw, Bolhuis, Bosch and Gerrits2008; da Silva et al., Reference da Silva, van den Borne, Gerrits, Kemp and Bolhuis2012). Furthermore, crude fibre decreases the production of stomach acid, which improves the stomach's health by reducing the risk of gastric ulcers (Di Martino et al., Reference Di Martino, Capello, Scollo, Gottardo, Stefani, Rampin, Schiavon, Marangon and Bonfanti2013; Holinger et al., Reference Holinger, Früh, Stoll, Graage, Wirth, Bruckmaier, Prunier, Kreuzer and Hillmann2018). In the gut, crude fibre stimulates the intestinal wall mechanically; hence, intestinal motility is improved. Moreover, crude fibre is not able to pass the intestinal wall. Due to the water-binding capacity of crude fibre, water is kept in the intestinal lumen (van Leeuwen and Jansman, Reference van Leeuwen and Jansman2007; da Silva et al., Reference da Silva, van den Borne, Gerrits, Kemp and Bolhuis2012). These two effects lead to improved faeces quality and prevent constipation (Wenk, Reference Wenk2001). Additionally, crude fibre serves as an energy source for positive gut bacteria such as lactobacilli or some coliform bacteria (Wenk, Reference Wenk2001). Especially the neutral detergent fibre fraction, which can be varied by selecting various crude fibre components, is needed for the microflora of the hind gut (Noblet and Le Goff, Reference Noblet and Le Goff2001). The pathogenic bacteria are inhibited competitively by supporting the positive gut bacteria (Wenk, Reference Wenk2001). This leads to lower production of toxins that damage the organism. Therefore, higher crude fibre content and optimized composition of crude fibre in the feed ration offers better conditions for gut microflora, prevents the genesis of gastric ulcers and reduces the production of toxins. Thus, it can lead to improved animal health. In this way, stress levels of the animals are lowered, which potentially reduces the risk of tail-biting processes (Newberry and Wood-Gush, Reference Newberry and Wood-Gush1988).
The aim of this study was to analyse the influence of crude fibre in the weaning pigs' ration on the occurrence of tail-biting during the rearing period. Therefore, two trials were conducted. In the first trial, a higher crude fibre content, and in the second, different crude fibre components were administered. We hypothesized that a higher content and optimized composition of crude fibre in piglets' ration reduces the stress level for the animals and thus leads to a reduction in tail-biting.
Material and methods
General aspects
The data collection was carried out on the Futterkamp agricultural research farm of the Chamber of Agriculture of Schleswig-Holstein from September 2016 until January 2017, whereby both trials were conducted consecutively. The animals were kept in accordance with the Council Directive 2008/120/EG, Council Directive 2010/63/EU and the ‘German Order for the Protection of Production Animals used for Farming Purposes and other Animals kept for the Production of Animal Products’ (TierSchNutztV, 2017).
Housing and animals
Five identical compartments inside the rearing area in the ‘Futterkamp farm’ were used for this study. Each compartment comprised eight pens (size: 1.70 × 2.80 m2, 0.4 m2 per piglet) for 12 weaning pigs each. The pens were equipped with the fully slatted plastic floor on which no permanently available bedding material was provided. One nipple drinker was mounted in each pen, allowing free access to water all time. The feeding system was mash feed ad libitum out of round troughs (diameter 50 cm) with an animal to feeding place ratio of 2:1, whereby a sensor measured the filling of the trough every 25 min and refilled it when needed between 6.00 a.m. and 10.00 p.m.. The temperature was automatically regulated in each compartment. It was set at 26.0°C at the beginning of the rearing period and afterwards decreased stepwise to 23.0°C over the first 30 days of the rearing period. Besides the natural light, the artificial lighting pursued no particular scheme; at night (between 7.00 p.m. and 6.00 a.m.) only an emergency illumination was available. The pens were each enriched with one cotton rope and one metal chain with a plastic element, as occupation material, which hung from the wall of each pen. Additionally, the piglets were given about 100 g chopped wheat straw per pen and day, which corresponded to a handful of material in the morning and the afternoon, placed on the bare pen floor.
Twelve undocked, non-castrated male and female crossbreed piglets (Pietrain × [Large White × Landrace]) were grouped together in one pen (space allowance: 0.40 m2 per piglet), resulting in 96 piglets in each compartment. The piglets were weaned after an average suckling period of 28 days with a weight of 7.9 kg (± 1.1 kg) on average. The piglets had increased their weight to 26.5 kg (± 4.3 kg) on average by the end of the 40-days rearing period.
Experimental design
The study was conducted during the rearing period and consisted of two trials: soya hulls were added to the piglets' ration to achieve different amounts of crude fibre for the first trial of the study. The second trial referred to different crude fibre components. The trials were planned identically and executed consecutively. Each trial consisted of five batches, which were performed simultaneously but with a shift of 1 week due to the farrowing rhythm. Thereby, each batch lasted 40 days. The batches included eight pens with twelve animals per pen. The compartments were subdivided into four treatment groups considering two pens per treatment group in each batch, which were randomly located inside the compartment for each batch. The piglets were randomly selected after weaning. Overall, 960 piglets were used in the present study with 480 piglets per trial and 96 piglets per batch. Each treatment group accounted for 120 piglets.
Until weaning, all piglets were fed the same pre-starter. During the rearing period, all piglets received the same three-phased basic diet (Table 1). The feed of phase I was fed from day 1 to 13, phase II lasted from day 17 to 28 and phase III from day 32 to 40. A three-day transition was performed at each change of feed, whereby 0.80 of the previous feed and 0.20 of the new feed were fed on the first day, 0.50 of each feed on the second day and 0.20 of the previous feed and 0.80 of the new feed on the third day. This basic diet contained a crude fibre content of 34 g/kg in phase I and 40 g/kg in phases II and III. Additionally, the basic diet was enriched with different feed supplements, depending on the treatment group. These were mixed into the ration, so the piglets were not able to select them.
Table 1. Analysed composition of the basic diet during the three feeding phases and the different feeding supplements
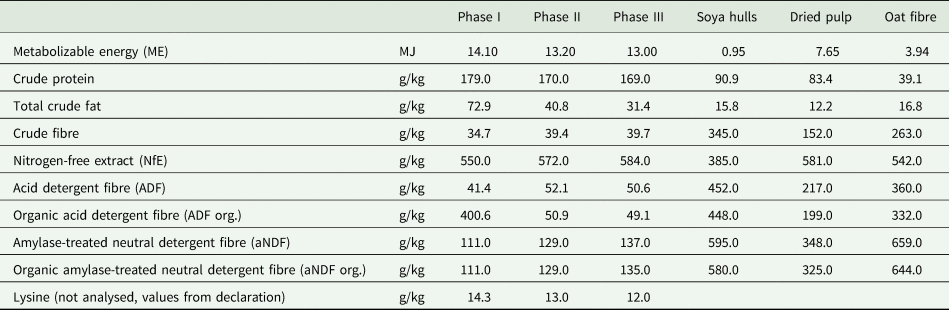
Values are given as a fed basis.
Trial one – different amounts of crude fibre
In the first trial, the fibre content was increased by admixing soya hulls (Table 1) to the basic diet, or alternatively by providing ad libitum in separate piglet bowls. The piglets of the control group (CG1) were given only the basic diet. Three treatment groups were used: G5, G6 and AL. The piglets of treatment group G5 were given 49 g/kg soya hulls in feeding phase I, and 33 g/kg admixed to their ration in feeding phases II and III to achieve a total crude fibre content of 50 g/kg. The piglets of treatment group G6 received 81 g/kg soya hulls in feeding phase I, and 66 g/kg admixed to their ration in feeding phases II and III, which resulted in total crude fibre content of 60 g/kg. The piglets of treatment group AL received soya hulls pressed to pellets ad libitum in separate piglet bowls as additional crude fibre provision. These piglet bowls were filled up twice a day to guarantee uninterrupted access to soya hull pellets.
Trial two – different components of crude fibre
The second trial referred to three different crude fibre components, so three treatment groups were formed in addition to the control group (CG2), which received the same ration as CG1. The different treatment groups received either soya hulls (SS), dried pulp (DP) from sugar beet or oat fibre (OF) admixed to their ration. A crude fibre content of 60 g/kg was achieved in all three groups (Table 1).
Intervention measures in case of tail-biting
Jute bags, which hung from the pen wall, were provided as additional manipulable material in case of tail-biting. Additionally, the biter was separated from the group if identified during the daily animal observation routine. In case of severe tail-lesions, the bitten piglet was medically treated and, if deemed necessary by farm staff, separated as well. Animals that were separated from the group were excluded from the study.
In the present study, different numbers of piglets were removed due to animal losses or to maintain animal protection after a tail-biting outbreak. One piglet died in the first trial in treatment group CG1 and one piglet was identified as a biter in treatment group G6 and therefore removed from the group. In the second trial, the animal losses spaced out mostly evenly across all treatment groups, whereby five to eight piglets were removed from the study per treatment group. Six piglets were removed as victims in treatment group CG2; one piglet was identified as a biter and one piglet died during the study. Four victims and one biter were separated in the treatment group SS, two victims and three biters in treatment group DP and six victims and two biters in treatment group OF. Therefore, 29 g/kg of all study animals were removed for all indications.
Data collection
Two observers were trained to evaluate tail-biting lesions reliably and in good agreement by examining the tails of the piglets. Each trial was observed completely by solely one of the observers. The tails of all piglets were scored once a week, starting on the first day of the rearing period after piglets had been moved and regrouped, by means of two parameters: tail-lesions and tail-losses, according to the ‘German Pig Scoring Key’ (German designation: Deutscher Schweine Boniturschlüssel) (Anonymous, 2016) that includes four categories for tail-lesions (0: no lesion visible, skin is intact; 1: superficial lesions, points or lines; 2: small lesions, deeper skin lesion of small size, (max. size = tail diameter at respective location); 3: large lesions, deeper skin lesion of large size, (larger than tail diameter at respective location)) and five categories for tail-losses (0: original length, tail has natural length (ideally with flattened end, possibly with tassel); 1: partial loss, up to one-third of length missing; 2: partial loss, up to two-thirds of length missing; 3: partial loss, more than two-thirds of length missing; 4: complete loss (stump of max. 1 cm in weaned piglets)). Tail-lesions and tail-losses were scored for each piglet identified by its individual ear tag.
Statistical analysis
The statistical analysis of both trials of this study was performed in the same way, using the statistical software R (R Core Team, 2017) version 3.3.3. For analysing, the data were transformed from an individual pig basis into a pen-level basis by summarising data from all piglets within a pen. To determine the statistical model, several fixed effects (batch, treatment group, rearing week, pen and the interaction between batch and treatment group) were added stepwise to the model and evaluated by the AICC ‘Akaike's information criterion corrected’ (Hurvich and Tsai, Reference Hurvich and Tsai1989) and the BIC ‘Bayesian information criterion’ (Schwarz, Reference Schwarz1978) values. The model with the smallest AICC and BIC was chosen for further analyses of the tail-lesions and tail-losses.
Tail-lesions
The data of the tail-lesions were surveyed as multinomial scores. Due to low frequencies of scores, small and large lesions corresponding to scores 2 and 3 were summarized to a score of 2. The multinomial data of tail-lesions (score 0–2) were evaluated with multinomial marginal binomial models (Pipper et al., Reference Pipper, Ritz and Bisgaard2012). The analysis of the AICC and BIC values led to a final model containing the fixed effects batch (1–5), treatment group (trial one: CG1, G5, G6, AL; trial two: CG2, SS, DP, OF respectively), rearing week (1–6) and the interaction between batch and treatment group. The glm function of the R package stats (R Core Team, 2017) together with the functions glht and mmm of the R package multcomp (Hothorn et al., Reference Hothorn, Bretz and Westfall2008) was used to apply post-hoc-comparisons (P < 0.05) between factor levels.
Tail-losses
The data of the tail-losses at the end of the rearing period were surveyed as multinomial scores as well. However, only two classes for tail-losses (0: no tail-losses; 1: all degrees of tail-losses) were used due to the low frequencies of scores. The model of tail-losses, now binomial distributed, contained the fixed effects batch (1–5), treatment group (CG1, G5, G6, AL and CG2, SS, DP, OF, respectively) and the interaction between batch and treatment group. Thus, temporal development was not considered. The model was analysed using the glm function of the R package stats (R Core Team, 2017) together with the functions glht and mmm of the R package multcomp (Hothorn et al., Reference Hothorn, Bretz and Westfall2008) to apply post-hoc-comparisons (P < 0.05) between factor levels.
Results
Trial one – different amounts of crude fibre
Tail-lesions
Figure 1 delineates the influence of the rearing week on the occurrence of tail-lesions during the first trial of the study. The highest number of uninjured tails occurred in the middle of the rearing period with proportions of 0.91 in the third and 0.89 in the fourth rearing week, while a minimum of uninjured tails could be observed with a proportion of 0.69 in the last rearing week. Most lesions were superficial (0.28) at the beginning of the rearing period, whereby the proportion of superficial lesions decreased to 0.08 in the following 2 weeks and then increased continuously to 0.15 in the last rearing week. From the fifth week on, the proportion of small and large lesions rose to 0.15 at the end of the rearing period.
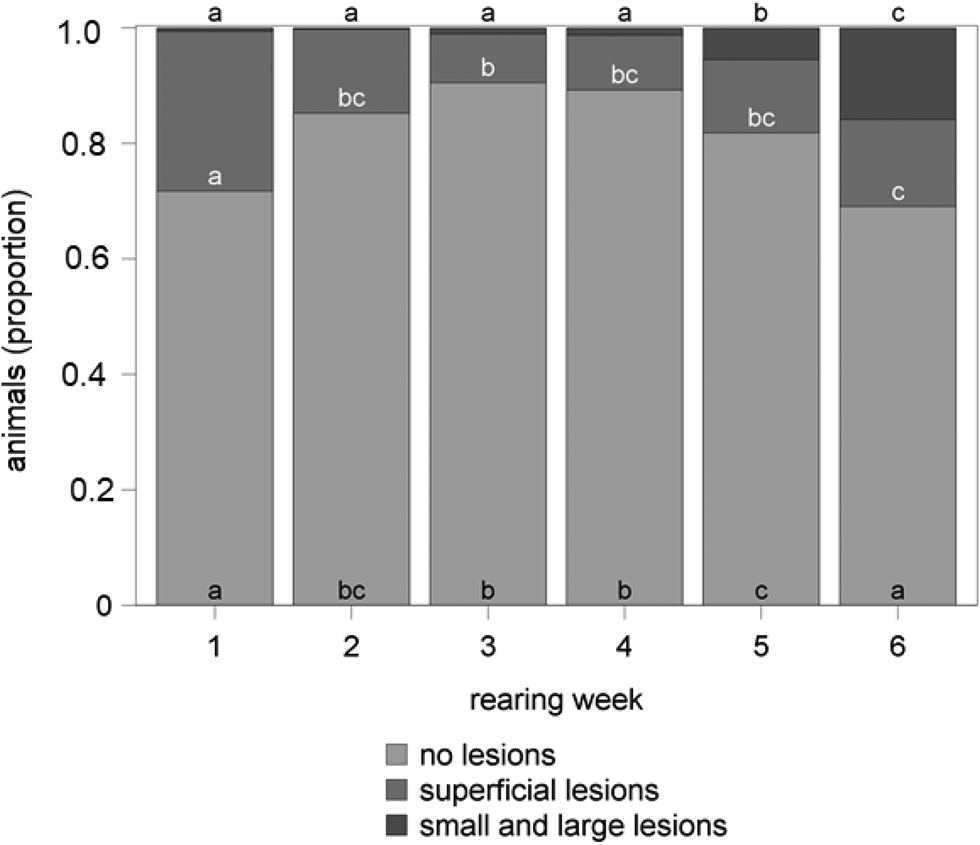
Fig. 1. Tail-lesions during the first trial of the study in accordance with the rearing week. Different letters mark significant differences (P < 0.05) between the rearing weeks.
Figure 2 delineates the tail-lesions in accordance with the interaction between batch and treatment group. Only a few significant differences could be found in the treatment groups. The highest proportion of uninjured tails (0.92) occurred in the 2nd batch, in treatment group G5. Focusing on the differences between the batches within one treatment group, it is noticeable that more differences occurred significantly between the batches than the treatment groups. Merely treatment group G5 had no significant differences concerning the uninjured tails and the superficial lesions. Focusing on small and large lesions, significant differences occurred merely in treatment group G6, whereby the 5th batch showed significantly more small and large lesions than the 1st and the 3rd batches.
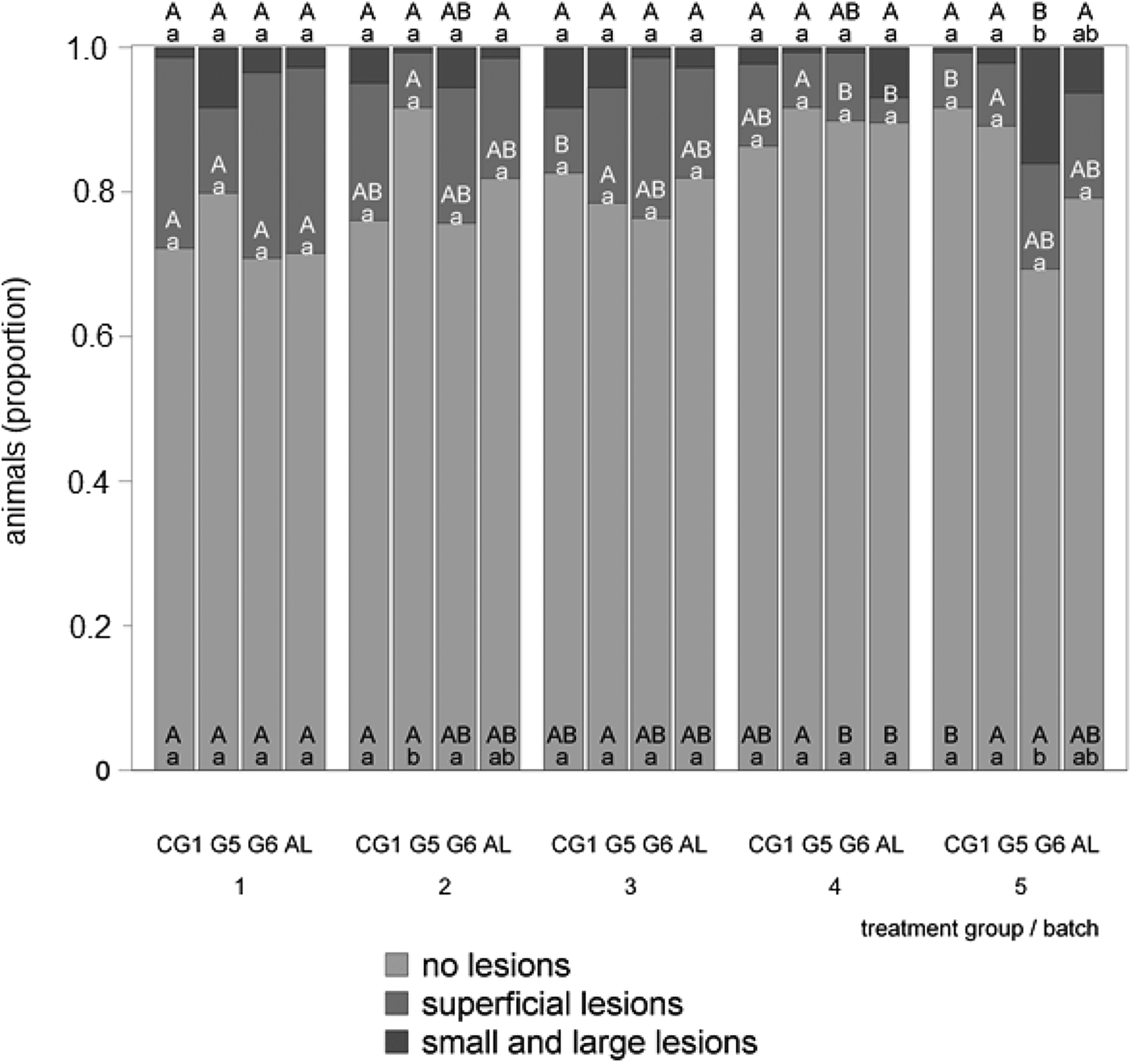
Fig. 2. Tail-lesions during the first trial of the study in accordance with the interaction between batch and treatment group. Lowercase letters mark differences between the treatment groups within one batch (P < 0.05) and capital letters mark differences between the batches within one treatment group (P < 0.05).
Tail-losses
The model investigating the influence of the interaction between batch and treatment group on the occurrence of tail-losses at the end of the rearing period showed that 0.08 of the piglets had tail losses in the 3rd batch in the control group CG1 and the 5th batch in treatment group AL, 0.21 in the 4th batch in treatment group AL and 0.46 in the 5th batch in treatment group G6. No tail-losses occurred in all treatment groups in the 1st and the 2nd batches. Treatment group G5 was the only group without tail-losses. Hence, no significant differences between the treatment groups and the batches were found.
Trial two – different components of crude fibre
Tail-lesions
Figure 3 delineates the influence of the rearing week on the occurrence of tail-lesions during the rearing period of the second trial of the study. The highest proportion of uninjured tails occurred in the second week after weaning (0.88). Afterwards, the rate of uninjured tails decreased to a minimum of 0.57 in the last rearing week. Figure 3 delineates that only a few small and large lesions occurred at the beginning of the rearing period. The highest proportion of small and large lesions occurred again at the end of the rearing period (0.38).

Fig. 3. Tail-lesions during the second trial of the study in accordance with the rearing week. Different letters mark significant differences (P < 0.05) between the rearing weeks.
Figure 4 delineates the number of tail-lesions in accordance with the interaction between batch and treatment group. Only a few significant differences could be detected in the treatment groups. The highest proportion of uninjured tails (0.95) occurred in the 1st batch in treatment group DP. However, no significant differences in superficial lesions existed. Significant differences in small and large lesions could be found merely in batches 1–3, whereby treatment group SS had the highest proportion of small and large lesions in the 1st batch (0.19), treatment group DP in the 2nd batch (0.28) and treatment group OF in the 3rd batch (0.24). More significant differences occurred between the batches than between the treatment groups as in the first trial of the study. In the control group CG2, the 1st batch showed significantly more uninjured tails (0.85) than batch 2 (0.61), as within treatment group DP where the 1st batch had significantly more uninjured tails (0.95) than all following batches. In the treatment group SS, the highest proportion of uninjured tails occurred in the 3rd batch (0.83) and the lowest in the 2nd batch (0.64). Significant differences in small and large lesions occurred in all treatment groups.
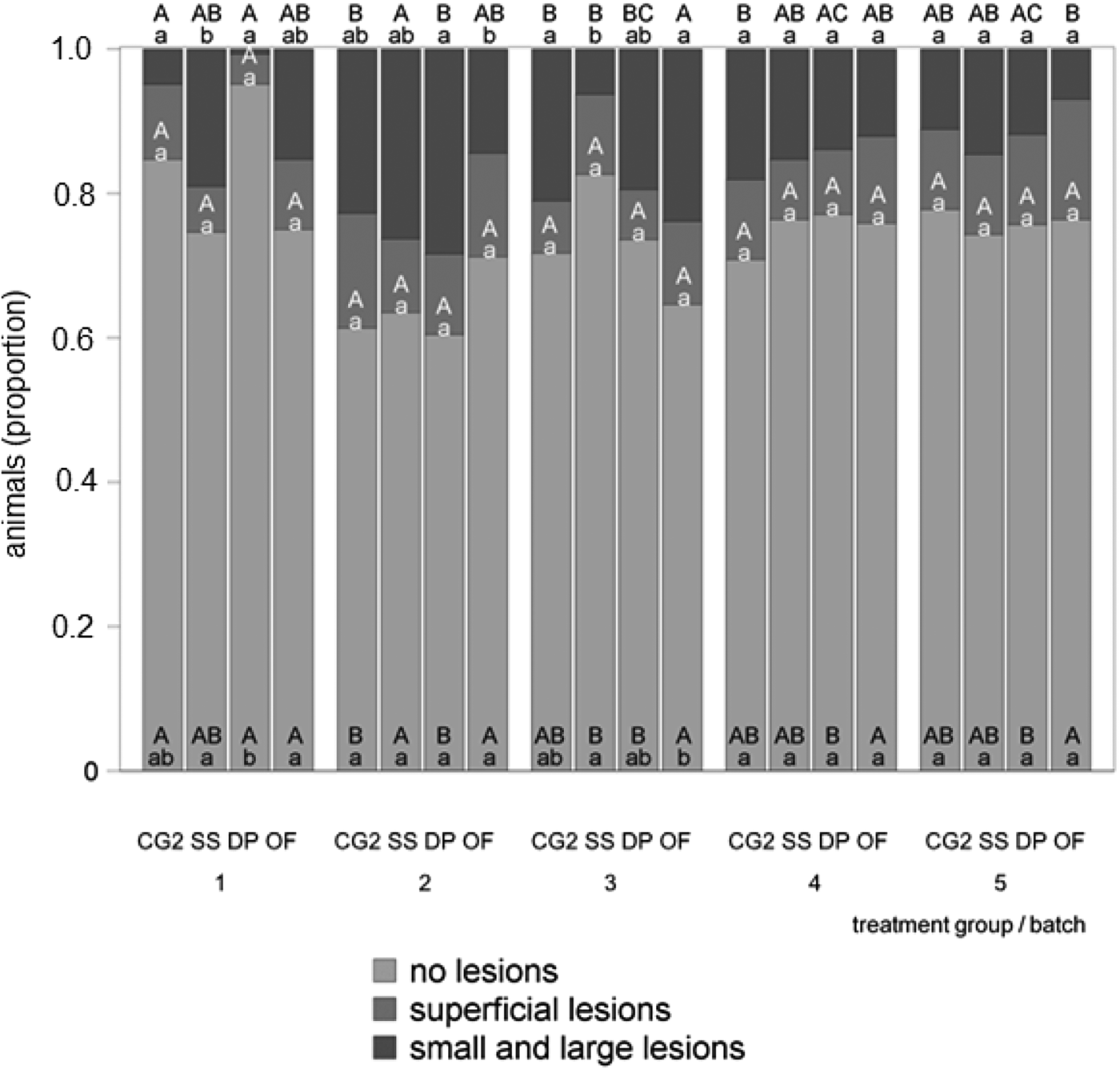
Fig. 4. Tail-lesions during the second trial of the study in accordance with the interaction between batch and treatment group. Lowercase letters mark differences between the treatment groups within one batch (P < 0.05) and capital letters mark differences between the batches within one treatment group (P < 0.05).
Tail-losses
The model investigating the influence of the interaction between batch and treatment group on the occurrence of tail-losses at the end of the rearing period showed that 0.28 (2nd batch), 0.32 (3rd batch), 0.04 (4th batch) and 0.00 (1st and 5th batch) of the piglets had tail losses in the control group CG2, 0.40 (2nd batch), 0.08 (4th batch), 0.04 (5th batch) and 0.00 (1st and 3rd batch) in treatment group SS, 0.31 (2nd batch), 0.22 (3rd batch) and 0.00 (1st, 4th and 5th batch) in treatment group DP and 0.22 (3rd batch), 0.08 (1st batch), 0.05 (2nd batch), 0.04 (5th batch) and 0.00 (4th batch) in treatment group OF. No significant differences between the treatment groups and the batches were found.
Discussion
In the current study, superficial lesions occurred from the beginning of the rearing period onwards (Figs 1 and 3). These tail-lesions could originate from the suckling period and therefore be evaluated in the first rearing week. However, many studies have not been able to observe tail damage during the suckling period (van Nieuwamerongen et al., Reference van Nieuwamerongen, Soede, van der Peet-Schwering, Kemp and Bolhuis2015), but 2–3 weeks after weaning (Abriel and Jais, Reference Abriel and Jais2013, Reference Abriel and Jais2014; Veit et al., Reference Veit, Traulsen, Hasler, Tölle, Burfeind, große Beilage and Krieter2016, Reference Veit, Büttner, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2017; Naya et al., Reference Naya, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2018). Another possible explanation is that these superficial tail-lesions are not caused by tail-biting but are scratches caused by rank fight during the first days of rearing. As literature shows, tail-biting often starts in the second to third week after weaning (Abriel and Jais, Reference Abriel and Jais2013, Reference Abriel and Jais2014; Veit et al., Reference Veit, Traulsen, Hasler, Tölle, Burfeind, große Beilage and Krieter2016, Reference Veit, Büttner, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2017; Naya et al., Reference Naya, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2018). The piglets were moved to new compartments during weaning, so they had to deal with a completely new environment. Furthermore, the feed changed (Lallès et al., Reference Lallès, Bosi, Smidt and Stokes2007) and the piglets were mixed into new groups, which could have led to a higher stress level (Hötzel et al., Reference Hötzel, de Souza, Costa and Machado Filho2011; Proudfoot and Habing, Reference Proudfoot and Habing2015) and therefore promoted fights during the first days (Parratt et al., Reference Parratt, Chapman, Turner, Jones, Mendl and Miller2006). After a couple of days, the rank order had been established and the superficial lesions had time to heal. Then, the second cause of superficial lesions arose in the middle of the rearing period during this study when the tail-biting process started (Fig. 1 and Fig. 3). In agreement with our observations, in this period, the superficial lesions appeared as predecessors of real bite marks, scored as small and large lesions.
In the second trial of the study, more tail-losses at the end of the rearing period occurred compared to the first trial of the study. This is because the tail-biting process started in the second trial about 2 weeks earlier than in the first trial. However, due to the overall low occurrence of tail-biting, especially in the first trial of the study, only a few tail-losses at the end of the rearing period occurred in both trials. Thereby, one prominent appearance is that no tail-losses occurred in the first trial of the study in the first two batches. A possible explanation could be that the animals of these two batches were marked twice a week for video analyses and were therefore handled more than the other animals. This increased handling led to a higher human–animal interaction (Büttner et al., Reference Büttner, Czycholl, Basler and Krieter2018) and additional variety during the everyday life of the piglets, which reduced the risk of tail-biting.
A huge batch effect occurred during this study, which could have overwhelmed the treatment group effect, as is shown in Fig. 2 and Fig. 4. This is a challenge which former studies also have had to deal with (Veit et al., Reference Veit, Traulsen, Hasler, Tölle, Burfeind, große Beilage and Krieter2016, Reference Veit, Büttner, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2017; Naya et al., Reference Naya, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2018). Possibly, a greater difference in the feed composition between the treatment groups could increase the treatment group effect. Moreover, conducting studies under different housing conditions could reduce extrinsic influences and clarify the influence of the treatment group. The variable occurrence of tail-lesions during this study suggests that tail-biting is influenced by many extrinsic and intrinsic factors as summarized by (EFSA, 2007). For example, the health status of piglets and climate changes during the course of a study could explain the batch effect and possibly overlay the effect of the treatment group. However, the animals were not medically treated for diseases during this study and the batches were conducted with a temporal offset of only 1 week and therefore took place during one season. Thus, tail-biting is likely to be unpredictable (McGlone et al., Reference McGlone, Sells, Harris and Hurst1990; D'Eath et al., Reference D'Eath, Jack, Futro, Talbot, Zhu, Barclay and Baxter2018). During this study, it seems that the animals were kept under conditions which the piglets can deal with. Therefore, tail-biting can be compared to a bucket which spills over when different factors come together (Benard et al., Reference Benard, Schuitmaker and de Cock Buning2013). If any additional stressors occur, the tolerable stress level could fast be reached and exceeded, which results in a tail-biting outbreak.
The first trial of the study was conducted with the hypothesis that the tail-biting process decreased in treatment groups with a higher amount of crude fibre in the ration. However, the hypothesis could not be confirmed during the observation period. One possible reason is that the difference in crude fibre intake between the treatment groups was too slight to generate significant differences. The treatment group AL, which had constantly free access to soya hull pellets, had a crude fibre intake of between 50 and 60 g/kg during the whole study, thus always between treatment groups G5 and G6. However, the used soya hulls were not analysed for the presence of mycotoxins, which could have a negative influence on tail-biting and therefore overlap the positive effects of soya hulls.
The second trial of the study was conducted to analyse whether different components of crude fibre influenced the tail-biting process. Therefore, all piglets received a high amount of crude fibre in their ration, but from different components. As during the first trial, no appreciable differences between the treatment groups occurred.
Overall, only a few tail-lesions and tail-losses occurred during the whole study, which led to an absence of significant differences and furthermore to an impossibility of generalising these results. This low level of tail-biting had various justifications due to the multifactorial genesis of tail-biting. A tail-biting outbreak is always a result of many influencing factors that are not optimized. If there are only a few not well-adjusted factors, the piglets are able to compensate for these suboptimal factors (Benard et al., Reference Benard, Schuitmaker and de Cock Buning2013). However, if the number of these not well-adjusted factors increases, the tolerance against them decreases which can result in a tail-biting outbreak. In the ‘Futterkamp farm’ the piglets were kept under well-adjusted conditions. Consequently, the risk of tail-biting was lower than on other conventional farms, which also masks the treatment group effect. Another important influencing factor is the group size. In this study, the piglets were kept in small groups with only 12 piglets per pen. Smaller groups often have a lower risk of a tail-biting outbreak (Jericho and Church, Reference Jericho and Church1972; Arey, Reference Arey1991; Vermeer et al., Reference Vermeer, de Greef and Houwers2014), because of more stable rank order and therefore a lower level of social stress. Furthermore, the piglets received a small amount of straw twice a day that served as additional occupation material and therefore reduced the risk of tail-biting (Zonderland et al., Reference Zonderland, Wolthuis-Fillerup, van Reenen, Bracke, Kemp, den Hartog and Spoolder2008; Galli et al., Reference Galli, Scollo, Contiero and Gottardo2018; Lahrmann et al., Reference Lahrmann, Hansen, D'Eath, Busch, Nielsen and Forkman2018). This additional straw provision was placed on the bare pen floor and therefore accessible to all piglets in the pen. Moreover, in contrast to the continuously available occupation material, this straw satisfied the rooting behaviour of the piglets and served as a new stimulus and variety (Trickett et al., Reference Trickett, Guy and Edwards2009) since it was exhausted after a few minutes and the piglets had to wait for the next straw offer.
The stable employee of the ‘Futterkamp farm’ executed a very intensive animal observation, which made it possible to recognize initial signs of unrest or stress within a group and react in a timely manner. Therefore, identified biters were separated from the group and jute bags (Ursinus et al., Reference Ursinus, van Reenen, Kemp and Bolhuis2014) were placed in the affected pens as additional occupation material. This conduct is very important to maintain animal welfare but reduces the tail-biting process and therefore disguises the effect of the treatment groups. While comparing the present study with former studies which were conducted in the ‘Futterkamp farm’ (Veit et al., Reference Veit, Traulsen, Hasler, Tölle, Burfeind, große Beilage and Krieter2016, Reference Veit, Büttner, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2017; Naya et al., Reference Naya, Traulsen, Gertz, Hasler, Burfeind, große Beilage and Krieter2018), it became evident that tail-lesions and especially tail-losses occurred less frequently in the present study. This could show that the employees gained experience throughout the various studies. Thus, this observation gives an indication of the importance of good management and practice in an emerging tail-biting process (Valros et al., Reference Valros, Munsterhjelm, Hänninen, Kauppinen and Heinonen2016).
Conclusion
Due to the low frequency of the tail-biting process during the present study, it is not easy to generalize the results achieved. However, crude fibre seems to have no major influence on tail-biting during this study though it must be kept in mind that the batch effect overwhelmed the treatment group effect. It would be useful to observe more batches, contingently under different housing conditions, to minimize the batch effect.
An important observation suggested by the current study is that intensive animal observation performed by well-trained staff seems to be very influential for minimizing the risk of tail-biting.
Financial support
This project was kindly financed by the Landwirtschaftliche Rentenbank.
Conflict of interest
None.
Ethical standards
The animals used in this study were housed according to the guidelines of the Council Directive 2008/120/EG, the Council Directive 2010/63/EU and the ‘German Order for the Protection of Production Animals used for Farming Purposes and other Animals kept for the Production of Animal Products’ (TierSchNutztV, 2017). No animal experiments were carried out.








