Introduction
Cereal straws are agricultural by-products of poor nutritive value for ruminants because of low digestibility and low protein concentration (Antongiovanni et al., Reference Antongiovanni, Acciaoli, Grifoni, Martini and Ponzetta1991). Although applying chemical treatments to low-quality forage such as wheat straw has been evaluated before (Horton et al., Reference Horton, Nicholson and Christensen1982; Antongiovanni et al., Reference Antongiovanni, Acciaoli, Grifoni, Martini and Ponzetta1991), there are some concerns related to animal and labour health while working with chemical compounds. Some other strategies to improve low-quality forage nutrient utilization provide enough ruminal degradable nitrogen (RDN) for microbial growth (Males, Reference Males1987; Salisbury et al., Reference Salisbury, Krehbiel, Ross, Schultz and Melton2004), in addition to providing adequate rumen undegradable protein levels (Batista et al., Reference Batista, Detmann, Titgemeyer, Valadares Filho, Valadares, Prates, Rennó and Paulino2016) to improve urea recycling into the gastrointestinal tract. Indeed, ammonia may be a first limiting nitrogen source for fibre-degrading bacteria (Griswold et al., Reference Griswold, Hoover, Miller and Thayne1996). Therefore, providing an adequate RDN source could positively affect the nutrient utilization of low-quality forage through providing ruminal ammonia nitrogen. It has also been determined that addition of peptide and amino acid nitrogen sources improve digestion of fibre (Griswold et al., Reference Griswold, Hoover, Miller and Thayne1996) and may have additional nutritional benefits for host animals (Reynal et al., Reference Reynal, Ipharraguerre, Liñeiro, Brito, Broderick and Clark2007). Males (Reference Males1987) reported that natural protein sources were more effective than non-protein nitrogen sources by providing more amino acids as well as higher peptide contents when high levels of wheat straw are fed to growing beef cattle. Soybean meal (SBM) and cottonseed meal (CSM) are solid natural protein sources and have not been compared with a liquid protein source, although some studies have evaluated the availability of liquid protein feeds in the rumen (Elwakeel et al., Reference Elwakeel, Titgemeyer, Drouillard and Armendariz2007) and evaluated the inclusion of liquid protein feeds in animal diets (Schingoenthe, Reference Schingoenthe1976; Bowman et al., Reference Bowman, Sowell and Paterson1995; Walker et al., Reference Walker, LaMay, Davis and Bandyk2013). However, there is a lack of data regarding the inclusion of liquid protein in the diets of animals fed with a low-quality forage diet. The comparison of a liquid protein feed and conventional protein sources used in ruminant nutrition is not well documented.
Maize steep liquor (MSL) is an inexpensive, palatable and abundant by-product of wet-maize milling, which has been evaluated in cattle (Ribeiro-Filho and Trenkle, Reference Ribeiro-Filho and Trenkle2002), dairy cows (Wickersham et al., Reference Wickersham, Shirley, Titgemeyer, Brouck, DeFrain, Park, Johnson and Ethington2004) and growing lambs (Azizi-Shotorkhoft et al., Reference Azizi-Shotorkhoft, Sharifi, Mirmohammadi, Baluch-Gharaei and Rezaei2016). This feedstuff is a viscous slurry, which has a high soluble protein content, but data regarding the comparison of its inclusion with other conventional protein sources is scarce. The current study hypothesized that inclusion of MSL as a liquid natural protein source could improve nutrient utilization of a low-quality forage basal diet.
The current study aims to evaluate the effects of including MSL as a liquid protein source in the diets of growing lambs, compared to two conventional solid protein sources (SBM and CSM), on growth performance, ruminal fermentation, microbial protein synthesis, blood metabolites and liver enzymes, and carcass characteristics of growing lambs fed high dietary levels of wheat straw.
Materials and methods
Lamb management and diets
The present study was conducted in the Animal Production Station of Arak University, Arak, Iran, according to the guidelines of the Iranian Council of Animal Care (1995). The study lasted 9 weeks, with the first week for adaptation of the animals to the experimental diets and conditions. Average environmental conditions in the farm throughout the study were: temperature ranging between 25 and 31 °C, relative humidity in the range 61–67% and day length of 16–17 h/day. Eighteen male Farahani lambs (36 ± 3.3 kg body weight, age 5.8 ± 0.23 months) were used in a complete randomized design with three different protein sources (six animals per experimental group). The total mixed ration (TMR) fed to the lambs was formulated according to National Research Council (NRC, 2007 – small ruminant requirement recommendations for lambs) and its ingredient and chemical composition is shown Table 1. The animals were housed in individual pens and had free access to water. The basal diet contained the same amount of wheat straw [400 g/kg; dry matter (DM) basis] in all treatments. To obtain iso-nitrogenous experimental diets, the MSL, SBM and CSM were included at 130, 120 and 150 g/kg (DM basis) of TMR, respectively. The MSL was provided by a Glucosan factory (Qazvin, Iran). The TMR was fed to animals in two equal meals at 08.00 and 16.00 h. Orts were collected and weighed once daily at 07.30 h and the amount of feed offered to each animal adjusted to attain refusals of 10% intake. The DM intake was calculated depending on the DM composition of feed and orts.
Table 1. Feed ingredients and chemical composition of experimental diets (g/kg dry matter; unless otherwise stated)

a Protein sources were: maize steep liquor (MSL), soybean meal (SBM) and cottonseed meal (CSM) feeding in growing lambs fed high level of wheat straw diet [400 g/kg dry matter (DM) basis].
b Maize steep liquor contained 420 g/kg crude protein (DM basis). Dry matter content of MSL was 520 g/kg and its soluble protein content was 360 g per kg DM.
c Soybean meal solvent contained 450 g/kg crude protein (DM basis).
d Cottonseed meal contained 380 g/kg crude protein (DM basis).
e Contained per kilogram of supplement: 2 50 000 IU vitamin A, 40 000 IU vitamin D3, 1000 vitamin E, 750 g manganese, 110 g calcium, 2000 mg zinc, 45 g phosphorus, 20 g magnesium, 15 g sodium, 1000 mg iron, 8 mg cobalt, 500 mg copper, 20 mg iodine and 10 mg selenium.
f Calculated based on NRC (2001).
Sample collection, chemical and biochemical analysis
Body weights were recorded on the first day of the experiment and at 10 day-intervals thereafter until the end of the experiment (i.e. 9 weeks later). Animals were weighed before the morning meal to minimize the effect of digestive contents on body weight. Feed conversion ratio (FCR) was calculated as kg intake per kg of gain. The weight gain per 100 g of nitrogen (N) intake was considered as nitrogen conversion ratio into weight gain. Samples of each TMR were collected daily and DM was determined by drying at 60 °C for 48 h (AOAC, 1995). Composite samples of each TMR were ground in a Wiley mill through a 1 mm screen, and analysed to determine total nitrogen, ether extract and ash contents (AOAC, 1995). The methods of Van Soest et al. (Reference Van Soest, Robertson and Lewi1991) were used for the determination of neutral detergent fibre (NDF), which was assayed with a heat-stable amylase and expressed inclusive of residual ash. The soluble fraction of protein as the proportion of total protein (TP) in MSL was measured by a protein fractionation system (Licitra et al., Reference Licitra, Hernandez and Van Soest1996) with some modifications described by Eghbali et al. (Reference Eghbali, Kafilzadeh, Hozhabri, Afshar and Kazemi-Bonchenari2011) in using phosphate buffer solution.
On the last 5 days of the experiment, two faecal grab samples were collected daily from each animal at 6 and 18 h after the morning meal (ten samples for each lamb). Faecal samples were dried in a forced draft oven (60 °C; 72 h), and then ground in a Wiley mill through a 1 mm screen. Aliquots of all faecal samples collected for each lamb were mixed to obtain one composite sample for each animal. These faecal samples were analysed to determine total nitrogen, ether extract and NDF based on the procedures mentioned above for TMR samples. Apparent total tract digestibility coefficients of dietary components were measured using acid-insoluble ash as an internal marker (Van Keulen and Young, Reference Van Kuelen and Young1977). In this method, the acid-insoluble ash concentration was determined in feed and faeces by treating the ash with a 2 N hydrochloric acid (HCl) solution.
Samples of rumen fluid were collected using a stomach tube at 4 h after the morning feeding on days 28 and 56 of the experiment. The first 5 ml was eliminated because of possible saliva contamination and ruminal pH was measured immediately (HI 8314 membrane pH meter, Hanna Instruments, Villafranca, Italy). The rumen samples were squeezed through four layers of cheesecloth. Two samples of rumen fluid (8 ml each) were mixed with 0.2 ml sulphuric acid 50% and stored at −20 °C. Just before analysis, samples were thawed, centrifuged at 10 000 g (4 °C, 20 min). The clarified supernatant was then decanted and analysed for ruminal ammonia-N using a modified phenol-hypochlorite method adapted from Broderick and Kang (Reference Broderick and Kang1980). In this method, phenol-hypochlorite and ninhydrin colorimetric procedures were used to measure ammonia concentration in the ruminal fluid of animals. The other sample was used for the analysis of short chain fatty acids (SCFA) using a gas chromatograph (model CP-9002; Chrom-pack, Delft, The Netherlands) with a 50 m (0.32 mm ID) silica-fused column (CP-Wax Chrompack Capilary Columnl Varian, Palo Alto, CA, USA) and using helium as the carrier gas, as described previously (Kazemi-Bonchenari et al., Reference Kazemi-Bonchenari, Mirzaei, Jahani-Moghadam, Soltani, Mahjoubi and Patton2016).
The spot urine sampling technique was used for the estimation of daily urine output from creatinine concentration (Chen et al., Reference Chen, Mejia, Kyle and Ørskov1995). On days 29 and 58 of the experiment, spot urine samples were collected from each animal during the morning (between 09.00 and 11.00 h). Samples were collected when lambs urinated spontaneously. An aliquot of 10 ml of each sample was diluted immediately with 90 ml of 0.036 N sulphuric acid and stored at −20 °C for analysis. Later, urine samples were thawed at room temperature and analysed to determine the creatinine, uric acid and allantoin as described previously (Kazemi-Bonchenari et al., Reference Kazemi-Bonchenari, Salem and Lopez2017). In this method, total urine volume was estimated based on daily creatinine excretion, due to the close relationship between body mass and creatinine excretion. An average of 9.79 mg/kg BW for creatinine excretion was used for total urine volume estimated as previously found for sheep (David et al., Reference David, Poli, Savian, Amaral, Azevedo and Jochims2015). Thereafter, the total excretion of allantoin and uric acid was calculated from estimated daily urine output and determined metabolite concentrations. The synthesis of microbial N in the rumen was calculated from daily urinary purine derivative (PD) output using the following equation described by Chen and Gomes (Reference Chen and Gomes1992);

where X is the microbial purine absorbed (mmol/day), 70 is the nitrogen content of purines coefficient (mg N/mmol), 0.116 is the ratio of purine nitrogen to total nitrogen in mixed rumen microbes which is 11.6:100, and 0.83 is the average digestibility of microbial purines.
Blood was sampled from the jugular vein of each lamb twice throughout the study (on days 30 and 60 of the experiment), 4 h after the morning feed. Blood samples were heparinized (Na-heparin) and stored at 2 °C; then centrifuged at 3000 g (4 °C, 15 min) and the plasma stored at −20 °C. After thawing, the samples were analysed to determine the concentrations of glucose (Kit No. 9308), albumin (Kit No. 9307), TP (Kit No. 9304), blood urea nitrogen (BUN) (Kit No. 93013), aspartate aminotransferase (AST) (Kit No. 11840) and alanine aminotransferase (ALT) (Kit No. 11940) using commercial kits in accordance with the manufacturer's instructions (Pars Azmoon Co., Tehran, Iran).
At the end of the experiment (day 70), lambs were slaughtered and carcass characteristics measured. Dressing percentage (the ratio of hot carcass weight to live body weight) as well as different organ weights such as liver, heart and gastrointestinal tract were measured. Fat distribution in different deposition sites throughout the body was also measured. The deposited fat in fat-tail, mesenteric and abdominal sites was recorded by weighing the fat mass. However, the back-fat thickness was measured by fat depth (mm) coverage between ribs 12 and 13, measured by callipers (Esquivelzeta et al., Reference Esquivelzeta, Casellas, Fina and Piedrafita2012). Moreover, the total fat deposited in the liver was measured by the method of Folch et al. (Reference Folch, Lees and Sloane-Stanley1957): briefly, fat deposited in the liver was extracted by a detergent containing chloroform, methanol and water in the ratio 85:14:1 (by volume).
Statistical analysis
The data were analysed applying SAS (version 9.1; SAS Inst. Inc., Cary, NC, USA) and using PROC MIXED using POLYANOVA routine for performance variables where there were repeated measurements over time (feed intake, daily gain, FCR, nitrogen intake and nitrogen conversion ratio) using the following model:
where Y ijkl is the dependent variable, μ is the overall mean, A i is the effect of animal, Prj is the effect of protein source j (MSL, SBM and CSM), T k is the effect of sampling time k (different sampling times), PrT jk is the interaction between time k and protein source j, β (X i−X̅) is the covariate variable (used for BW with initial BW as covariate) and ε ijkl is the residual error. The POLYANOVA model was considered for performance data because there were six time repetitions for each of these variables. Linear and quadratic contrasts were reported from this statistical analysis route output. The variables with two repetitions throughout the study (ruminal fermentation metabolites, microbial protein synthesis and blood metabolites) were analysed based on the above-mentioned model in PROC MIXED and linear contrast was reported as a general P value. The data for variables that did not have repeated measurements over time (apparent digestibility coefficient of components and carcass characteristics) were analysed using PROC GLM using the following model:
where Y i is the dependent variable, μ is the overall mean, Pri is the effect of protein source i (MSL, SBM and CSM) and ɛ i is the residual error. The model contained lamb effect within the treatment as a random effect. Differences between least squares means were considered significant at P ⩽ 0.05, and differences were considered to indicate a trend towards significance at 0.05 < P ⩽ 0.10. In addition to overall treatment comparisons, orthogonal contrasts were constructed to compare (1) MSL v. SBM and CSM and (2) SBM v. CSM.
Results
Chemical analysis, animal growth and components digestibility coefficient
The crude protein (CP) contents for MSL, SBM and CSM were 420, 450 and 380 g/kg (DM basis), respectively. The DM content of MSL was 520 g/kg and the soluble protein content for MSL was 360 g/kg DM.
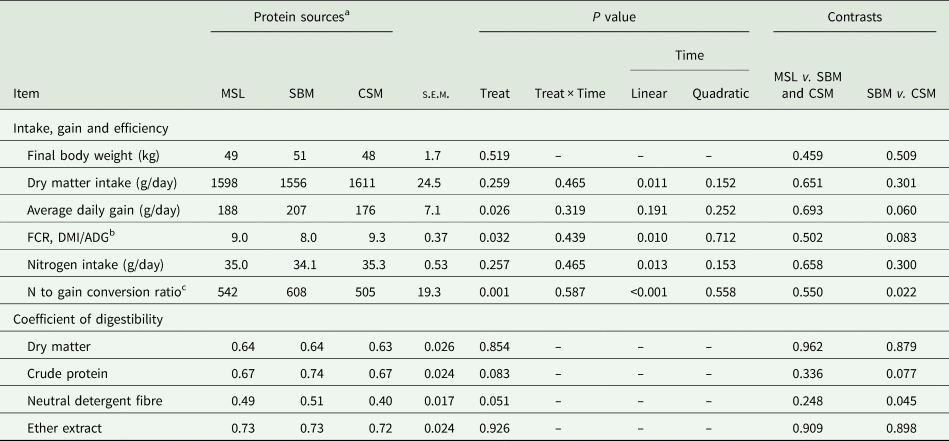
Feed intake did not differ (P = 0.259) among different protein sources, while the average daily gain (ADG) (P = 0.026) and FCR were improved (P = 0.032) in SBM-fed lambs compared with CSM and MSL animals (Table 2). The lowest daily gain was observed in CSM-fed lambs (176 g/day), and in contrast, the greatest gain was seen in SBM-fed lambs (207 g/day). The FCR tended towards significance in SBM-fed lambs compared to CSM (P = 0.083). Although the nitrogen intake was constant among different protein sources in the current study (P = 0.257), the nitrogen conversion ratio (g weight gain per 100 g nitrogen intake) was greater in SBM (P = 0.022) v. CSM lambs. Dry matter intake, FCR, nitrogen intake and nitrogen conversion ratio were influenced with time (P = 0.024); however, the daily gain was not affected by time in the current study.
Table 2. Effects of maize steep liquor inclusion in growing lambs' diet fed high level of wheat straw on growth and component digestibility coefficient and compared to soybean meal and cottonseed meal (n = 18)

a Protein sources were: maize steep liquor (MSL), soybean meal (SBM) and cottonseed meal (CSM) feeding in growing lambs fed high level of wheat straw diet (400 g/kg DM basis).
b Feed conversion ratio is calculated as kg dry matter intake per kg of weight gain.
c Nitrogen to gain conversion ratio calculated as the amount of weight gain per 100 g of N intake.
The total tract digestibility coefficient data showed an improvement in fibre digestibility for SBM compared with MSL and CSM lambs (P = 0.051) (Table 2). Digestibility of CP tended to be greater in SBM v. CSM lambs (P = 0.077). The other nutrient digestibility measurements (DM; P = 0.854; and EE; P = 0.926) were not affected by dietary protein sources (Table 2).
Rumen fermentation pattern
Ruminal fluid pH was not different (P = 0.334) among different protein sources fed to growing lambs (Table 3). Ruminal ammonia nitrogen was enhanced (P = 0.010) in both CSM and MSL v. SBM lambs. Total SCFA production was different among different protein sources, with SBM shown to have the greatest concentration (P = 0.026). With the exception of isovalerate, which has relatively constant concentration among different protein sources in the current study (P = 0.561), the remaining individual SCFAs were shown to have a greater concentration in SBM-fed lambs v. the other experimental diets. The statistical contrasts showed that MSL-fed lambs produced less butyrate (P = 0.001) and had lower acetate to propionate ratios (P = 0.034) compared with either SBM or CSM diets (Table 3). Effect of time did not differ for rumen fermentation variables in the current study (P = 0.846).
Table 3. Effects of maize steep liquor inclusion in growing lambs' diet fed high level of wheat straw on ruminal fermentation pattern and microbial protein synthesis and compared to soybean meal and cottonseed meal (n = 18)

a Protein sources were: maize steep liquor (MSL), soybean meal (SBM) and cottonseed meal (CSM) feeding in growing lambs fed high level of wheat straw diet (400 g/kg dry matter basis).
b A:P is the proportional ratio of acetate to propionate in the ruminal fluid.
c Branched chain volatile fatty acids (BCVFA) are the molar proportion of valerate + molar proportion of isovalerate.
d Total purine derivatives (TDP) is allantoin + uric acid.
e Microbial crude protein synthesis calculated through urinary TDP excretion based on Chen and Gomes (Reference Chen and Gomes1992).
Urinary purine derivatives excretion and microbial protein synthesis
The allantoin concentration was increased (P = 0.011) in SBM v. CSM- and MSL-fed lambs. Although uric acid concentration did not differ among different protein sources, total PD concentration was greater in SBM-fed lambs (P = 0.001). Microbial protein synthesis estimated through PD excretion was greater (P = 0.001) in SBM-fed lambs rather than those fed CSM or MSL (Table 3). Although MSL-fed lambs had lower PD excretion and lower microbial crude protein (MCP) production compared to SBM-fed lambs, the above-mentioned parameter values for this protein source were similar or greater rather than those observed for CSM-fed lambs (Table 3). Microbial protein synthesis variables were not influenced by sampling time in the current study (P = 0.714).
Blood metabolites and liver enzymes activities
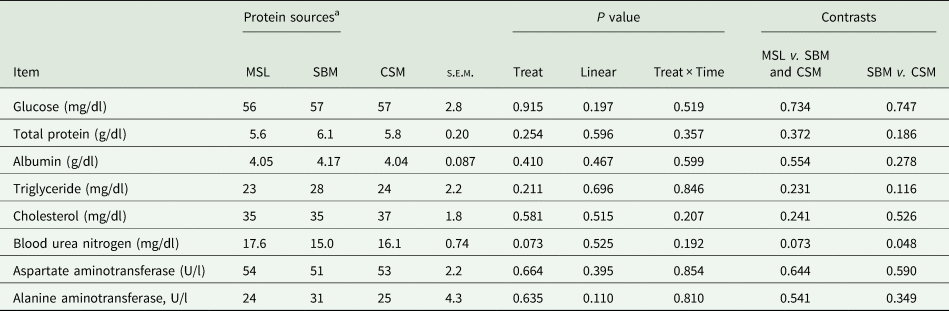
The blood concentrations of glucose (P = 0.915), TP (P = 0.254), albumin (P = 0.410), triglyceride (P = 0.211) and cholesterol (P = 0.581) were not different among dietary protein sources. Liver enzymes concentrations (AST and ALT) as well as other blood metabolites were not different among protein sources in the present study; however, the blood urea concentration was numerically increased in CSM and MSL compared to SBM-fed animals (P = 0.073) (Table 4). Time had no effect on blood biochemical variables in the present study (P = 0.197).
Table 4. Effects of maize steep liquor inclusion in growing lambs' diet fed high level of wheat straw on blood metabolites and liver enzymes and compared to soybean meal and cottonseed meal (n = 18)

a Protein sources were: maize steep liquor (MSL), soybean meal (SBM) and cottonseed meal (CSM) feeding in growing lambs fed high level of wheat straw diet (400 g/kg dry matter basis).
Carcass characteristics and fat deposition sites
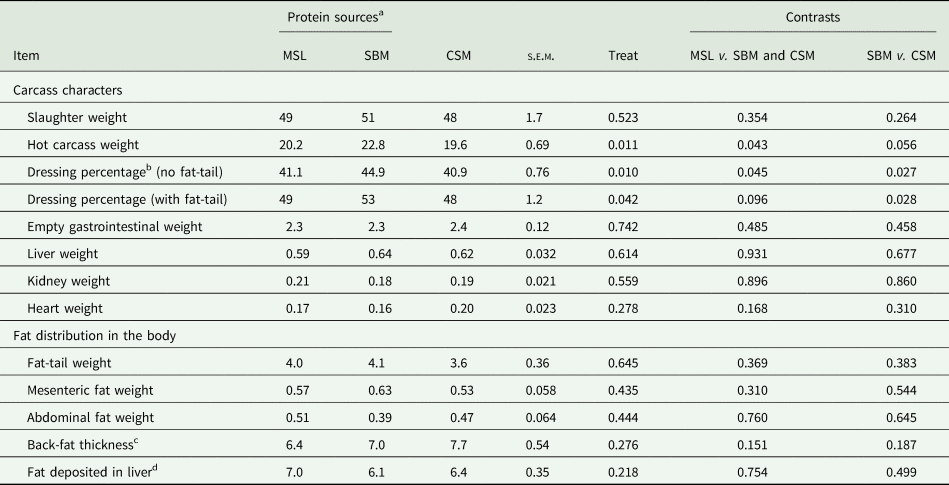
Slaughter weight was no different among dietary protein sources (P = 0.523); while hot carcass weight (P = 0.011) and dressing percentage were greater in SBM-fed lambs (P = 0.010). Results indicated an improvement in carcass weight and efficiency when comparing MSL with SBM and CSM (P = 0.042). Internal organ weights [liver (P = 0.614); kidney (P = 0.559); heart (P = 0.278)] as well as gastrointestinal weight (P = 0.742) did not differ among different protein sources. There was no difference among different fat deposition sites for different protein sources in the current study (P = 0.310) (Table 5).
Table 5. Effects of maize steep liquor inclusion in growing lambs' diet fed high level of wheat straw on carcass characteristics and fat deposition (kg) and compared to soybean meal and cottonseed meal (n = 18)

a Protein sources were: maize steep liquor (MSL), soybean meal (SBM) and cottonseed meal (CSM) feeding in growing lambs fed high level of wheat straw diet (400 g/kg dry matter basis).
b Dressing percentage was calculated as hot carcass weight divided by slaughter weight.
c Back-fat depth (mm) was considered as fat thickness covered between ribs 12 and 13 measured by callipers (Esquivelzeta et al., Reference Esquivelzeta, Casellas, Fina and Piedrafita2012).
d As the per cent of liver weight which was measured based on Folch et al. (Reference Folch, Lees and Sloane-Stanley1957).
Discussion
Growth, total tract digestibility coefficients and ruminal fermentation pattern
Energy is the main factor controlling feed intake in animals (Allen et al., Reference Allen, Bradford and Oba2009); therefore, the similar energy contents of the experimental diets in the current study probably resulted in similar intakes in different treatments. Despite the similar intake among different protein sources, the greater gain was observed with SBM compared to CSM or MSL-fed lambs. This could be related to rumen fermentation patterns as well as the total tract digestibility coefficient. Ruminal SCFA concentration was greatest for lambs fed SMB and least for CSM, with MSL-fed lambs having intermediate SCFA concentrations. This shows greater energy extracted from the diet towards SCFA production, and as stated previously the main energy source for ruminant performance is SCFA (Bergman, Reference Bergman1990) which could subsequently increase gain and improve FCR in the SBM diet. The greater individual volatile fatty acids, especially propionate and butyrate, could also influence gain and efficiency from the stoichiometry point of view (Ørskov and Ryle, Reference Ørskov and Ryle1990) which was observed in the SBM diet in the current study. The improved fibre digestion in SBM-fed lambs could also be an effective factor in improving gain. Soybean meal is a protein source which could produce relatively high peptide levels (Wang et al., Reference Wang, Cui, Liu, Zhao, Wang, Liu and Xie2017). Addition of a peptide source in continuous culture has been reported to increase branched chain volatile fatty acid (BCVFA) production (Griswold et al., Reference Griswold, Hoover, Miller and Thayne1996), which has potential for increasing fibre digestion (Gorosito et al., Reference Gorosito, Russell and Van Soest1985). In the present study, BCFVA was increased with SBM feeding in comparison with the other two protein sources. The results of the present study revealed that feeding SBM resulted in greater BCVFA as well as greater fibre digestibility compared to CSM and MSL. It seems that greater fibre digestibility supplies more organic matter as a substrate for the production of SCFA in the SBM diet. Regardless of the positive effects of SBM on growth and ruminal fermentation pattern in the current study, comparison of the MSL and CSM protein sources indicated that both ruminal SCFA as well as propionate concentrations were greater in MSL-fed lambs, which may partly explain the greater gain and improved FCR in this diet (8.96 v. 9.31 for MSL and CSM, respectively). Improved fibre digestion in MSL compared to CSM (0.49 v. 0.40 for MSL and CSM fibre digestibility coefficients, respectively) may be partly related to greater RDN for microbes, supplied through greater RDP in the diet, which had a positive influence on cellulolytic bacterial activity. The related mechanism for greater RDN effect on cellulolytic bacteria activity has been stated in previous works (Griswold et al., Reference Griswold, Hoover, Miller and Thayne1996, Reference Griswold, Apgar, Bouton and Firkins2003). Considering the rumen fermentation pattern and nutrient digestibility, these indicate that greater fibre digestibility resulted in greater SCFA concentration, and in turn, greater BCVFA caused greater fibre digestibility. Results show that although MSL as a liquid protein feed was not as profitable as SBM in lambs' growth, it was more efficient than CSM in parameters such as rumen fermentation profile and fibre digestibility. Considering the effect of time on performance in the current study, it is clear that DM intake, FCR, nitrogen intake and nitrogen conversion ratio were influenced by different sampling times. The above-mentioned traits responded to time throughout the study. This clarifies that regardless of protein source in the current study, intake level, FCR and nitrogen efficiency may be influenced by animal age, which is mostly related to whole-body metabolism of the animal at different ages (Atti and Mahouachi, Reference Atti and Mahouachi2011). Zhao et al. (Reference Zhao, Gordon, O'Connell and Yan2016) stated that younger lambs have better nitrogen efficiency in comparison with older ones, which could consequently affect whole-body efficiency towards better FCR.
Ruminal pH fluid did not differ among different protein sources in the current study and all recorded pH values were in range for healthy animals (>5.8) (Penner et al., Reference Penner, Beauchemin and Mutsvangwa2007). Greater ruminal ammonia nitrogen concentration in both MSL and CSM diets showed lower nitrogen efficiency in these protein sources compared with SBM-fed animals. In agreement with rumen fermentation profile results in the current study, looking at the nitrogen conversion ratio into a gain (weight gain per 100 g of nitrogen intake) shows that this variable was greater for the SBM diet compared to MSL or CSM. Regardless of the greater nitrogen efficiency observed for SBM-fed lambs, comparing the results obtained for MSL and CSM indicates a greater nitrogen conversion ratio for MSL in comparison with CSM-fed lambs (542 v. 505 for MSL and CSM, respectively). The greater nitrogen conversion ratio for MSL compared to CSM-fed lambs may relate to improved fibre digestibility and increased ruminal SCFA production in this protein source.
Urinary purine derivatives excretion and microbial protein synthesis
Urinary allantoin was increased in lambs fed SBM and, because the main portion of excreted PD is allantoin, the urinary excreted PD was also greater for this protein source. The results of the current study indicated that SBM was the source of protein preferred by ruminal microbes in comparison with MSL and CSM. Based on the previous literature, optimal ammonia nitrogen as well as optimum peptide nitrogen in the ruminal fluid are necessary to have adequate MCP production (Griswold et al., Reference Griswold, Hoover, Miller and Thayne1996). Based on the findings of the current study, it could be suggested that SBM provided enough ammonia nitrogen and peptide nitrogen to obtain greater MCP. Regarding the microbial protein synthesis, comparing the MSL and CSM diet results suggests that neither was highly preferred by ruminal microbes. Lower MCP observed in both MSL and CSM diets in comparison with SBM may be partly related to greater ruminal ammonia nitrogen concentration in these two treatments compared to SBM animals. The current results suggest that incorporating SBM with low-quality forage fed to growing lambs caused better nitrogen capturing than either MSL or CSM; therefore, we observed greater N efficiency in lambs fed SBM. The results of the current study indicate that ruminal ammonia concentration ~14 mg/dl was enough for optimum microbial growth when a high dietary level of wheat straw was fed to lambs and the greater MCP obtained in SBM was probably due to greater peptide concentration in ruminal fluid released from SBM. Future studies may need more consideration from the nitrogen efficiency point of view when liquid protein feed is supplemented to ruminants.
Blood metabolites and liver enzymes
Among the metabolites, BUN concentration was lower in SBM-fed lambs in comparison with other protein sources. As indicated previously, BUN can be used as an indicator of rumen nitrogen capture and associated with rumen ammonia concentration (DePeters and Ferguson, Reference DePeters and Ferguson1992). Lower BUN concentration observed in SBM-fed lambs revealed greater nitrogen capturing and subsequently its efficiency in this diet. The greater BUN in MSL and CSM diets compared to SBM-fed animals was in line with greater ruminal ammonia concentrations observed for these protein sources. Propionate concentration changed among diets and similar changes in blood glucose level were expected because propionate is the main glucose precursor in the liver (Bergman, Reference Bergman1990), but blood glucose concentration was similar among different protein sources. Even though blood glucose is a function of ruminal propionate concentration, it is an indicator of the energy status of the body and is a function of intake level that provides more energy to metabolism (Reynolds et al., Reference Reynolds, Aikman, Lupoli, Humphries and Beever2003), which was constant in the current study. Total protein and albumin were also constant among experimental diets, partly due to similar protein levels of the experimental diets. Similarly, due to the relatively constant amounts of ether extract in the diets, cholesterol and triglycerides concentrations were not different among dietary protein sources in the current study. Similar blood metabolite ranges were found for MSL- and CSM-fed lambs, indicating that MSL as a liquid protein source has similar effects to a solid protein source on body energy indicators; this was probably due to similar intake in lambs fed different protein sources. No blood variables were influenced by sampling time in the current study. Previous works have shown that sampling time can affect some blood metabolite concentrations in pre-weaned ruminants compared to mature ruminants (Kazemi-Bonchenari et al., Reference Kazemi-Bonchenari, Falahati, Poorhamdollah, Heidari and Pezeshki2018). However, the experimental lambs used in the current study were mature ruminants and blood metabolites were mostly affected by experimental treatments rather than their physiological variations.
There were no differences between AST and ALT enzyme concentrations among lambs fed different experimental diets. Liver function is influenced by different situations such as reduced appetite and high fat deposition (Cebra et al., Reference Cebra, Garry, Getzy and Fettman1997), or when feeding high-grain diets through reducing rumen pH (Nagaraja and Lechtenberg, Reference Nagaraja and Lechtenberg2007). The results of the present study show that liver function was not negatively influenced with either liquid or solid protein source in the current study.
Carcass characteristics and fat deposition sites
No differences were found regarding most carcass characteristics in different protein sources fed to animals. However, the hot carcass weight and dressing percentage were increased in SBM-fed lambs. Comparing the MSL and CSM results in carcass characteristics indicated a slight improvement in carcass weight with MSL-fed lambs. Previously, it has been stated that energy (Craddock et al., Reference Craddock, Field and Riley1974) and protein level (Prado et al., Reference Prado, Campo, Muela, Valero, Catalan, Olleta and Sañudo2014) are the most effective factors influencing carcass characteristics in meat production animals. However, the energy and protein levels were similar among different experimental diets in the current study and the only variable was the protein source included in the diet. Examining SCFA produced in the rumen of the animals indicated that the greatest value was observed for SBM-fed lambs. Indeed, SCFA is the main source of energy in ruminants (Bergman, Reference Bergman1990) that can improve animal body gain. In addition, the greatest nitrogen conversion ratio to gain was observed in the SBM diet, indicating better nutrient partitioning into carcass deposition. Higher digestibility of amino acids in SBM than found in previous studies (Khorasani et al., Reference Khorasani, Sauer, Ozimek and Kennelly1990) could also be an issue in the better dressing percentage found in SBM diet. Dabiri and Thonney (Reference Dabiri and Thonney2004) found the best growth rate and efficiency in growing lambs fed SBM, compared with fish meal. Finally, better fibre digestibility was observed in SBM-fed lambs v. MSL and CSM diets, suggesting an improvement in dressing percentage. For all these reasons, it was found that SBM has the potential to improve dressing percentage compared to MSL and CSM in growing lambs fed low-quality forage diet.
It has been revealed in ruminants that greater energy intake (Beauchemin et al., Reference Beauchemin, McClelland, Jones and Kozub1995) or greater glucose concentration could influence fat deposition throughout the body (Hocquette et al., Reference Hocquette, Ortigues-Marty, Pethick, Herpin and Fernandez1998). In the current study, both intake level and blood glucose concentration were in similar ranges among experimental diets; hence, fat accumulation in different deposition sites was relatively homogenous among different protein sources. The results indicate that a liquid protein source could change neither fat content nor fat deposition site in growing lambs in comparison with solid protein sources.
Conclusion
Based on the current results, SBM could be a better dietary protein source for lambs fed low-quality forage (wheat straw) in comparison with MSL and CSM. However, results show that liquid protein feed (MSL) is recommended v. CSM when low-quality forage is included in growing lambs' diets. Greater ruminal volatile fatty acid production and enhanced nitrogen efficiency were seen by feeding MSL compared to CSM, showing the profitability of a liquid protein source while feeding low-quality forage diet in growing lambs.
Acknowledgments
Appreciations to the management board and staff of sheep production farm to help in collecting the data throughout the study.
Financial support
The data in this study were developed as a part of the first author thesis (Grant No. 94-5103). Special thanks to the deputy of research and technology in Arak University for covering the financial supports of the present study.
Conflict of interest
None.
Ethical standards
The experiments in this work were carried out according to the guidelines of the Iranian Council of Animal Care (1995).