Introduction
Changing meat fatty acid profiles in order to reduce the proportion of saturated fatty acids (SFA) is of paramount importance in the production of healthier meat for consumers (Ladeira et al., Reference Ladeira, Santarosa, Chizzotti, Ramos, Machado Neto, Oliveira, Carvalho, Lopes and Ribeiro2014). Thus, improving the nutritional quality of beef is a key challenge for the beef industry. The current challenge of global livestock production is to increase the productivity and quality of the end product, such as milk and meat, and reduce environmental impact. Thus, an alternative suggested for use in livestock to mitigate methane (CH4) emissions and improve meat quality is the inclusion of lipids in diets of ruminants (Cottle et al., Reference Cottle, Nolan and Wiedemann2011; Moloney et al., Reference Moloney, Kennedy, Noci, Monahan and Kerry2012). This strategy could be an attractive target for manipulation because the reduction of CH4 is usually linked to increased productivity (Leng, Reference Leng1993) and improvement in meat fatty acid profiles.
Soybean is one of the most commonly used lipid sources in ruminant diets in Brazil due to its availability, low cost and high nutritive content (Barletta et al., Reference Barletta, Rennó, Gandra, Freitas Júnior, Verdurico, Mingoti and Vilela2012). Brazil is currently one of the world's largest soybean producers (USDA, 2018). The lipids present in soy beans contain approximately 850 g/kg of unsaturated fatty acids (UFA), especially oleic, linoleic and linolenic acids. The inclusion of lipid sources in cattle feed may increase the energy density of the diet, resulting in increased energy intake, decreased methane emission and modification of the fatty acid profile of meat from these animals (Fiorentini et al., Reference Fiorentini, Berchielli, Santana, Dian, Reis, Sampaio and Biehl2012, Reference Fiorentini, Carvalho, Messana, Castagnino, Berndt, Canesin, Frighetto and Berchielli2014, Reference Fiorentini, Lage, Carvalho, Messana, Canesin, Reis and Berchielli2015a). According to Grainger and Beauchemin (Reference Grainger and Beauchemin2011), the addition of 10.0 g fat/kg dry matter (DM) in the diet reduces the emission of methane by 1.0 g/kg DM intake by cows. In contrast, the high quantity or availability of fatty acids in the rumen may interfere with rumen fermentation, causing adverse effects on the digestibility and intake of nutrients (Fiorentini et al., Reference Fiorentini, Carvalho, Messana, Canesin, Castagnino, Lage, Arcuri and Berchielli2015b). However, the effects of fat in the rumen, the digestion of diet components (e.g. fibre) and the deposition of fatty acids in tissues may vary greatly because they are determined by the degree of saturation and availability of fat in the diet, the amount of supplement provided and composition of the basal diet (Jenkins and McGuire, Reference Jenkins and McGuire2006).
Thus, the hypothesis of the current study is that the addition of different forms of soybean lipids in the diet could reduce methane emissions, increase animal performance and improve the profile of UFA in the meat and the response will depend on the form in which the lipids are fed. So, the objective of the current study was to evaluate the effect of different forms of soybean lipids in diets on enteric methane emission, intake, performance, digestibility and meat quality of feedlot young Nellore bulls.
Materials and methods
The study was carried out at the animal nutrition experimental sector of the Departamento de Zootecnia at FCAV/UNESP, Jaboticabal, São Paulo, located at 21°15′22″ S, 48°18′58″ W and 595 m asl.
Experimental design and animals
A total of 40 Nellore young bulls with an initial average body weight (BW) of 444 ± 10.2 kg (after the adaptation period and at 24 ± 2.1 months) were housed in individual 8 m2 (4 × 2 m2) pens and provided with a feed trough and a water trough. The animals spent 28 days acclimating to the diets, facilities and management. After this period, they were confined for 84 days (totalling 112 days – acclimatization plus experimental period), during which intake, weight gain and CH4 emissions were evaluated. At the beginning of the trial (0 day – after 28 days of acclimatization period), all the animals were weighed after a 16-h fast and before the first feeding in the morning. Average daily weight gain (ADG) was obtained by weighing animals at the beginning and the end of the experiment (84 days), always after a 16-h fast.
Diets, sampling and data collection
Young bulls were fed with one of the following experimental diets: NF = no dietary additional fat (46.0 g ether extract (EE)/kg diet); SO = soybean oil (62.0 g EE/kg diet); SB = soy beans (without any processing; 62.0 g EE/kg diet); and RPF = rumen protected fat based on soybean oil (62.0 g EE/kg diet; Megalac-E® – Church and Dwight, affiliate Química Geral do Nordeste S/A, Camacari, Brazil). The RPF (Megalac-E®) is manufactured by a saponification process where fatty acids (based on soybean oil) react with calcium hydroxide. The diets were mixed weekly to avoid potential rancidity problems.
All four diets were composed of maize silage (300 g/kg DM), used as the only source of roughage and concentrate (700 g/kg DM). Concentrates were composed of maize, soybean meal, urea, crude glycerin (803 g/kg glycerol; 15.9 g/kg EE; 50.3 g/kg ash; 120 g/kg water and 0.20 g/kg methanol). The crude glycerin was acquired from a soybean oil-based biodiesel production company (ADM, Rondonópolis, Brazil; Table 1). All diets were formulated to provide 160 ± 4.4 g/kg of crude protein (CP) to meet the nutritional requirements of Nellore bulls with an average initial weight of 400 kg and ADG of 1.25 kg/day (Valadares Filho et al., Reference Valadares Filho, Paulino and Magalhães2006).
Table 1. Composition of the experimental diets
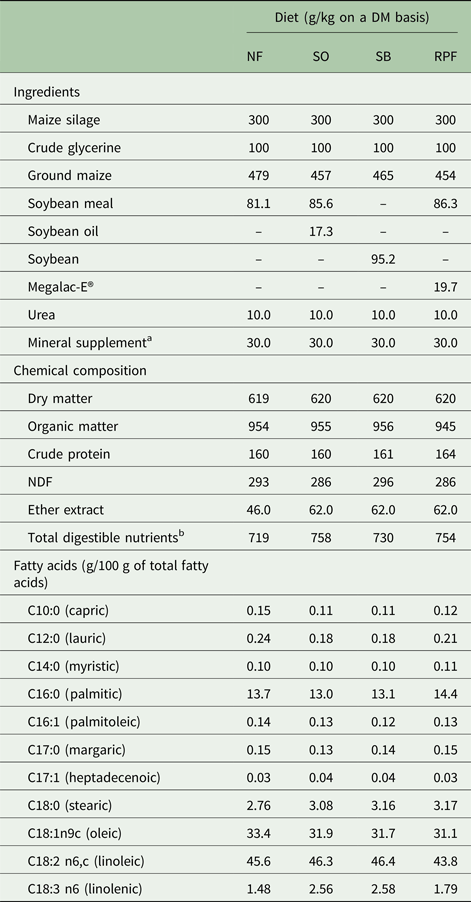
NF, no additional fat; SO, soybean oil; SB, soy beans; RPF, rumen-protected fat; NDF, neutral detergent fibre. The guarantee levels of the RPF (Megalac-E®): 850 g/kg ether extract; linoleic acid (C18:2): 450 g/kg; Linolenic acid (C18:3): 60 g/kg.
a Composition of product expressed in g or mg per 100 g of supplement: Calcium, 210 g; Phosphorus, 20 g; Sulphur, 37 g; Sodium, 80 g; Copper, 490 mg; Manganese, 1.424 mg; Zinc, 1.830 mg; Iodine, 36 mg; Cobalt, 29 mg; Selenium, 9 mg; Fluorine (maximum), 333 mg.
b Estimated by NRC (2001).
Cattle were fed with maize silage and the experimental concentrates twice per day at 08:00 and 16:00 h as a total mixed ration. Throughout the entire experimental period, the quantities provided were adjusted to allow a surplus of approximately 100 g/kg in relation to the total amount consumed on the previous day. Daily weights and samplings were taken for the diet quantities provided. To estimate intake, samples of feed offered (maize silage and concentrates) and feed refusals from each animal were first composited weekly and then composited every period (every 28 days). Feed samples were frozen at −20 °C for later analysis. Samples (feed offerings and feed refusals) were dried at 55 °C for 72 h and ground in a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA) to pass through a 1 mm screen and analysed according to AOAC (1990) to determine DM (method 934.01) and mineral matter (MM; method 942.05) and to obtain EE (method 954.02). Nitrogen was determined using a LECO FP-528 nitrogen analyser (LECO Corp., St. Joseph, MI, USA). Neutral detergent fibre (NDF) was determined using α-amylase and without the addition of sodium sulphite following Van Soest et al. (Reference Van Soest, Robertson and Lewis1991) and adapted for the Ankom200 Fibre Analyser (Ankom Technology, Fairport, NY, USA).
The fatty acid composition of feed offerings was determined using approximately 1 g of dried sample. The frozen sample was homogenized in 20 ml of a chloroform and methanol solution (2:1) using a Turrax homogenizer, disintegrator and emulsifier (Folch et al., Reference Folch, Lees and Sloane Stanley1957). In the next step, the lipid extract aliquot was methylated using the protocol of Kramer et al. (Reference Kramer, Fellner, Dugan, Sauner and Mossoba1997). Fatty acids were quantified using a gas chromatograph (GC 2010, Shimadzu Corp., Kyoto, Japan) with an SP-2560 capillary column (100 m × 0.20 mm internal diameter with a 0.02-μm film thickness; Supelco, Bellefonte, PA, USA). The initial temperature was set to 70 °C for 4 min (13 °C/min) until it reached 175 °C and then held for 27 min. After this, the temperature was increased by 4 °C/min until it reached 215 °C and held there for 31 min. Hydrogen was used as the carrier gas with a flow of 40 cm3/s (Table 1).
Total tract diet digestibility coefficients were determined for all animals using the indigestible NDF (iNDF) marker technique, as described by Cochran et al. (Reference Cochran, Adams, Wallace and Galyean1986). There were three in vivo digestibility measurement periods (every 28 days = 3 periods) with ten animals from each treatment sampled during each period. Faecal samples were collected directly from the rectum over 3 days (end each period – 26th day), at 07:00, 11:00 and 16:00 h, the first, second and third day of collection, respectively (Ferreira et al., Reference Ferreira, Valadares Filho, Marcondes, Paixão, Paulino and Valadares2009). Faecal samples were dried at 55 °C for 72 h, ground and composited proportionately (on DM basis) for each of the 3 days of sampling, for each animal. The iNDF was obtained via an in-situ methodology after 240 h (Casali et al., Reference Casali, Detmann, Valadares Filho, Pereira, Henriques, de Freitas and Paulino2008) with incubated samples of feed offered, feed refusals and faeces (Wiley mill, 2 mm screen). The iNDF was analysed using an Ankom200 Fibre Analyser (Ankom Technology Fairport, NY, USA).
Methane measurements
Methane emissions were assessed using the sulphur hexafluoride (SF6) tracer technique (Johnson et al., Reference Johnson, Huyler, Westberg, Lamb and Zimmerman1994), where each animal was sampled daily for 5 consecutive 24-h days, beginning at 77 days of feeding. Twenty-four animals (six per treatment) were fitted with gas collection halters at 10 days before methane sampling to allow animals to adapt and facilitate sampling. The selection criterion utilized was the animal's tameness and ease of handling in the management centre (stockyard). The enteric methane was measured simultaneously on the 24 animals.
The release rate of gas from a permeation tube was known before its insertion into the rumen. The permeation tubes were maintained in a water bath at 39 °C and weighed for 6 weeks in the laboratory. The average release rate was 2.0 ± 0.52 mg SF6/day, mean ± s.d. Brass permeation tubes filled with SF6 and known release rates were administered orally to each of the 20 animals 72 h before methane sampling to allow the tracer gas to equilibrate in the rumen. The animals were fitted with gas collection halters connected to pre-evacuated polyvinyl chloride canisters designed to fill halfway over 24 h. Sampling started at 06:00 h daily (one by one), when the animals were removed from the pens and was conducted at the management centre (stockyard) to facilitate sampling.
The collection canisters were located above each animal to reduce the risk of equipment damage and were connected to the halter by tubing inside airline flexible-coil tubing. Canister pressure was measured after 24 h of collection and the halter replaced if the final pressure was beyond the expected range (from −12 000 PSI (initial pressure) to −6000 PSI (final pressure)); higher pressure indicated probable blockage of the halter, whereas lower pressure indicated a probable leak in the system. In both situations, a new halter was placed on the animal to achieve an average absorption rate within the stipulated range; filling halfway over 24 h. After sampling (approximately 30 min), each animal was taken to the original pen for feeding. The pressure readings were recorded and the canisters pressurized using pure nitrogen gas (N2). The ambient air samples in the feedlot were collected twice daily to determine the background concentrations of methane and SF6. These values were subtracted from the animal's values to calculate the net output in the expired breath. At the end of sample collection, concentrations of CH4 and SF6 were determined at the EMBRAPA Environment laboratory, located in Jaguariúna, Sao Paulo State, Brazil, by a GC HP6890 (Agilent, Delaware, USA), equipped with flame ionization detector at 280 °C, column megabore (0.53 mm × 30 m × 15 µm) Plot HP-Al/M (for CH4), electron capture detector at 300 °C and for cleaning purposes. Soon after the collection period and prior to the determination of CH4 and SF6, the canisters were pressurized to between 1.3 and 1.5 PSI (g) using special nitrogen 5.0, and then readings of the initial and final dilution pressures were taken with a portable digital manometer (±0.01), certified for reading range from −1 to +2 bar (g) (model DPI705, Druck, Groby, UK), in order to obtain the dilution factor. Calibration curves were established using standard gases certified by the White Martins development laboratory (Jaboticabal, São Paulo, Brazil), with the concentration for CH4 in ppm (5 ± 0.3, 10 ± 0.2 and 19 ± 0.7 ppm) and for SF6 in ppt (34 ± 9.0, 91 ± 9.0 and 978 ± 98.0 ppt), according to Westberg et al. (Reference Westberg, Johnson, Cossalman and Michal1998). The emission of methane (kg/year) was calculated by multiplying the daily emission by 365 days. Methane emissions (g CH4/kg DM intake (DMI, g/kg)) were calculated by dividing the daily CH4 output of each animal by daily intake (during methane sampling).
Slaughter, carcass data and sample collection
At the end of the trial period (84 days), young bulls were fasted for 16 h on the day prior to transportation to the slaughterhouse. The slaughterhouse (Minerva Foods, Barretos, Sao Paulo, Brazil) was 90 km and 01:30 h transport time away from the feedlot. On arrival at the slaughterhouse, they were kept in resting pens and slaughtered after 6 h. Pre-harvest handling was in accordance with good animal welfare practices and slaughtering procedures followed the Sanitary and Industrial Inspection Regulation for Animal Origin Products (Brasil, Reference Brasil1997). After slaughter, carcasses were weighed and then refrigerated at 0 °C for approximately 24 h. After the post-mortem chill period, the cold carcass weight (CCW), final carcass pH (using a Testo 205 pH meter 205 coupled penetration electrode − Testo Inc., Sparta, NJ, USA), 12th rib subcutaneous fat thickness (SFT) and 12th rib longissimus loin-eye area (LEA) were measured on the left side of each carcass. The LEAs were traced on transparencies and measured later with a compensating polar planimeter; SFT measurements were taken at ¾ of the ventral length over the longissimus muscle (Steiner et al., Reference Steiner, Vote, Belk, Scanga, Wise, Tatum and Smith2003). Cold carcass yield (CCY) was calculated using CCW divided by final slaughter weight and then multiplying the result by 100.
A boneless longissimus section was removed from the posterior end of the wholesale rib (between the 10th and 13th ribs on the right half of the carcass). Longissimus muscle samples were vacuum-packed individually and held at −20 °C for 2 days. After that, each frozen longissimus sample was standardized from the posterior end into one 2.54 cm thick steak sample (AMSA, 1995) for Warner–Bratzler shear force (WBSF) measurement and other analyses as described later. All steaks were vacuum-packed and held at −20 °C for 10 days, then they were thawed at 4 °C for 24 h prior to analysis.
Meat and subcutaneous fat colour
The determination of meat and fat colour was performed as described by Houben et al. (Reference Houben, van Dijk, Eikelenboom and Hoving-Bolink2000), using a Minolta colorimeter (Model CR 300, Minolta Camera Co. Ltd., Osaka, Japan) evaluating lightness (L*), redness (a*) and yellowness (b*). The colour aspects were assessed by the International Commission on Illumination L*a*b* colour system with a 25-mm-diameter measurement area using a D65 illuminant (which corresponds roughly to the average midday light in Western Europe/Northern Europe) and 0° as the standard observing point. The samples were thawed before colour evaluation. Cross sections were made at the sample surface 30 min prior to the assessment to expose the myoglobin to oxygen; the same steps were performed for the fat colour measurement. After this step, the colour was measured at three different points and the average values calculated. The colorimeter was calibrated before analysing the samples against black and white standards.
Warner–Bratzler shear-force measurement, cook loss and sarcomere length
Warner–Bratzler shear-force steaks were thawed at 4 °C for 24 h and oven-broiled in an electric oven (Layr, Luxo Inox, Rio de Janeiro, Brazil) preheated to 150 °C. Internal steak temperatures were monitored by 20-gauge copper-constantan thermocouples (Omega Engineering, Stamford, CT, USA) placed in the approximate geometric centre of each steak and attached to a digital monitor. When the internal steak temperature reached 35 °C, the steak was turned over and allowed to reach an internal temperature of 70 °C before removal from the oven. Cooked WBSF steaks were cooled for 24 h at 4 °C (AMSA, 1995). Five round cores (1.27 cm diameter) were removed from each steak parallel to the long axis of the muscle fibres (AMSA, 1995). Each core was sheared once through the centre, perpendicular to the fibre direction, by a Warner–Bratzler shear machine (G-R Manufacturing Company, Manhattan, KS, USA). Cook loss was evaluated on the steaks used for the WBSF measurement. The total cooking loss was calculated as the difference between the weight of the steaks before and after oven-broiling, thawing loss was calculated as the difference between the weights of the steaks before and after thawing, and total loss was calculated by adding the cooking loss and thawing loss. Sarcomere length (SL) was measured by laser diffraction using a 05-LHR-021 laser, Melles Griot, (Carlsbad, CA, USA) and calculated as described by Cross et al. (Reference Cross, West and Dutson1981). From each cube, the SL of six fibre samples was determined and used for calculation of average SL.
Proximate analysis
After thawing at room temperature, the meat samples were lyophilized for 36 h to obtain homogeneous and moisture-free samples, ground in a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA) to pass through a 1-mm screen and chemical composition determined according to AOAC (1990). Crude protein was quantified by the Kjeldahl method, EE was extracted by the Soxhlet method and the ashes were obtained through a muffle furnace at 550 °C.
Lipid oxidation
For determination of thiobarbituric acid reactive substances (TBARS), meat samples (50 g) were collected from between the 10th and 13th ribs on the right half of the carcass, identified and vacuum-packed in polyethylene bags. A 10-g sample was ground in a multiprocessor and 0.2 ml of antioxidant butylated hydroxytoluene (0.30 g/kg) was added along with 50 ml of distilled water and 1 ml of an antifoaming solution (Sigma A5758, São Paulo, Brazil). The samples were then ground again and homogenized for 1 min. After homogenization, the samples were transferred to a 250 ml volumetric flask containing pieces of porcelain, to which 50 ml of a 4 M hydrochloric acid (HCl) solution was added. Subsequently, the samples were distilled in a blanket heater at 100 °C until 50 ml of the distillate was collected. From the distillate, 5 ml was transferred to a test tube and 5 ml of a 0.02 M thiobarbituric acid solution was added. The test tubes were kept in a boiling water bath for 35 min and the absorbance was measured at 530 nm in a spectrophotometer (Hitachi High Technologies America, Inc., model U-2,900, Pleasanton, USA). The TBARS value, expressed in mg malonaldehyde (MDA)/kg meat, was obtained using a conversion factor based on a standard curve using 1, 1, 3, 3-tetraethoxypropane.
Fatty acid profile
To determine the fatty acid composition of fresh meat, samples of the transverse section were collected from the longissimus muscle, freeze-dried and frozen for lipid extraction and methylation. The fatty material was extracted using a mixture of chloroform–methanol, as reported by Bligh and Dyer (Reference Bligh and Dyer1959) and the fatty acid methyl esters were obtained by ISO method 5509 (ISO, 1978). The transmethylated samples were analysed with a GC (model Focus CG-Finnigan, Thermo Finnigan, San Jose, CA, USA) with a flame-ionization detector and a capillary column (CP-Sil 88; Varian, Palo Alto, CA, USA) measuring 100 m × 0.25 mm internal diameter with a thickness of 0.20 µm (Supelco, Bellefonte, PA, USA). Hydrogen was used as the carrier gas at a flow rate of 1.8 ml/min. The initial temperature of the oven was 70 °C and was increased by 13 °C/min to 175 °C, where it was maintained for 27 min. The temperature was then increased by 4 °C/min to 215 °C, where it was maintained for 9 min, followed by another increase of 7 °C/min to 230 °C, where it remained for 5 min. The temperature of the injector was 250 °C and of the detector was 300 °C. Identification of the fatty acids was performed by comparison of retention times with standards of fatty acids from butter, and the percentage of fatty acids was obtained using Chromquest 4.1 software (Thermo Electron, Milan, Italy).
Statistical analysis
The experimental design was completely randomized. Intake, digestibility, weight gain and quantitative carcass trait data were collected from 40 animals over four treatments with ten replications. Initial BW was used as a covariate for the statistical analysis of intake, digestibility, weight at slaughter, ADG, feed efficiency (FE), hot carcass weight (HCW), hot carcass yield (HCY), LEA, relative LEA and back fat thickness. The mathematical model was represented by:
where Y ij = observation of animal j subject to treatment i; μ = the overall mean; t i = effect of treatment i; i = 1 to 4; iw j = initial weight of the animal j; and e ij = the residual experimental error. The CH4 emission data were analysed without the use of the covariate. Statistical analysis was conducted using the GLM procedure of SAS (Statistical Analysis System, version 9.1). Treatment means were compared using the Tukey test and adopting P < 0.05. The standard errors of the mean derived from the model are reported in tables.
Results
The intake of DM, organic matter (OM), CP and NDF was greater (P = 0.010) in the SO diet than those fed with NF (Table 2). The SO diet decreased digestibility of NDF (P = 0.010) when compared with the NF and RPF diets. The diets did not affect the digestibility of DM, OM or CP. The animals fed with SO diet showed greater final weight, average daily gain and FE (P < 0.05) than those fed with the other diets (Table 3). Emission of enteric methane (g/d, kg/year and g/kg DM intake) was not affected by different forms of soybean lipids (Table 3). The animals with SO diet had greater slaughter weight (P < 0.001) when compared with the NF and SB diets. The HCW and CCW values were greater (P < 0.001) in SO diets compared with NF diet. The HCYs (P = 0.040) and CCYs (P = 0.020) were greater in RPF compared with the NF diets. No significant differences were observed among the treatments on carcass shrink loss, LEA and final pH of the meat. The animals fed with SO diet had thicker subcutaneous fat (P = 0.040) than those from the NF diet (Table 4).
Table 2. Effects of feeding diets containing different forms of soybean lipids on component intake and apparent total tract digestibility in young Nellore bulls (n = 10)
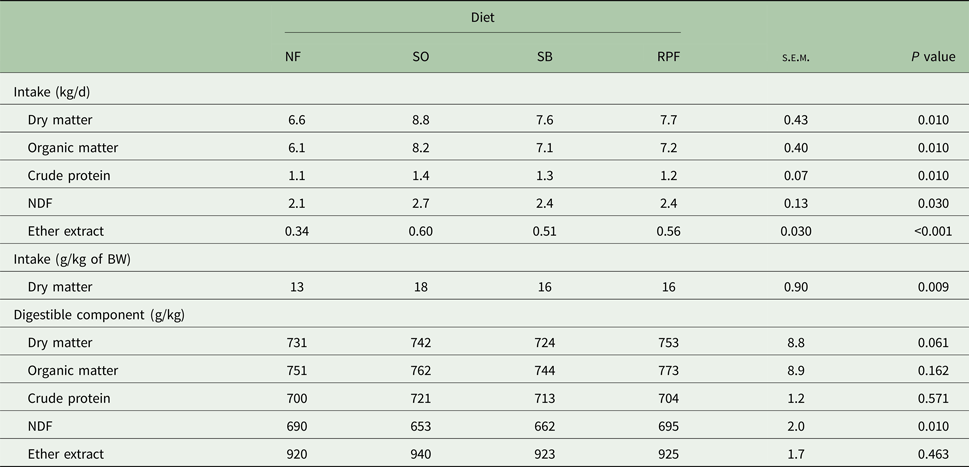
NF, no additional fat; SO, soybean oil; SB, soy beans; RPF, rumen-protected fat; NDF, neutral detergent fibre.
Table 3. Effects of diets containing different forms of soybean lipids on the initial weight (IW), slaughter weight (SW), average daily weight gain (ADG), feed efficiency (FE) and methane (CH4) emission in young Nellore bulls (n = 10 for SW, ADG and FE; n = 6 for methane emission)
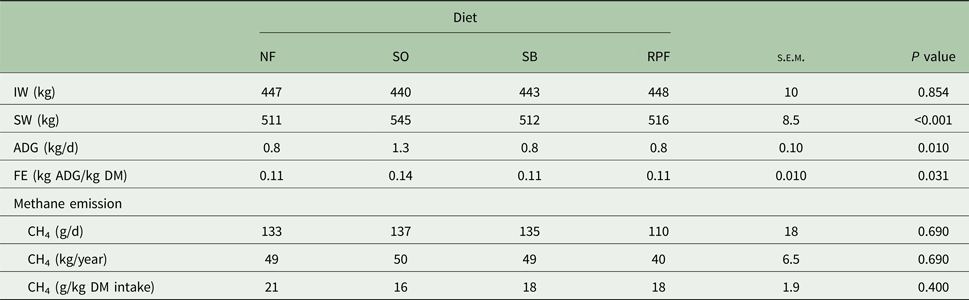
NF, no additional fat; SO, soybean oil; SB, soy beans; RPF, rumen-protected fat; DM, dry matter. The feed efficiency (FE) was calculated as follows: Average daily weight gain (kg)/Dry matter intake (kg).
Table 4. Hot carcass weight (HCW), hot carcass yield (HCY), cold carcass weight (CCW), cold carcass yield (CCY), shrink loss (SL) and loin-eye area (LEA) expressed as cm2 and cm2/100 kg of cold carcass, subcutaneous fat thickness (SFT) and final pH of feedlot-finished young Nellore bulls fed with diets containing different forms of soybean lipids (n = 10)
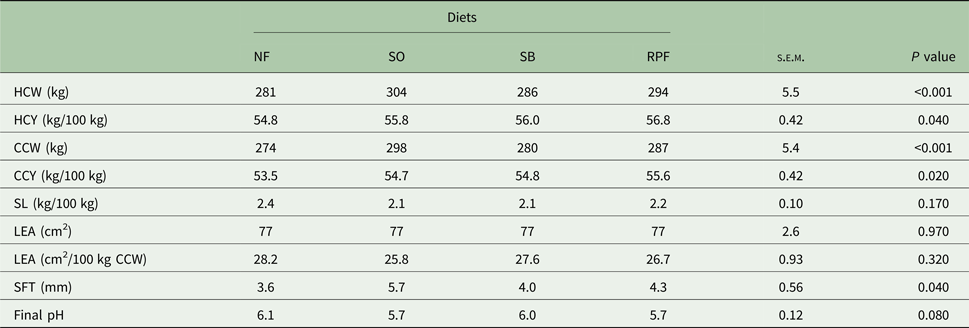
NF, no additional fat; SO, soybean oil; SB, soy beans; RPF, rumen-protected fat.
The young bulls fed with SO and RPF diets had greater SL in the longissimus muscle (P = 0.020) than the SB supplemented animals (Table 5). No significant differences were observed for moisture, protein, ash or fat (Table 5). The lightness (L*) of fat was greater (P = 0.030) in the RPF compared with SO diets (Table 5). The proportions of saturated and unsaturated, mono and polyunsaturated and n-3 and n-6 fatty acids were not affected significantly by the fat sources (Table 6).
Table 5. Warner–Bratzler Shear Force (WBSF), cooking loss (CL), sarcomere length (SAL), thiobarbituric acid reactive substances (TBARS) and chemical composition of the longissimus muscle, and colour of meat and fat from the tenderloin of feedlot-finished young Nellore bulls fed with diets containing different forms of soybean lipids (n = 10)
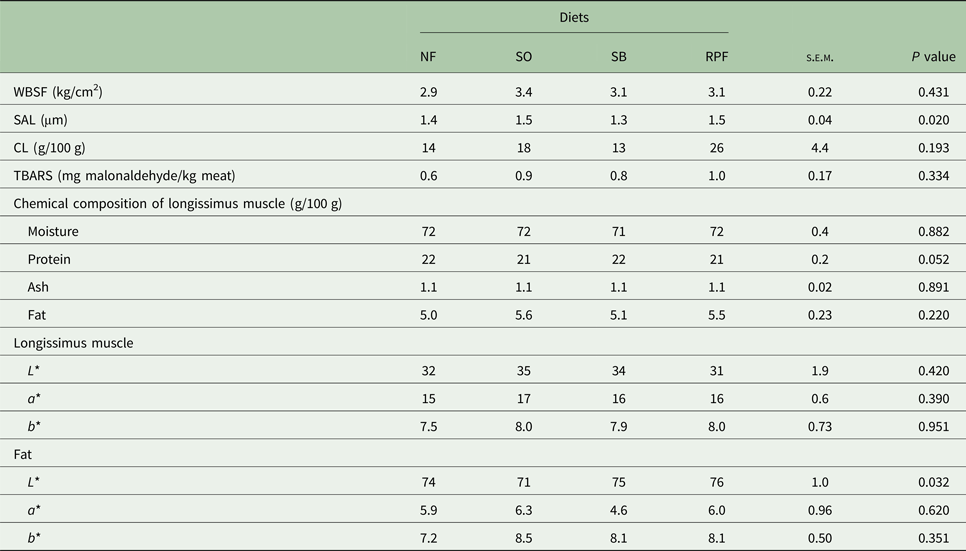
NF, no additional fat; SO, soybean oil; SB, soy beans; RPF, rumen-protected fat; L* = lightness; a* = red colour intensity; b* = yellow colour intensity.
Table 6. Fatty acid profile of the longissimus muscle of feedlot-finished young Nellore bulls fed with diets containing different forms of soybean lipids (n = 10)

NF, no additional fat; SO, soybean oil; SB, soy beans; RPF, rumen-protected fat; SFA, total saturated fatty acids; UFA, total unsaturated fatty acids; MUFA, total monounsaturated fatty acids; PUFA, total polyunsaturated fatty acids; UFA:SFA, ratio between UFA and SFA; PUFA:SFA, ratio between PUFA and SFA; n-6, fatty acids of the omega-6 series; n-3, fatty acids of the omega-3 series; n-6:n-3, ratio between n-6 and n-3.
Discussion
The inclusion of fat may cause adverse effects on rumen fermentation and alter ruminant intake (Fiorentini et al., Reference Fiorentini, Carvalho, Messana, Canesin, Castagnino, Lage, Arcuri and Berchielli2015b). The fatty acids of the SO diet were probably more readily available in the rumen, and thus more prone to impair rumen functions. The reduction of fibre digestibility in SO diets compared with NF may be associated with unsaturated fat inclusion (Harvatine and Allen, Reference Harvatine and Allen2006), which may have toxic effects on gram-positive bacteria, especially in the cellulolytic population (Nagaraja et al., Reference Nagaraja, Newbold, Van Nevel, Demeyer, Hobson and Stewart1997). Diets containing 70 g/kg or more of EE in the DM can cause negative effects on feed degradation, especially if there are large proportions of UFA contained within the EE. In addition to being toxic to ruminal microorganisms, fatty acids will adhere to food particles and create a physical barrier that prevents the action of microorganisms and microbial enzymes, which consequently impairs the performance of growing and finishing animals (Sullivan et al., Reference Sullivan, Bernard, Amos and Jenkins2004). Nevertheless, Zinn et al. (Reference Zinn, Gulati, Plascencia and Salinas2000) and Hess et al. (Reference Hess, Moss and Rule2008) reported that supplementing 50–60 g/kg of EE in the DM affected DMI. Thus, contrary to expectations, there was no negative effect with the addition of different forms of soybean lipids in diets (DMI and digestibility), with the exception of reduced NDF digestibility for the SO and SB diets. On the other hand, the SO diet increased DMI and consequently the performance of animals. Therefore, it can be concluded that there is no absolute amount of added dietary lipid that would be appropriate across all feed sources. The amount is variable and depends on the particular source used, the type of animal studied, the supply methods and other characteristics.
It has been postulated that high levels of fat can be used as supplements for beef cattle without deleterious effects in the form of saturated or protected fat (Fiorentini et al., Reference Fiorentini, Carvalho, Messana, Castagnino, Berndt, Canesin, Frighetto and Berchielli2014; Carvalho et al., Reference Carvalho, Fiorentini, Berndt, Castagnino, Messana, Frighetto, Reis and Berchielli2016). This statement supports the results found in the present study, in which the SB diet, with grain as a physical barrier that reduces the release of triglycerides present within the cells, did not affect intake or digestibility (Doreau and Ferlay, Reference Doreau and Ferlay1995). The nutrient digestibility of RPF diet, whose triglycerides are partially protected from ruminal effects due to the saponification process, did not differ from the NF diet. Changes in fibre digestibility can have a great impact on intake in high fibre diets. The increase in fibre retention could result in lower total passage rates and affect intake. Jordan et al. (Reference Jordan, Kenny, Hawkins, Malone, Lovett and O'Mara2006) verified similar DM intake between the control diet and a diet supplemented with soybean oil, using fat supplementation at levels >60 g/kg of EE in high grain diets with the addition of soy beans or refined soybean oil in diets of young bulls. These authors attributed the absence of harmful effects on intake to the low portion of roughage in the diets (<120 g/kg). However, the lower NDF digestibility coefficients in the current experiment apparently contributed to increasing the intake of animals fed the SO diet.
Supplementation of lipid sources is linked with a reduction of methane emissions (Martin et al., Reference Martin, Morgavi and Doreau2010; Patra, Reference Patra2013; Fiorentini et al., Reference Fiorentini, Carvalho, Messana, Castagnino, Berndt, Canesin, Frighetto and Berchielli2014; Rossi et al., Reference Rossi, Fiorentini, Vieira, José Neto, Messana, Malheiros and Berchielli2017). However, the current results differed from these statements and this may be explained by the similar intakes of DM and NDF. Some studies show that intensifying production system can reduce the enteric emission of CH4, although the emission of nitrous oxide (N2O) can be increased by the use of nitrogen fertilizers, either for grain cultivation or for pasture fertilization. This reduction of greenhouse gases per unit of product is mainly related to better utilization of feed and reduction of age at slaughter. The overall estimation of gas balance is complex and requires much research, including more efficient production systems such as silvopastoral and agrosilvopastoral that improve the recycling of gases emitted by the activities of beef cattle production.
Furthermore, glycerol may inhibit ruminal lipolysis (Edwards et al., Reference Edwards, Anderson, Miller, Taylor, Hardin, Smith, Krueger and Nisbet2012) and the amount of fatty acid released in the rumen. The lower concentration of free UFA may reduce deleterious effects in the Archaea group. It is reported that this corresponds to no change in methane production (g/kg DMI) in diets with soybean oil combined with crude glycerin (Jose Neto et al., Reference Jose Neto, Messana, Ribeiro, San Vito, Rossi and Berchielli2015). However, decreases in CH4 emissions have been reported in in vitro studies when increasing levels of glycerol were added to the diet (Lee et al., Reference Lee, Lee, Cho, Kam, Lee, Kim and Seo2011) or combined with soybean oil (Castagnino et al., Reference Castagnino, Messana, Fiorentini, de Jesus, San Vito, Carvalho and Berchielli2015). In the current study, CH4 emissions remained constant regardless of soybean lipid source. A possible explanation for this lack of effect is that the current data refer to in vivo studies, in which the microbial population had the possibility of adapting to the new environment, and also because some of the added glycerol could be absorbed through the rumen wall.
The changes in carcass characteristics are associated with the greater slaughter weight of animals supplemented with fat sources. Slaughter weight is an indicator of carcass yield in animals; therefore, heavier animals usually have greater HCW and CCW. Carcass yield depends directly on carcass weight, but indirectly on the BW of the animal. Feed type may cause changes in the size of organs and the gastrointestinal tract, which affects the animal's BW and carcass yield (Lage et al., Reference Lage, Paulino, Valadares Filho, Souza, Duarte, Benedeti, Souza and Cox2012).
Loin eye area is indicative of the muscle development of animals, especially when expressed as cm2/100 kg of the carcass. As this index increases, the edible portion of the carcass also increases. In this way, it can be inferred that the fat sources utilized did not affect the animals’ muscle development. The similarities found in the current study for LEA, expressed as cm2 (76.8 cm2) and as 100 kg of cold carcass (27.1 cm2/100 kg cold carcass) are in agreement with Aferri et al. (Reference Aferri, Leme, Luz e Silva, Putrino and Pereira2005), who also found no differences in LEA and LEA percent (average 68.4 and 28.3, respectively) of feedlot finished animals fed cottonseed and protected fatty acids.
The subcutaneous fat tissue acts as a thermal insulator and is an important quality factor, directly affecting the chilling time and conversion of muscle to meat (Savell et al., Reference Savell, Mueller and Baird2005; Zuin et al., Reference Zuin, Buzanskas, Caetano, Venturini, Guidolin, Grossi, Chud, Paz, Lobo and Munari2012). For all the treatments, SFT met the guidelines of the Brazilian market, which requires that the meat has between 3 and 6 mm of fat (Eiras et al., Reference Eiras, Marques, do Prado, Valero, Bonafé, Zawadzki, Perotto and do Prado2014) to prevent muscle fibres from shortening due to the cold.
The differences found among the treatments for SFT might have influenced shortening of the sarcomeres in the longissimus muscle. The SO and RPF diets presented greater SLs than the SB treatment, and thus lower WBSF values were expected for SO and RPF, given that shear force decreases exponentially as SL increases for lengths shorter than 2.00 µm (Wheeler et al., Reference Wheeler, Shackelford and Koohmaraie2000), such as the average of 1.44 µm in the present study. However, the difference found in the current study for SL did not influence meat tenderness.
The association of glycerin and fat in diets did not affect the WBSF. According to Belew et al. (Reference Belew, Brooks, McKenna and Savell2003), meat can be considered very tender when the shear force is below 3.20 kg/cm2; in the current study, all treatments showed meat with highly satisfactory average results for WBSF (3.13 kg/cm2). The higher frequency of problems related to meat toughness usually coincides with the final pH of the muscle being near 5.8–6.0 (Wulf et al., Reference Wulf, Emnett, Leheska and Moeller2002), but this was not observed in the present study, where the final meat pH (not affected by the fat inclusion in the diets) averaged 5.9.
Colour is the parameter most affected by the slaughter technique, feeding type and management (Dunne et al., Reference Dunne, Monahan and Moloney2011), but it is well reported that fat supplementation does not have an influence on the meat colour (Fiorentini et al., Reference Fiorentini, Berchielli, Santana, Dian, Reis, Sampaio and Biehl2012; Moloney et al., Reference Moloney, Kennedy, Noci, Monahan and Kerry2012; Oliveira et al., Reference Oliveira, Sampaio, Henrique, Pivaro, Rosa, Fernandes and Andrade2012), as also observed in the present study.
Although the diets with soybean had different forms of protection, there was no modification in the fatty acid composition of meat, which can be explained by the similar profile of all saturated and UFA fed to animals. Of the commonly present SFA in ruminant meat, myristic and palmitic acids have a high hypercholesterolemic potential (Daley et al., Reference Daley, Abbott, Doyle, Nader and Larson2010); myristic acid has the greatest potential (Scollan et al., Reference Scollan, Hocquette, Nuernberg, Dannenberger, Richardson and Moloney2006) but is present at a lower concentration than palmitic acid.
Despite constant criticism about the level of SFA in beef, they account for <0.50 of the total fatty acids; 0.30 consists of stearic acid (C18:0), which although saturated, because of its long chain and absence of a double bond, does not behave as such; it is therefore not related to heart disease and does not affect the level of circulating cholesterol (Scollan et al., Reference Scollan, Hocquette, Nuernberg, Dannenberger, Richardson and Moloney2006). The addition of fat did not change the proportion of stearic acid (14.3 g/100 g fatty acid) in the longissimus muscle: this result is in line with Fiorentini et al. (Reference Fiorentini, Berchielli, Santana, Dian, Reis, Sampaio and Biehl2012) and Oliveira et al. (Reference Oliveira, Sampaio, Henrique, Pivaro, Rosa, Fernandes and Andrade2012), who found no differences between the concentrations of stearic acid in the meat from zebu (Nellore) cattle and crossbred heifers with the inclusion of fat in their feedlot-finishing diets, obtaining mean values of 14.3 and 14.5 g/100 g fatty acid, respectively.
In conclusion, supplemental soybean oil at <0.02 of DM diet does not modify the emission of enteric methane and meat quality of young Nellore bulls. However, soybean oil diets have been demonstrated to be more beneficial for animal performance than diets without supplemental fat.
Financial support
The authors thank the Sao Paulo Research Foundation (FAPESP, grants #2011/00060-8; 2011/03757-0; 2013/02418-2) for providing financial support.
Conflicts of interest
None.
Ethical standards
The protocol used in the current experiment was in accordance with the Brazilian College of Animal Experimentation (COBEA – Colégio Brasileiro de Experimentação Animal). A guideline was approved by the Ethics, Bioethics and Animal Welfare Committee (CEBEA – Comissão de Ética e Bem Estar Animal) of FCAV-UNESP – Jaboticabal campus (protocol number 012799). Pre-harvest handling was in accordance with good animal welfare practices, and slaughtering procedures followed the Sanitary and Industrial Inspection Regulation for Animal Origin Products (Brasil, Reference Brasil1997).









