Introduction
The plumpness of a rice (Oryza sativa L.) grain is correlated closely with its position on the panicle. Superior grains are the caryopses that are usually located on apical primary branches in a panicle and generally flower earlier, fill faster and produce heavier grains (Peng et al., Reference Peng, Lü, Zhao, Sun, Han, Du, Zhang, Li, Wang and Zhao2015). The later-flowering inferior grains, which are usually located on proximal secondary branches, fill slowly and poorly and consequently produce lighter grains (Yang and Zhang, Reference Yang and Zhang2010). The grain weight of inferior grains is significantly lower than that of superior grains after ripening.
Germination is the first key phase in the plant lifecycle. It is a complex process during which the imbibed mature seed must shift quickly from a maturation to a germination-driven programme of development and prepare for seedling growth (Rajjou et al., Reference Rajjou, Duval, Gallardo, Catusse, Bally, Job and Job2012). This growth depends on the quality of components stored in mature grains, and on those components’ ability to be utilized during germination (Nonogaki et al., Reference Nonogaki, Bassel and Bewley2010). The difference in grain weight between superior and inferior grains might, therefore, be affecting germination vigour. Several reviews discuss aspects of seed physiology in relation to germination, including how diffusible signals from the embryo, such as hormone and chemical stimulants, promote the production of hydrolytic enzymes in the aleurone layer surrounding the starchy endosperm, and that the products of hydrolysis released from the endosperm are absorbed by the scutellum and utilized by the growing embryo (Thomas and Rodriguez, Reference Thomas and Rodriguez1994; Appleford and Lenton, Reference Appleford and Lenton1997). Previous research has investigated seed germination by studying amylase activity, carbohydrate metabolism, endogenous hormones, signal transduction, gene expression and redox system repair, and has shown that the activity of α-amylase plays an important role in regulating the germination rate of rice grains (Bewley, Reference Bewley1997; Zhao and Wang, Reference Zhao and Wang2001). It has also been demonstrated that soluble sugar produced by starch metabolism could depress the gene expression of amylase (Perata et al., Reference Perata, Matsukura, Vernieri and Yamaguchi1997; Umemura et al., Reference Umemura, Perata, Futsuhara and Yamaguchi1998). Plant hormones gibberellin (GA), abscisic acid (ABA), auxin and cytokinin have profound effects on plant development, including the germination stage, even at extremely low concentrations (Koornneef et al., Reference Koornneef, Bentsink and Hilhorst2002; Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005). Meanwhile, the ratio of ABA/GA is essential for metabolic transitions in germination (Yamaguchi, Reference Yamaguchi2008; Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). Expression of the rice α-amylase gene (OsRAmy1A) in the endosperm depends on the de novo synthesis of GAs (O'Neill et al., Reference O'Neill, Kumagai, Majumdar, Huang, Sutliff and Rodriguez1990; Kaneko et al., Reference Kaneko, Itoh, Ueguchi-Tanaka, Ashikari and Matsuoka2002). Moreover, OsGAMYB, a transcription factor, which could respond to GA signals, could regulate the induced expression of OsRAmy1A in aleurone (Kaneko et al., Reference Kaneko, Inukai, Ueguchi-Tanaka, Itoh, Izawa, Kobayashi, Hattori, Miyao, Hirochika, Ashikari and Matsuoka2004; Park et al., Reference Park, Park, Yoon, Yu and An2010). However, reports about germination of superior and inferior grains are scarce and the effects of dynamic changes in endogenous hormones during early stage germination are unknown.
In the present study, the conventional Japonica rice cultivar Xinfeng2 was used to study the dynamic changes of endogenous hormones, relative water content, seed germination related α-amylase activity and gene expression at the early stages of germination. The present study also assessed the characteristics of seedlings from superior and inferior grains obtained from the rice panicle. The results indicated that relative water content, GA, soluble sugar and the expression level of the α-amylase gene were higher in inferior grains than in superior grains in the first 24 h of germination. This led to higher germination vigour of inferior grains at 24 h. However, owing to its initial lower grain weight and greater consumption of dry matter, the seedlings of inferior grains were eventually weaker. But the final thousand-grain weight, yield per plant and other agronomic traits of superior plants and inferior plants showed no significant differences.
Materials and methods
Experimental design
The Japonica rice cultivar Xinfeng2 was selected as the research material in the present study. Rice grains were germinated in plastic breeding trays in late April and the seedlings were transplanted into paddies at the research farm of Henan Agricultural University, Henan Province, China (34°53′N, 113°35′E, 94 m asl) in mid-June. Water and insect pests were managed according to requirements. As described previously, grains located on the upper primary branches, excluding the penultimate ones, were taken as superior grains and those on the lower secondary branches, excluding the distal ones, were taken as inferior (Peng et al., Reference Peng, Lv, Zhang, Li, Du and Zhao2011). Both superior and inferior grains used in the experiment were stored for 2 months after-ripening at room temperature. Grains were hulled and imbibed in distilled water and 15% polyethylene glycol (PEG) and 20% PEG at 26 °C for 0, 12, 24 and 48 h, with the water changed every 24 h (He et al., Reference He, Han and Yang2011). Samples used for enzyme activity, plant hormone and gene expression assays were stored at −80 °C.
Measurement of thousand-grain weight, relative water content and relative dry matter content
Superior and inferior grains were collected from mature rice and dried completely at room temperature. Five hundred randomly selected grains with three individual biological repeats were measured to calculate their thousand-grain weights. Fifty grains at germination (0 h), 6, 12, 24 and 48 h, with three replications, were placed in an oven at 70 °C to obtain constant weight, and total weights were assayed to calculate individual dry weights. The relative water content and relative dry matter content were calculated as below:
Quantification of endogenous hormones
Gibberellin and abscisic acid quantification was determined by enzyme-linked immunosorbent assay (ELISA) at the Phytohormone Research Institute, China Agricultural University, China (Zhang et al., Reference Zhang, Tan, Yang, Yang, Zhang and Zhao2009). Briefly, samples including hulled grains were placed in an ice-cold mortar containing 5–10 ml 80% (v/v) methanol extraction medium with 1 mm butylhydroxytoluene as an antioxidant. The extract was incubated at 4 °C for 4 h and centrifuged at 5000 g for 15 min at the same temperature. Approximately 5 ml of the purified supernatant fraction containing GAs, and ABA was collected, dried under liquid nitrogen and dissolved in 2 ml phosphate buffered saline containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5) for analysis by ELISA.
α-amylase activity
Isolation and determination of α-amylase activity and measurement of soluble sugar content was achieved using 3,5-dinitrosalicylic acid. α-amylase was extracted from rice grains at germination (0 h), 6, 12, 24 and 48 h and incubated in 8 ml distilled water with stirring for 15–20 min. After centrifugation at 1000 g for 10 min, the extract was transferred into a 100 ml volumetric flask and labelled as enzyme solution I. This solution was diluted 5-fold and labelled enzyme solution II. Both enzyme solutions I and II were incubated in boiling water for 10 min. The absorbance of the supernatant was measured at 540 nm using a TU-1810 UV–vis spectrophotometer (PERSEE, Beijing, China) and the units of activity were determined from a standard curve prepared using malt sugar.
Soluble sugar content
Grains at different germination stages, with three replications, were homogenized using 5 ml distilled water. The extraction was completed in a boiling water bath for 30 min, and the extract transferred into a 25 ml volumetric flask after filtering. Then, 0.5 ml extract with 1.5 ml distilled water was placed into a 10 ml tube. Finally, 1 ml 2% anthrone colorimetric reagent and 5 ml concentrated sulphuric acid were added. After stopping the reaction, the absorbance of the supernatant was measured at 630 nm using a TU-1810 UV–vis spectrophotometer (PERSEE). Units of soluble sugar content were determined from a standard curve prepared using purified sugar.
Expression analysis of starch degradation related genes
The cDNA sequences of OsGAMYB and OsRAmy1A were acquired from the National Center for Biotechnology Information database (http://www.ncbi.nlm.gov). Total RNA of superior and inferior grains at germination (0 h), 6, 12, 24 and 48 h were extracted by RNA extraction kit (TransGen Biotech, Beijing, China). Reverse transcription was conducted using a HiFi-MMLV cDNA kit (TOYOBO, Shanghai, China). cDNA was diluted 20-fold and 5 µl was used as a template for real-time quantitative polymerase chain reaction with 10 µl UltraSYBR (TOYOBO) in a total mixture of 20 µl. The β-actin gene was used as internal reference. The reaction was carried out on an iQ5 (BioRad, USA) using the following procedure: 95 °C warm-up for 10 min, 40 cycles of 95 °C denaturation for 30 s, annealing at 60 °C for 15 s and elongation at 72 °C for 30 s. There were three technical replications for each reaction and the relative expression level was calculated using the 2−▵▵Ct method as described previously (Peng et al., Reference Peng, Du, Zhang, Li, Liu, Zhao, Sun and Zhao2013). All gene-specific primers are listed in Table 1.
Table 1. Primers used in the present study

Starch granule structure analysis
Sample grains were fractured in liquid nitrogen after washing with 0.2 mol/l phosphate buffer (pH 7.2). They were then fixed using 2.5% glutaraldehyde and dehydrated using serial ethanol dilutions and finally oven drying. Gold was sprayed on sections using an Ion sputter E-1010 (HITACHI). The structure of sections at 0 and 48 h was observed by scanning electron microscopy using an S-3400N-II scanning electron microscope (HITACHI, Japan) under 5.0 kV accelerating voltage (5000×).
Statistical analysis
All the experimental data were analysed by SPSS 18.0 and significant differences were identified using Student's t-test.
Results
As shown in Table 2, thousand-grain weight of superior grains was as almost twice as that of inferior grains, while the relative water content showed no obvious difference. In the case of germination, there was no difference in germination vigour between superior and inferior grains within 12 h. At 24 h, all the inferior grains showed white radicles that denoted visible germination, while no radicles appeared among superior grains (Fig. 1(a)). At 48 h, the germination rate had reached 100% in both superior and inferior grains. To observe the post-germination growth of seedlings from these two types of grain, seedlings were cultivated on 1/2 Murashige and Skoog medium and the phenotypic index was measured at the two-leaf stage (Fig. 1(d)). This showed that plant height, basal culm thickness, root numbers and root/shoot ratio of inferior grains were significantly (P < 0.01) lower than that of superior ones (Table 3). These results indicated that inferior grains had higher germination vigour in the first 24 h but weaker seedling growth after germination.
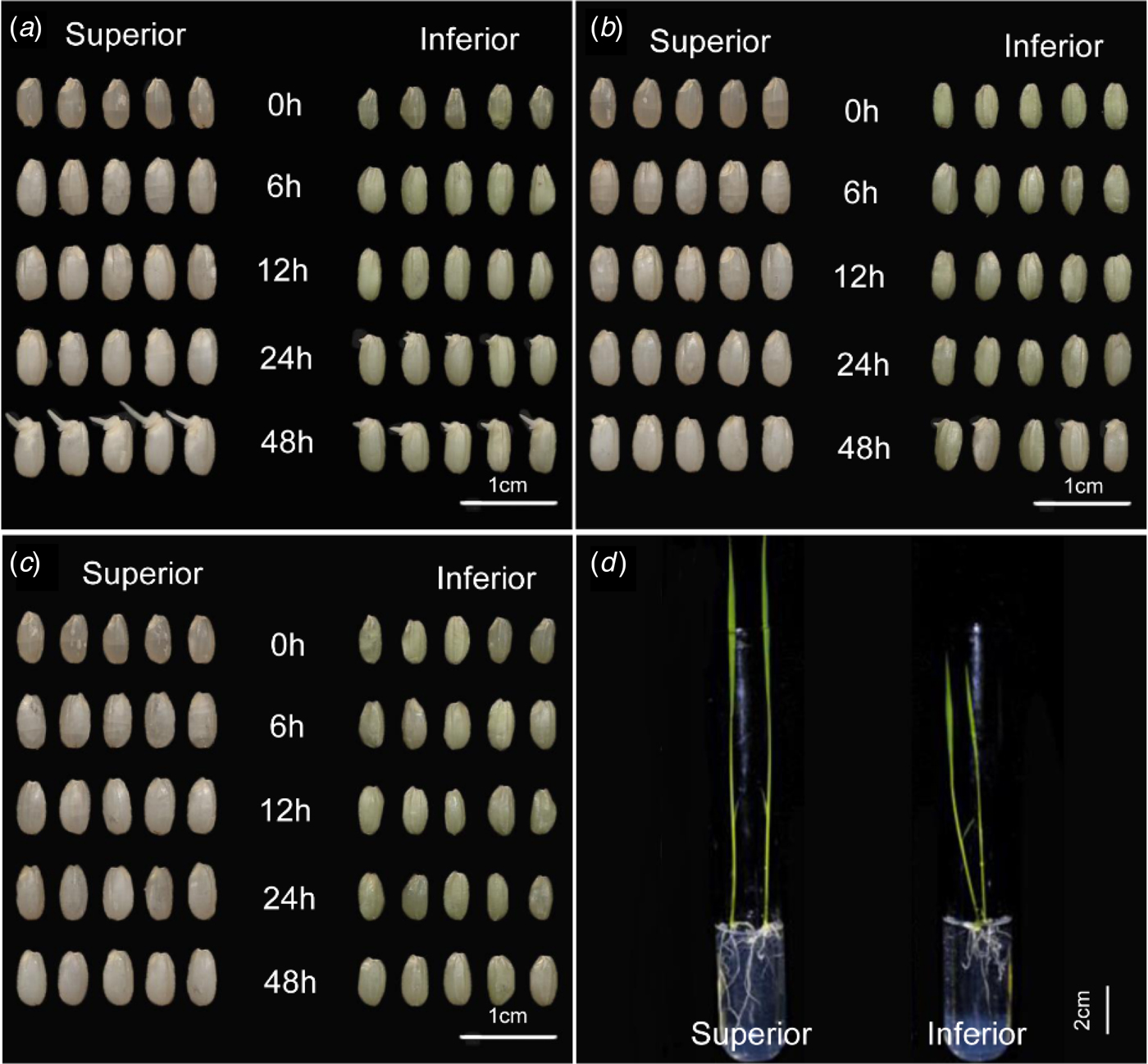
Fig. 1. Germination differences between superior and inferior grains. Superior and inferior grains at different germination stages under normal condition (a), 15% polyethylene glycol (PEG) (b) and 20% PEG (c); (d) Comparison of seedlings cultivated on 1/2 Murashige and Skoog medium for 1 week. Colour online.
Table 2. Thousand-grain weight of superior and inferior grains

Values shown are the means ± sd (n = 1000 grains, n = 3). Significant differences were identified using Student's t-test.
Table 3. Phenotypic index of seedlings cultivated on 1/2 Murashige and Skoog medium 1 week after transfer

Values shown are the means ± sd (n = 60 plants, n = 3). Significant differences were identified using Student's t-test.
The structure of the starch granules of superior and inferior grains differed at 0 h. In superior grains, the granule structure was a closely packed, regular angular polyhedron (Fig. 2(a)). In inferior grains, the arrangement was loose, with starch granules packed irregularly (Fig. 2(b)). At 48 h, the starch granules of both superior and inferior grains absorbed water and swelled. However, the change in starch granules of superior grains was not as obvious because of their densely packed structure (Fig. 2(c)). It was found that the loosely arranged starch granules in inferior grains may contribute to quick water uptake and swelling, leading to rapid enlargement of starch granules (Fig. 2(d)).

Fig. 2. Scanning electron microscopy images of starch granules in germinating superior and inferior grains (5000×). (a) Superior grains at 0 h of germination; (b) Inferior grains at 0 h of germination; (c) Superior grains at 48 h of germination; (d) Inferior grains at 48 h of germination.
To further investigate whether the higher vigour at the beginning of germination in inferior grains was due to the quick uptake of water, treatments of drought stress were conducted using 15% and 20% PEG6000. Compared with the normal condition, germination of both superior and inferior grains was inhibited under these treatments. More importantly, inferior grains still showed stronger germination vigour than superior grains under 15% PEG treatment at 48 h after germination (Fig. 1(c)). However, germination of both superior and inferior grains was inhibited almost completely under the 20% PEG treatment (Fig. 1(d)).
After removing the shells of grains after ripening, the thousand-grain weight of superior grains was twice that of inferior grains, but there was no significant difference between their relative water contents (Fig. 3(a)). Seeds absorb water mainly through imbibition prior to germination. The relative water content in both superior and inferior grains increased sharply in the first 6 h of germination. Specifically, the relative water content in superior grains at 6 h was 22.44% and in inferior grains was 32.53% (P < 0.05). Moreover, it was still significantly higher (P < 0.01) in inferior grains at 12, 24 and 48 h (Fig. 3(a)). Consistent with the mobilization of major storage reserves, dry matter (including the radicle after 24 h) decreased during germination, with the rate of decline highest in the first 6 h. In the following hours, the rate of decline gradually reduced but was still higher in inferior grains (P < 0.05) (Fig. 3(b)). At 48 h, the final dry matter content was 32.78 and 61.82% when compared with the initial dry weight of inferior and superior grains, respectively.

Fig. 3. Changes in the relative water content (a) and relative dry matter (b) of superior and inferior grains during germination. Error bars are the means ± sd (n = 3).
Gibberellin plays an important role in stimulating the germination of seeds and breaking seed dormancy. It reached its minimum level at 12 h and then increased. By comparing GA levels in superior and inferior grains at the same time, it was found to be higher in inferior grains than superior grains at 0 and 12 h (P < 0.05). At 6, 24 and 48 h, the trends were similar, but the differences were not significant (Fig. 4(a)). Abscisic acid acts as an inhibitory hormone during seed germination. In dry grains, the ABA content of inferior grains was 2.27 times that of superior ones. As germination progressed, ABA in superior and inferior grains decreased and the differences between them reduced (Fig. 4(b)). From 0 to 12 h it dropped by 75.94 mg/g in inferior grains while in superior grains it dropped by only 27.03 mg/g. The difference in ABA content between superior and inferior grains was significant except at 48 h (P < 0.05).

Fig. 4. Changes in hormonal content during germination of superior and inferior grains. (a) Gibberellin (GA) content; (b) Abscisic acid (ABA) content. Error bars are the means ± sd (n = 3).
The amount of soluble sugar in dry superior grains was higher than in inferior ones (Fig. 5(a)). After seed imbibition, the soluble sugar content in both reduced at first and then increased. Soluble sugar content was significantly (P < 0.05) higher in inferior than superior grains at all times except at 0 h. Both superior and inferior dry seeds showed the presence of α-amylase activity (Fig. 5(b)): it decreased over the first 24 h of germination but increased thereafter. At 24–48 h, α-amylase activity was higher in inferior grains than in superior ones (P < 0.01) (Fig. 5(b)).

Fig. 5. Changes in soluble sugar content (a) and activity of α-amylase (b) during germination of superior and inferior grains. Error bars are the means ± sd (n = 3).
OsGAMYB was expressed throughout germination and its expression level in inferior grains increased sharply from 12 to 24 h before reducing again. In superior grains, OsGAMYB expression was stable until 6 h, where it decreased slightly before increasing up to 48 h (Fig. 6(a)). There was no significant difference between expression in inferior and superior grains in the first 12 h. Moreover, OsGAMYB expression level in inferior grains was higher than in superior ones at 24 h. The expression level of OsRAmy1A was comparable in superior and inferior grains in the first 12 h. It increased rapidly from 12 to 24 h and decreased from 24 to 48 h in inferior grains. A much weaker increase from 12 to 24 h was observed in superior grains. As a whole, the expression level of OsRAmy1A was much higher in inferior grains than in superior grains from 24 to 48 h (Fig. 6(b)).

Fig. 6. Relative expression level of (a) OsGAMYB and (b) RAmy1A during germination of superior and inferior grains. Error bars are the means ± sd (n = 3).
Considering the stronger germination vigour but weaker seedlings of inferior grains, agronomic traits including plant height, tiller number, thousand-grain weight, yield per plant and so on of superior and inferior seedling were measured at harvest, with no significant differences for the characteristics (Table 4).
Table 4. Grain yield of superior and inferior plants in the field

PH, Plant height; TN, Tiller number; EGNPP, Effective grain numbers per panicle; SSR, Seed setting rate (i.e. percentage of effective grains); TGW, thousand-grain weight; YPP, yield per plant. Values are the means ± sd (n = 60 plants, n = 3). Significant differences were identified by using Student's t test.
Discussion
Generally, germination of a grain commences with the uptake of water and in most species is completed with protrusion of the radicle through the surrounding tissue (Bewley, Reference Bewley2001). Previous studies have shown that dry grains initially absorb water rapidly until all of the grain matrices and cell contents are full of water (Nonogaki et al., Reference Nonogaki, Bassel and Bewley2010). However, the kinetics of uptake are influenced by the surrounding structure of grains, meaning that water may not enter all regions equally (Bewley, Reference Bewley2001). In the present study, it was clear that the arrangement of starch granules in the endosperm of inferior grains was looser than in superior ones, which might make it easier for water to enter inferior grains at the imbibition stage. The present study showed that the relative water content in inferior grains was 1.6 times higher than in superior grains from 0 to 6 h, which might make it easier for inferior grains to set in motion the metabolic events essential for germination to commence, and reduced radicle breaking resistance to complete germination. Furthermore, the weight of superior grains was almost twice that of inferior grains on the same rice panicle. The energy required for germination and seedling growth before the two-leaf stage is derived mainly from the metabolism of nutrients stored in the endosperm. In the present study, the rate of reduction of dry matter, or nutrients, in inferior grains was much higher than in superior grains from 0 to 6 h. The final weight of superior grains was 3.5 times as high as inferior ones, indicating that inferior grains contained less initial nutrients and consumed these faster during germination, ultimately resulting in weaker seedlings.
Plant hormones, especially GAs, play an essential role in completing seed germination, while release or deactivation of ABA is also important (Appleford and Lenton, Reference Appleford and Lenton1997; Seo et al., Reference Seo, Nambara, Choi and Yamaguchi2009). In the present study, ABA levels were much higher in inferior dry grains and declined more rapidly in inferior grains than in superior ones during the first 24 h of germination. A correlation between ABA reduction and the ability of grain to germinate has been observed previously (Nonogaki et al., Reference Nonogaki, Bassel and Bewley2010). It was, therefore, speculated that the stronger germination vigour of inferior grains was partially due to the higher rate of ABA decline. Gibberellin biosynthesis is required for Arabidopsis thaliana seed germination (Koornneef and Van der Veen, Reference Koornneef and Van der Veen1980), with homologs of the gene involved in GA biosynthesis found in rice (Zhu et al., Reference Zhu, Ye and Zhang2009). Before 48 h, GA levels in inferior grains were higher than in superior ones at all times. Therefore, higher GA levels in dry inferior grain might also contribute to its stronger ability to complete germination in the first 24 h.
It is well established that GA stimulates the synthesis and secretion of hydrolases such as α-amylase, by aleurone cells that lead to mobilization of the stored endosperm reserves (Higgins et al., Reference Higgins, Jacobsen and Zwar1982; Jacobsen and Beach, Reference Jacobsen and Beach1985). In the Hordeum vulgare aleurone, the effect of GA on the expression of α-amylase genes is mediated through HvGAMYB, whose expression is up-regulated by GA treatment (Gubler et al., Reference Gubler, Chandler, White, Llewellyn and Jacobsen2002). In the present study, the expression of α-amylase gene OsRAmy1A increased from 12 h, with the highest expression at 24 h, before decreasing along with a reduction in GA levels. OsGAMYB was also most highly expressed at 24 h. This might indicate that, in rice germination, GA stimulates transcription and translation of α-amylase through the expression OsGAMYB. In the present study, the expression of OsRAmy1A in superior and inferior grains coincided with increased α-amylase activity in the aleurone and in the whole grain when treated with exogenous GA. Therefore, the rate of GA transportation from scutellum to aleurone cells and α-amylase production were both faster in inferior grains, at least to a certain degree. This might account for the higher activity of α-amylase in inferior grains. Consequently, the metabolic rate of starch hydrolysis in the endosperm was faster in inferior grains and could lead to the production of more soluble sugar in these grains. Finally, more energy required for germination was provided by soluble sugar in inferior grains, resulting in stronger germination vigour.
Conclusion
The current research found that the structure of starch granules was looser in inferior grains after ripening. Dry matter reduced and relative water content increased faster in inferior grains. Higher GA content, α-amylase activity, soluble sugar content, and expression of germination-related genes at the early stages of germination in inferior grains resulted in superior germination vigour in the first 24 h. The lower grain weight and faster consumption of dry matter, however, resulted in inferior grains presenting weaker seedlings at the transplantation stage. But according to the current statistical data at harvest, agronomic traits including plant height, tiller number, thousand-grain weight, yield per plant, etc. showed no significant differences between superior and inferior grain plants. Therefore, it could be concluded that inferior grains had higher vigour at the beginning of germination partially due to the quick uptake of water. Considering the weaker seedling vigour of inferior grain after germination, it might be better to sow plump grains including superior grains in the field to obtain strong seedlings for transplantation. However, inferior grains could also be used for sowing in practice when necessary because of its potential high yield in rice.
Acknowledgements
We are grateful to the editors and reviewers for their suggestions.
Financial support
This work was supported by the National Natural Science Foundation of China (grant no. 31271651), Major Science and Technology Project of Henan Province (grant no. 141100110600), the Support Plan of Science and Technology Innovation Team in Universities of Henan province (grant no. 17IRTSTHN015) and Modern Agricultural Industry Technology System Projects of Henan Province (grant no. S2012-04-02).
Conflicts of Interest
None.
Ethical standards
Not applicable.













