Introduction
The major challenge facing rice production in China is how to increase crop yields in order to feed a growing population. As an essential nutrient for rice growth and development, nitrogen (N) is a major determinant of grain yields in rice cropping systems worldwide (Fageria and Barbosa Filho, Reference Fageria and Barbosa Filho2001; Habtegebrial et al., Reference Habtegebrial, Mersha and Habtu2013). In flooded lowlands in China, farmers generally top-dress N fertilizers using manual broadcast application (BA). However, this mode reduces N use efficiency because the fertilizer is readily lost from the topsoil through chemical transformation, and the mode and timing of its application may cause denitrification and N leaching (Xing and Zhu, Reference Xing and Zhu2000). To overcome these shortcomings, some N management programmes have attempted to achieve optimum yields by using alternative N fertilization modes, including different forms of fertilizers and deep soil placement techniques (Bacon and Lewin, Reference Bacon and Lewin1990; Sommer et al., Reference Sommer, Schjoerring and Denmead2004; Liu et al., Reference Liu, Fan, Zhang, Chen, Li and Cao2015). Most rice farmers in China apply N as a panicle fertilizer before panicle initiation (growth stage [GS] 30-31 according to Zadoks et al., Reference Zadoks, Chang and Konzak1974). The appropriate administration of panicle N fertilizer greatly aids rice production through improved inflorescence development and an increased number of flowers per panicle (Yang et al., Reference Yang, Mu, Li, Ye and Yu2003; Wu et al., Reference Wu, Zhang, Zhao, Wu, Li and Xia2007). Applying N fertilizer before the panicle initiation stage also helps to improve total N accumulation and rice grain yields (Bacon and Lewin, Reference Bacon and Lewin1990; Peng et al., Reference Peng, Buresh, Huang, Zhong, Zou, Yang, Wang, Liu, Hu, Tang, Cui, Zhang and Dobermann2010).
Approximately 0.75 of all rice production is in irrigated lowlands, which are traditionally kept continuously flooded with 5–10 cm of water for the duration of rice growth (Belder et al., Reference Belder, Bouman, Cabangon, Guoan, Quilang, Yuanhua, Spiertz and Tuong2004). This practice results in heavy clay soils and poor water permeability, and eventually leads to low fertilizer-use efficiency. Deep digging or tillage in paddy soil with organic manure not only breaks up the compact layer and loosens the surface soil but also increases rice yields (Shen et al., Reference Shen, Zhang, Liu and Lü2000; Kato et al., Reference Kato, Kamoshita, Abe and Yamagishi2007).
Deep fertilizer application has been explored as a more efficient means of N management in flooded lowland rice cultivation (Fageria et al., Reference Fageria, Baligar and Jones2010), because the sub-surface placement of N fertilizer can increase its efficiency as a result of reduced surface losses. Deep layer fertilizer application can increase rice grain yields by increasing the numbers of spikelets (Wada and Kudo, Reference Wada and Kudo1966; Wang et al., Reference Wang, Katayama and Takeda1991) and panicles, as well as tiller height, compared with the surface broadcast application, particularly in the wet season (Bandaogo et al., Reference Bandaogo, Bidjokazo, Youl, Safo, Abaidoo and Andrews2015). Another advantage of deep fertilizer application is that the root systems of crop plants (Chancy and Kamprath, Reference Chancy and Kamprath1982; Kundu et al., Reference Kundu, Ladha and Lapitan-de Guzman1996) and the rhizosphere environment can be improved by soil management practices (Liu et al., Reference Liu, Fan, Zhang, Chen, Li and Cao2015). The deep mechanized application of a super-specialized rice fertilizer during sowing not only increases the soil nutrient content and proportion of macroaggregates but also enhances soil enzyme activity (Shu et al., Reference Shu, Tang, Luo, Zhang, Li, Duan, Li, Chen, Liao, Yao and Ye2013). Similarly, N placement at a depth of 10 cm increases N use efficiency, urease activity in the topsoil and grain yield in no-tillage paddy fields (Liu et al., Reference Liu, Fan, Zhang, Chen, Li and Cao2015). As a biological indicator for assessing soil fertility, soil enzyme activity provides information about soil nutrient transformations (Zantua et al., Reference Zantua, Dumenil and Bremner1977; García-Ruiz et al., Reference García-Ruiz, Ochoa, Hinojosa and Carreira2008).
In China, panicle N application is vital during rice planting shortly after the fields sun-dry. Although increased N fertilizer recovery and deep root proliferation are associated with the practice of deep tillage in crops (Chancy and Kamprath, Reference Chancy and Kamprath1982; Kundu et al., Reference Kundu, Ladha and Lapitan-de Guzman1996), few studies have investigated the compaction of paddy soil after sun-drying and other methods of increasing N fertilization efficiency. Moreover, soil enzymes have been used to characterize responses to changes in soil management (Bandick and Dick, Reference Bandick and Dick1999) and their roles in the mineralization of carbon (C), N, phosphorous (P) and sulphur (S) at the fundamental nutrient cycling level (Dick, Reference Dick, Doran, Coleman, Bezdicek and Stewart1994; Cookson and Lepiece, Reference Cookson and Lepiece1996; Klose and Tabatabai, Reference Klose and Tabatabai1999; Sinsabaugh et al., Reference Sinsabaugh, Lauber, Weintraub, Ahmed, Allison, Crenshaw, Contosta, Cusack, Frey, Gallo, Gartner, Hobbie, Holland, Keeler, Powers, Stursova, Takacs-Vesbach, Waldrop, Wallenstein, Zak and Zeglin2008; Hartmann et al., Reference Hartmann, Schmid, van Tuinen and Berg2009; Finkenbein et al., Reference Finkenbein, Kretschmer, Kuka, Klotz and Heilmeier2013). Consequently, soil enzyme activity is an important indicator for evaluating the performance of different management practices intended to improve soil fertility in paddy fields. Therefore, the current study sought to clarify the effects of N top-dressing and ditching before panicle initiation on rice yields and soil enzyme activity and to develop a more efficient mode of panicle N application for rice cultivation.
Materials and methods
Treatment design
A field study was conducted at the Scientific and Educational Farm of Henan Agricultural University (34°75′N, 113°66′E, 0 m a.s.l.), Zhengzhou, in mid-eastern China during the 2011 and 2012 rice growing seasons (from May to October). The soil was a sandy loam containing 9.7 g/kg of organic matter, 26.5 mg/kg of available N, 34.8 mg/kg of Olsen-P, and 75.0 mg/kg of exchangeable potassium (K) with an average pH of 7.2. Seedlings of Yunongjing-6, a traditional japonica cultivar, were raised on nursery plates under humid conditions for 25 days at a seeding rate of about 2400 plants/m2. Then, the seedlings were transplanted to the study plots, with one seedling planted per hill. The total application of N fertilizer was 137.2 kg N/ha, supplied as urea as basal, tillering and panicle fertilizer at a ratio of 3.5:3.5:3. Calcium superphosphate and potassium chloride were applied only as a basal fertilizer, and the final proportions of N, phosphorus and potassium (N: phosphorus pentoxide [P2O5]: potassium oxide [K2O]) were 1.0:0.5:0.8.
Panicle N fertilizer was applied at the beginning of the inverted fourth leaf stage, 1 day before the recovery phase after field drying, i.e. the stem elongation stage or jointing stage (GS 30-31, Zadoks et al., Reference Zadoks, Chang and Konzak1974). Three modes of application were tested: manual BA of urea on the soil surface as a control, deep urea application during ditching at a depth of 15 cm (DD) and manual BA of urea on the soil surface during deep ditching (BAD). All treatments were conducted in a randomized block design with three replicates for each condition. In BA plots, urea was spread manually on the soil surface. In DD plots, urea was applied using a furrowing machine (3WG-3, Xia Jin Colorful Cotton, China) into 15-cm-deep ditches between the lines of rice plants. In BAD plots, the soil was ditched to a depth of 15 cm between the lines of rice plants with the same furrowing machine, but the urea was then broadcast on the surface. Each plot measured 40 m2 (4 × 10 m2) and the hill density was one per 30 × 13 cm2. To prevent fertilizer from flowing into adjacent plots, the ridges between each plot were covered with plastic film. The water depth in plots was maintained at 2–3 cm during the heading stage. After anthesis, the plots were irrigated to a depth of 2–3 cm whenever the paddy field dried naturally. Pests and diseases were controlled intensively to avoid yield losses, with herbicides usually applied before the jointing stage and pesticides applied whenever an infestation appeared.
Examination of rice yield and its components
Five hills were chosen at random from each plot and harvested at maturity in 2011 and 2012. The panicle number per plot, spikelet number per panicle, percentage of filled grains and 1000-grain weight were determined. Rice plants in fixed areas (5 × 4 m2) of each plot were harvested at maturity to measure crop yield.
Determination of soil enzyme activity
Each sample consisted of six cores taken at random from each plot between rows from the top 20 cm of the soil surface using a soil auger at the jointing (GS 32), booting (GS 45) and grain-filling stages (GS 85) in 2011 and 2012. These stages occurred 7, 20 and 40 days, respectively, after top-dressing with N fertilizer and ditching soil. After being air-dried at room temperature, the soil samples were sieved using a 1-mm mesh and stored at 4 °C until enzyme activity analysis. Air-dried soil samples were considered to be representative of moist field conditions in determining urease, phosphatase and invertase (Frankeberger and Johanson, Reference Frankeberger and Johanson1983; Guan, Reference Guan and Guan1986). Soil invertase, urease, phosphatase and catalase activities were measured spectrophotometrically, as described previously (Guan, Reference Guan and Guan1986) with minor modifications. Invertase activity was determined following Guan (Reference Guan and Guan1986): 5 g of air-dried soil were incubated with 15 ml of 8% (w/v) sucrose solution, 5 ml of phosphate buffer (pH 5.5) and 1 ml of toluene at 37 °C for 24 h in the dark. Then, 2 ml of the filtrate of the soil suspension after incubation was used to measure the amount of glucose. Urease activity was determined following Guan (Reference Guan and Guan1986): 5 g of air-dried soil were incubated with 10 ml of 10% (w/v) urea solution, 20 ml of phosphate buffer (pH 6.7) and 1 ml of toluene at 37 °C for 24 h in the dark. Then, 3 ml of the filtrate of the soil suspension after incubation was used to measure the concentration of ammonium ions. Invertase and urea activities were expressed as mg glucose/g/day and mg ammonia (NH3)–N/g/day, respectively. Soil phosphatase activity was measured as follows: 5 g of air-dried sample was incubated with 20 ml of 0.5% (w/v) disodium phenyl phosphate and 1 ml of toluene at 37 °C for 24 h in the dark. Phosphatase activity was expressed in mg nitrophenol/g/day. Catalase activity was determined by monitoring the hydrogen peroxide decomposition rate and expressed as ml 0.1 mol/l potassium permanganate (KMnO4)/g/day. Briefly, a 2 g air-dried sample was mixed with 40 ml of distilled water and 5 ml of 0.3% hydrogen peroxide (H2O2). After oscillation for 20 min, 5 ml of 1.5 mol/l sulphuric acid (H2SO4) was added to the mixture. For each soil sample, the geometric mean of the assayed enzyme activities (hereafter GMea) was calculated as follows (García-Ruiz et al., Reference García-Ruiz, Ochoa, Hinojosa and Carreira2008):
where Inv, Ure, Pho and Cat are the invertase, urease, phosphatase and catalase activities, respectively.
Detection of bleeding sap intensity
Bleeding sap was collected from de-topped plant stumps (Schurr, Reference Schurr1998). The bleeding intensity is indicated with the weight of sap bled per unit time after de-topping. Starting 30 days after treatment (about 7 days after flowering), bleeding sap intensity was measured every 5–8 days in three hills per plot under each of the BA, DD and BAD treatments (three biological replicates each) in 2012. Bleeding sap was collected following described methods (Zhao et al., Reference Zhao, Gao, Huang and Ling1999, Reference Zhao, Gao, Huang and Ling2001). The bleeding sap of basal internodes was collected from stems cut about 10 cm above the soil surface from 18:00 to 07:00 h using a sealed bag filled with degreasing cotton. The bleeding sap of neck internodes was collected from the neck-panicle internodes using a bleeding tube with degreasing cotton from 18:00 to 07:00 h.
Statistical analysis
All data were analysed with SPSS 17.0. Comparisons between means were made using Duncan's test at P < 0.05.
Results
Effects of panicle nitrogen application on soil enzyme activities
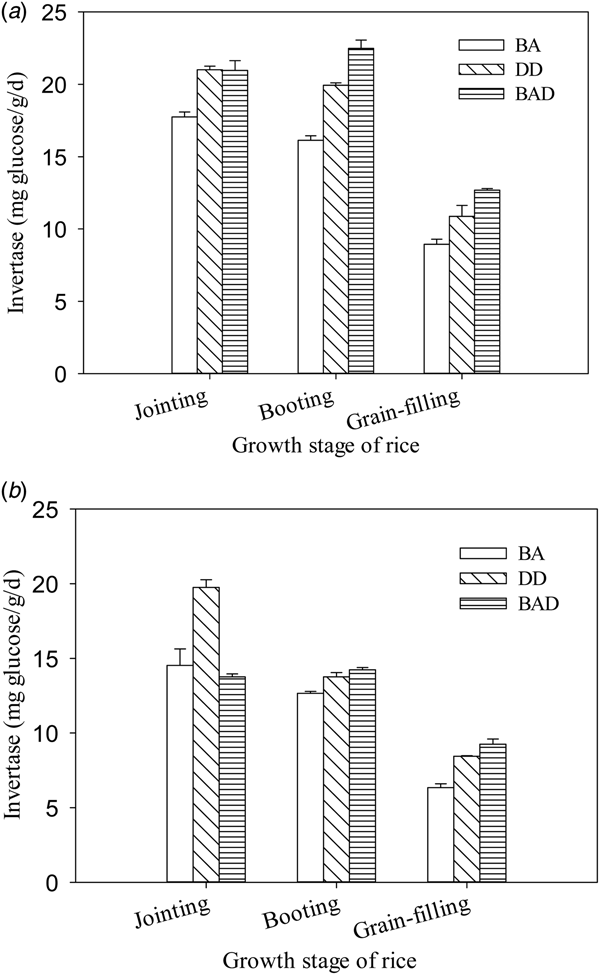
To understand the effects of N top-dressing before panicle initiation on soil biochemical properties, the enzyme activities in soil samples collected from each plot were examined. The soil invertase activity in DD was significantly higher than that in the BA group (P < 0.05, Figs 1(a) and (b)). In 2011, soil invertase activity was significantly greater at the jointing, booting and grain-filling stages in the DD and BAD groups than in the BA group (P < 0.05). A similar pattern was observed in 2012, except that there was no clear difference between the BA and BAD groups at the jointing stage. These results suggest that tillage had positive effects on soil invertase activity.

Fig. 1. Soil invertase activity in paddy plots under three different panicle fertilization modes. a, 2011 season; b, 2012 season. Values are means ± s.e. of three replicates. BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
Similarly, at all three critical growth stages of rice, the soil urease activities in the DD and BAD groups were significantly higher (0.025–0.048 mg NH3-N/g/day) than in the BA group at the jointing and grain-filling stages in both 2011 and 2012 (P < 0.05, Fig. 2). This indicates that the soil management practice also significantly enhanced soil urease activity.

Fig. 2. Soil urease activity in paddy plots under three different panicle fertilization modes. a, 2011 season; b, 2012 season. Values are means ± s.e. of three replicates. BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
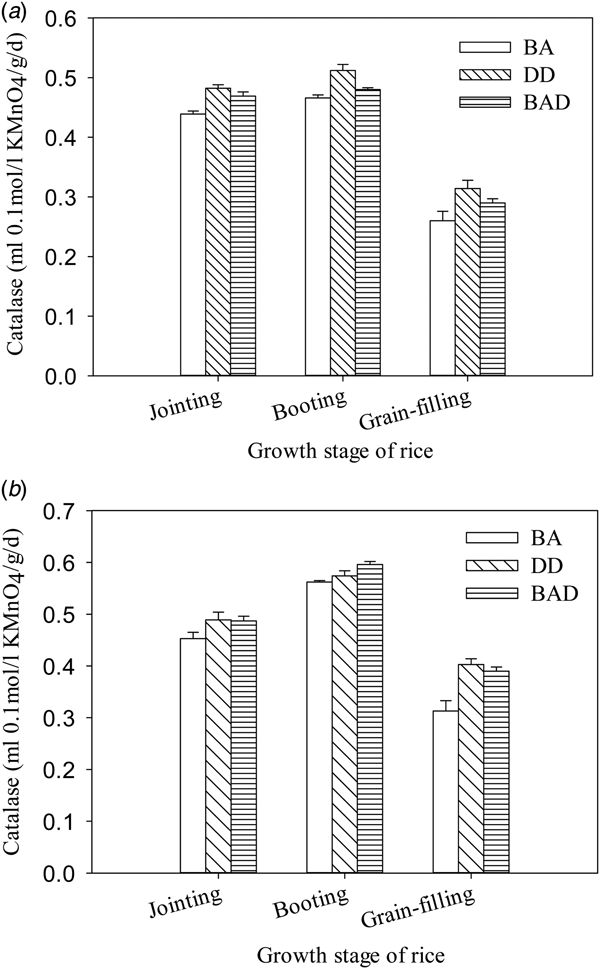
Soil catalase activity in 2011 and 2012 followed a pattern similar to those of invertase and urease (Fig. 3). Catalase activity in the DD and BAD groups at the booting and grain-filling stages was greater (by 0.01–0.09 ml 0.1 mol/l KMnO4/g/day) than in the BA group.

Fig. 3. Soil catalase activity in paddy plots under three different panicle fertilization modes. a, 2011 season; b, 2012 season. Values are means ± s.e. of three replicates. BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
By contrast, the differences in soil phosphatase activity among the three treatment groups and growth stages were complex. Soil phosphatase activity in the DD group was greater than in the BA group in most cases, except at the booting stage (Fig. 4(a)). At this stage, the difference was insignificant in 2011, while the DD group showed much more activity than the BA group in 2012. Similarly, the effects of BAD treatment on phosphatase activity at the jointing and grain-filling stages were not consistent in 2011 and 2012. At the jointing stage, there was only a slight difference in soil phosphatase activity between the BA and BAD groups in 2011, while in 2012 BAD treatment was associated with significantly more activity. At the grain-filling stage, soil phosphatase activity was much greater in the BAD group in 2011, while there was no significant difference in 2012 (Fig. 4(b)). Generally, soil phosphatase activity in the BAD and DD groups was greater than in the BA group at all three critical rice growth stages, indicating that tillage practice also affected phosphatase activity.

Fig. 4. Soil phosphatase activity in paddy plots under three different panicle fertilization modes. a, 2011 season; b, 2012 season. Values are means ± s.e. of three replicates. BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
Furthermore, GMea was calculated as a comprehensive indicator of soil biological quality. Compared with the BA control, GMea was improved significantly in the BAD and DD groups (P < 0.05, Fig. 5). At the jointing stage, GMea of the DD and BAD groups increased by 0.10 and 0.07, respectively, in 2011 and by 0.20 and 0.09 in 2012. A significant difference was also observed between the DD and BAD groups (P < 0.05). At the booting stage, DD treatment and BAD treatment enhanced GMea by 0.12 and 0.20, respectively, in 2011 and by 0.15 and 0.12 in 2012. At the grain-filling stage, GMea increased by 0.14–0.19 in the DD and BAD groups in both 2011 and 2012.

Fig. 5. Geometric means of the assayed enzyme activities (GMea) under three different panicle fertilization modes. a, 2011 season; b, 2012 season. Values are means ± s.e. of three replicates. BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
Effects of panicle nitrogen application on bleeding sap intensity
The root basal internode bleeding intensity was affected significantly by deep ditching and panicle N fertilization (P < 0.05, Fig. 6). The BA treatment was associated with the lowest bleeding intensity among the three treatments. Compared with BA, the DD and BAD treatments increased bleeding intensity by 24.99 and 5.52 mg/stem/h, respectively, 19 days after anthesis. Moreover, bleeding intensity with the DD treatment was 10.41 and 5.27 mg/stem/h higher than that with BA at 25 and 31 days after anthesis, respectively.

Fig. 6. Fluctuations in bleeding sap intensity at root basal internodes under three different panicle nitrogen application modes in 2012. Values are means ± s.e. of three replicates. BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
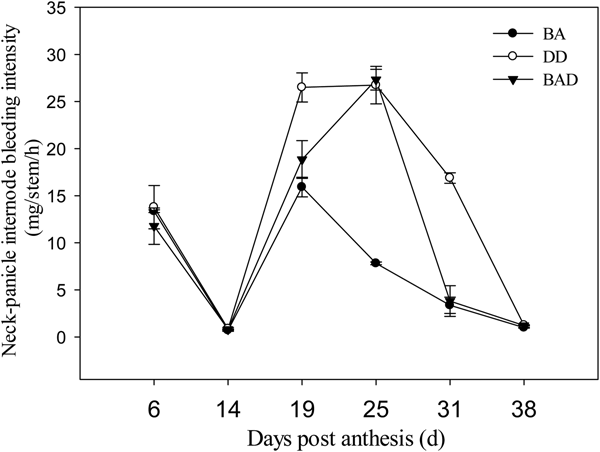
Except for 25 days after anthesis, trends in the neck-panicle internode bleeding intensity (Fig. 7) were consistent with those in root basal internode bleeding intensity (Fig. 6). Bleeding intensities in the DD and BAD groups were 10.59 and 2.93 mg/stem/h higher, respectively, than in the BA group 19 days after anthesis. Compared with BA, DD and BAD increased the bleeding intensity by 2.42 and 2.50-fold, respectively, 25 days after anthesis. At 31 days after anthesis, bleeding intensity in the DD group was 3.02-fold higher than that with BA. This indicates that deep ditching and panicle N fertilization can increase the root basal internode and neck-panicle internode bleeding intensity in rice.

Fig. 7. Fluctuations in bleeding intensity at neck-panicle internodes under three different panicle nitrogen application modes in 2012. Values are means ± s.e. of three replicates. BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
Effects of panicle nitrogen application on rice yield and its components
Both the grain weight and yield were significantly higher in the DD group than in the BA group in 2011 and 2012 (P < 0.05). The yield difference was mostly caused by more rainy days (14 days) in September 2011 than in 2012 (6 days), during the grain filling stage. Compared with BA, the yields of plants in the DD and BAD groups increased by 0.63 and 0.16 t/hm2 in 2011 and by 0.31 and 0.20 t/hm2 in 2012, respectively (Table 1). No significant differences were observed among the three treatment groups for panicle number, number of spikelets per panicle, or seed setting rate. A significant increase in the 1000-grain weight explained the majority of the improvement in yield associated with panicle fertilization combined with moderate tillage (P < 0.05).
Table 1. Rice yield and its components under three different panicle nitrogen application modes in 2011 and 2012

BA, manual broadcast application; DD, deep application of urea into 15-cm-deep ditches; BAD, manual broadcast application on the soil surface during deep ditching.
Data are represented as means ± .s.e. of three replicates.
Discussion
In China, panicle N fertilizer is usually applied to rice fields after sun-drying. To increase fertilization efficiency, in the current study N was applied during soil ditching on the final day of field drying. In a previous study, it had been observed that deep ditches and ridges enhanced rice grain yield by increasing the transport of nutrients to sinks (i.e. grains) enhancing the grain-filling process during the middle growth stage (Zhang et al., Reference Zhang, Zhang, Du, Li, Sun and Zhao2012). In the present study, the effects of ditching combined with panicle N fertilizer application after sun-drying fields of sandy loam paddy soil were investigated based on soil microbe activity, the physiological activity of roots and their relationships with rice yield.
It was observed that soil enzyme activity was generally higher in the ditching and panicle N fertilizer treatment groups than in the control group, indicating that these practices increased soil microbial activity, consequently enhancing soil nutrient availability. Panicle N fertilization combined with ditching increased the activity of invertase, which catalyses the hydrolysis of sucrose to yield glucose and fructose (Frankeberger and Johanson, Reference Frankeberger and Johanson1983). The increased invertase activity observed in the ditching treatments may have resulted partly from the cutting of roots during the practice of ditching, which enhances the leaking of invertase from the roots. However, it was difficult to control the extent of root cutting, which resulted in several values of invertase activity that were higher in the BAD group than in the DD group.
It has been reported that N fertilization triggers phosphorous demand and hence increases the activity of phosphatases (Allison et al., Reference Allison, Nielsen and Hughes2006; Pandey et al., Reference Pandey, Agrawal and Bohra2014). Both acid and alkaline phosphatase activities vary widely among different soil management, fertilizer and tillage routines (Dick et al., Reference Dick, Rasmussen and Krele1988; Gupta and Germida, Reference Gupta and Germida1988; Dick, Reference Dick, Doran, Coleman, Bezdicek and Stewart1994; Kandeler et al., Reference Kandeler, Tscherko and Spiegel1999). In the present study, it was observed that acid phosphatase activity was generally low in all three groups in 2011, possibly due to the soil pH (7.2) (Iyyemperumal and Shi, Reference Iyyemperumal and Shi2008). In 2012, soil alkaline phosphatase activity was re-assessed and similar results to those in 2011 were observed. The higher levels of phosphatase activity in the DD and BAD groups are expected to increase soil phosphorus availability.
Crop roots not only respond to water and fertilizer use but also adapt to changes in the soil environment (Jackson et al., Reference Jackson, Manwaring and Caldwell1990; Zhang and Forde, Reference Zhang and Forde1998). The bleeding intensity of roots can provide an accurate indicator of root physiological activity (Yoshida and Oritani, Reference Yoshida and Oritani1974; Soejima et al., Reference Soejima, Sugiyama and Ishihara1992; Fan et al., Reference Fan, Zhang, Turner, Duan, Wang and Shen2010). Deep fertilizer application can promote root foraging (Chancy and Kamprath, Reference Chancy and Kamprath1982; Kundu et al., Reference Kundu, Ladha and Lapitan-de Guzman1996), and in the current study root bleeding intensity was consequently higher in the DD and BAD groups. Furthermore, an active rice root system releases substances to either disturb or support microbial populations and enzyme activity in soil (Lu et al., Reference Lu, Watanabe and Kimura2002; Högberg and Read, Reference Högberg and Read2006; Spohn and Kuzyakov, Reference Spohn and Kuzyakov2014). In the cropping system described in the present study, increased root physiological activity may contribute to enhanced soil enzyme activity.
In rice plants, sap bleeding from basal internodes and neck-panicle internodes contains N and amino acids, which influence N uptake and grain yield (Zhao et al., Reference Zhao, Gao, Huang and Ling2001; Sakaigaichi et al., Reference Sakaigaichi, Morita, Abe and Yamaguchi2007; Fan et al., Reference Fan, Zhang, Turner, Duan, Wang and Shen2010). Yin et al. (Reference Yin, Yang, Du, Zhang, Li, Sun, Peng and Zhao2013) found that the bleeding intensity of the neck–panicle internodes was positively related to inferior grain plumpness at 10, 15, 25 and 35 days after anthesis in japonica rice, which also suggests a close relationship between the bleeding intensity of neck–panicle internodes and rice yield. With regard to the rice yield components, increased grain yield was mostly due to the 1000-grain weight rather than the panicle number or spikelet number per panicle in the deep ditching and deep fertilizer placement treatments.
Conclusions
The current study shows that synchronous ditching and panicle N fertilizer application improved soil biochemical properties by enhancing soil invertase, urease, catalase and phosphatase activity. The practice of ditching also increased rice root physiological activity, which further increased soil enzyme activity. Furthermore, the observed increases in grain yield were mostly due to increases in the bleeding intensity of basal root and neck-panicle internodes, which in turn increased grain weight. These results suggest that the deep application of a panicle N fertilizer combined with simultaneous ditching using a furrowing machine offers an effective approach for increasing rice yields. Moreover, given the reduction in labour achieved by synchronous ditching and deep N fertilizer application after sun-drying rice paddies, the method described in the current study offers an efficient process suitable for panicle N fertilizer application in loam paddy soil.
Financial support
This work was supported by the National Key RandD Program of China (2016YFD0300900 and 2016YFD0300505), the Special Fund for Agro-scientific Research in the Public Interest of China (201303102), and the earmarked fund for Henan Agriculture Research System (S2012-04-02). J. Zhang thanks the China Scholarship Council and Basic and Frontier Technology Research Project of Henan province (162300410159) for their support.
Conflict of interest
None.
Ethical standards
Not applicable.