Introduction
A large proportion of the phosphorus (P) found in grains and oilseeds is bound as phytate-P (Eeckhout & De Paepe Reference Eeckhout and De Paepe1994; Harland & Morris Reference Harland and Morris1995; Sandberg Reference Sandberg2002). In most experiments and commercially, phytase is utilized at dietary concentrations below 1000 FTU/kg diet to liberate dietary phytate-P and allow for the reduction of inorganic P supplementation due to improved phytate-P utilization (Ravindran et al. Reference Ravindran, Selle, Ravindran, Morel, Kies and Bryden2001; Onyango et al. Reference Onyango, Bedford and Adeola2005; Persia & Saylor Reference Persia and Saylor2006; Watson et al. Reference Watson, Matthews, Southern and Shelton2006). Current commercial concentrations of phytase are utilized to release a predicted amount of inorganic P and not to facilitate the rapid degradation of phytate in the upper gastrointestinal tract (GIT). Previous reports have shown that high-concentration phytase feed can increase broiler performance regardless of dietary P reductions (Persia Reference Persia and Zimmermann2010; dos Santos et al. Reference dos Santos, Srinongkote, Bedford and Walk2013). Mechanisms for explaining the increased growth performance have been limited.
One explanation of the positive effects of phytase on the performance has been the theory that phytate, the substrate that phytase degrades, may irritate the gut wall directly or indirectly by enhancing the growth of intestinal microflora, causing inflammation and further immune response (McKay & Baird Reference McKay and Baird1999). Cowieson et al. (Reference Cowieson, Acamovic and Bedford2004) demonstrated that phytic acid increases excretion of endogenous amino acids, minerals and sialic acid (SA) in broiler chickens. Sialic acid is a component of some mucins and has been utilized as an indirect measurement of overall mucin production. Dietary phytate has been shown to increase mucin secretion in broiler and duck intestines (Onyango et al. Reference Onyango, Asem and Adeola2009; Onyango & Adeola Reference Onyango and Adeola2012). Pirgozliev et al. (Reference Pirgozliev, Oduguwa, Acamovic and Bedford2007) reported that 2500 FTU/kg phytase influences endogenous loss including SA from the GIT. Liu et al. (Reference Liu, Ru, Cowieson, Li and Cheng2008) found that the application of 500 FTU/kg phytase in marginal P diets (2.8 g/kg non-phytate P) enhances lymphocyte numbers and mucosal antibodies of 28-day-old broilers. Although this response cannot be directly attributed to phytase or breakdown of phytate, it does suggest that intestinal immunity may be altered by dietary phytase.
Current commercial concentrations of phytase may only partially ameliorate the adverse effects of phytate on the GIT of broilers (Cowieson et al. Reference Cowieson, Acamovic and Bedford2004; Cowieson & Ravindran Reference Cowieson and Ravindran2007). Therefore, it is of interest to understand the effects of high concentrations of phytase on the performance, health and immune status of the GIT in broilers. In the current experiment, a high concentration of phytase was supplemented (5000 FTU/kg or approximately 10× the commercial dose) to diets that were formulated to contain adequate amounts of non-phytate P to examine the extra-phosphoric responses on the performance, intestinal health and immunity. The response of commercial broilers, a line selected for improved growth performance, was compared with that of an unimproved native Fayoumi line. Chick performance (average daily gain, average daily feed intake (ADFI) and feed efficiency) was measured, and mRNA expression of three immune-related genes, mucin-2, interleukin (IL)-1β and IgA, were determined in the tissue or mucosal layer of the duodenum and ileum.
Materials and methods
Live animal phase
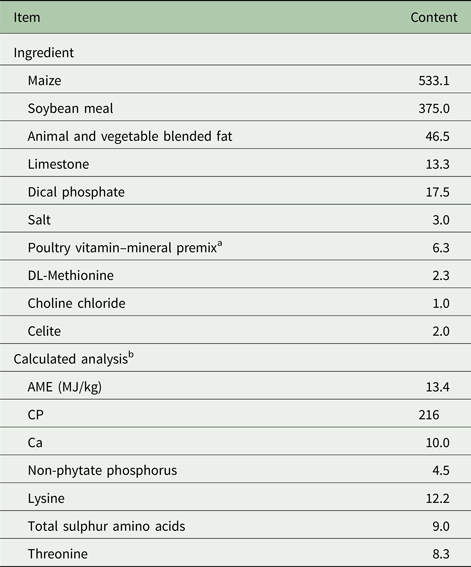
All animal procedures were approved by the Iowa State University Institutional Animal Care and Use Committee. Ross 308 broiler chicks were obtained from a local commercial hatchery (Welp Hatchery, Bancroft, IA, USA) and Fayoumi chicks (from an inbred population maintained at the Iowa State University) were set and hatched at the Iowa State University Poultry Research and Teaching Unit. In total, 192-day-old male chicks, with equal numbers from both the Fayoumi and Ross broiler lines were allocated to 24 cages (eight chicks per cage) according to line and initial body weight. The four treatments were each randomly allotted to six cages of eight chicks each. A 2 × 2 factorial arrangement of treatments was utilized including the two lines of chicks and dietary treatment. Dietary treatments consisted of a maize–soybean meal-based diet that was formulated to meet or exceed NRC (1994) starter chick requirements (Table 1) with or without 5000 FTU/kg of an Escherichia coli phytase (AB Vista, Marlborough, UK). There were no reductions in dietary calcium (Ca) or P, as the focus of the experiment was growth and immune responses independent of dietary Ca and P deficiency, not a response to the liberation of P to reach dietary adequacy. Chicks were maintained in raised wire battery pens (424.5 cm2 per bird) with continuous light in an environmentally controlled room, where all chicks had access to supplemental heat beginning at 35 °C on the day of hatch and decreasing 2 °C weekly. Chicks received no vaccinations over the duration of the experiment.
Table 1. Ingredients and composition of basal diet (g/kg, as-fed basis)
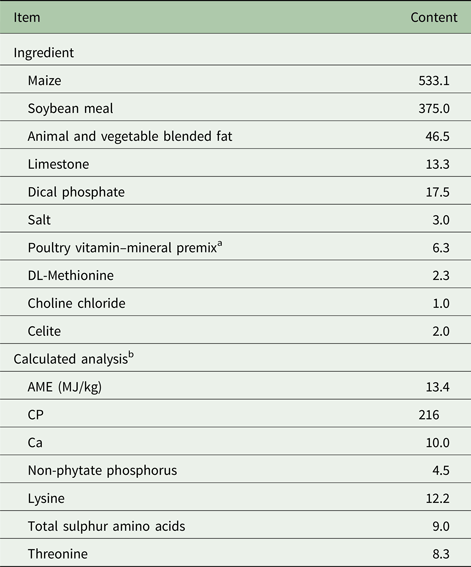
a Provided per kg of diet: vitamin A, 8250 IU; vitamin D3, 2750 IU; vitamin E, 17.9 IU; menadione, 1.1 mg; vitamin B12, 12 µg; biotin, 41 µg; choline, 447 mg; folic acid, 1.4 mg; niacin, 41.3 mg; pantothenic acid, 11 mg; pyridoxine, 1.1 mg; riboflavin, 5.5 mg; thiamine, 1.4 mg; iron, 282 mg; magnesium, 125 mg; manganese, 275 mg; zinc, 275 mg; copper, 27.5 mg; iodine, 844 µg; selenium, 250 µg.
b Values were calculated from data provided by Feed Database in NRC (1994).
Chicks were individually wingbanded and weighed on the day of hatch and were subsequently weighed (individually) on 7, 14 and 21 days of age. Birds were given ad libitum access to experimental starter diets and water throughout the experiment. Feed intake was recorded weekly on a cage basis. Mortality was recorded daily. Feed conversion ratio (FCR) was calculated by dividing total feed intake by the weight gain of all surviving birds plus the weight gain of any mortality/cull/sample bird.
Collection and processing of samples
On days 7, 14 and 21, one bird per cage was randomly selected for intestinal sample collection. After the selected chick was euthanized by carbon dioxide gas, intestinal segments were removed, including the pancreatic loop (duodenum), the segment between Meckel's diverticulum and the ileocecal junction (ileum). Tissue samples were taken from the midpoint of the duodenum, midway between Meckel's diverticulum and the ileocecal junction (ileum). For the intestinal mucosa preparation, intestines were cut open longitudinally, food debris was gently washed off using a phosphate-buffered saline solution (containing 8 g/l of sodium chloride, 0.2 g/l of potassium chloride, 1.44 g/l of sodium hydrogen phosphate, 0.24 g/l of monopotassium phosphate, pH 7.4). The mucosal linings of the duodenum and ileum were then immediately scraped from the intestinal tissue using a glass slide and placed in RNALater (Ambion, Austin, TX, USA), and stored at 4 °C. A full-wall thickness of tissue from the duodenum and ileum were also collected, minced and placed in RNALater, and stored at 4 °C. After removing from RNALater, all samples were stored at −80 °C until RNA isolation.
Gene expression assays
The gene expression levels of mucin-2 in the mucosal tissue layer of the duodenum and ileum, and IL-1β and IgA from the full-wall duodenum and ileum tissue were determined. Intestinal wall tissue (approximately 50 mg) and mucosa (approximately 20 mg) from each individual were homogenized. The RNA was isolated using the RNAqueous kit (Ambion, Austin, TX, USA) and processed as previously described by Abasht et al. (Reference Abasht, Kaiser and Lamont2008). The RNA concentration of the samples was quantified by using a spectrophotometer (Nanodrop 1000, Thermo Scientific, Wilmington, DE, USA) and samples were diluted to 50 ng/μl.
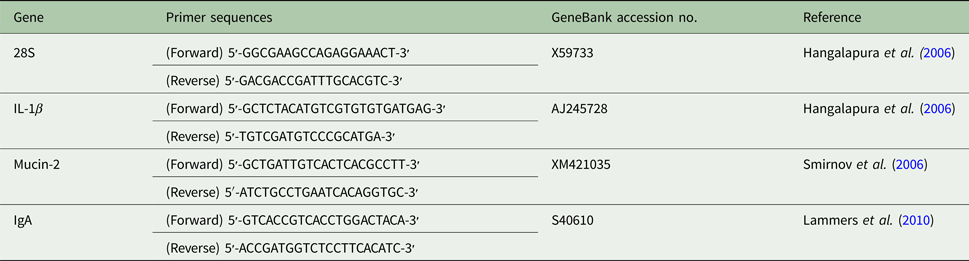
The relative mRNA expression of mucin-2, IL-1β and IgA were measured using the Quantitect SYBR-Green reverse transcription polymerase chain reaction (RT-PCR) kit (Qiagen, Waltham, MA, USA). The real-time RT-PCR protocol was conducted as previously described by Kaiser et al. (Reference Kaiser, Cheeseman, Kaiser and Lamont2006). An internal control of 28 s ribosomal RNA was used. Primers were synthesized (Table 2) at the DNA Facility of Iowa State University. Each reaction was run in triplicate on an Opticon 2 thermocycler (Bio Rad, Hercules, CA, USA) and consisted of a final concentration of 3 mM magnesium chloride, 0.25 µM of each gene-specific primer, 2 µl of cDNA template, 2 µl SYBR Green reaction mix and PCR-grade water to a total volume of 25 µl. Reactions were run for 30 min at 50 °C, 15 min at 95 °C, and were cycled 40 times through 15 s at 94 °C, 30 s at 59 °C, 30 s at 72 °C and a plate read.
Table 2. Primer sequences used in quantitative reverse transcription polymerase chain reaction

Statistical analysis
Growth and gene expression data were analysed using JMP Version 8.0.2 software (SAS Institute 2009). Each cage served as the experimental unit. Analysis of weight gain included line, diet and interaction of line and diet as fixed effects. Cycle threshold (C t) values for gene expression assays were adjusted before statistical analysis to account for individual sample RNA concentration and reaction efficiency by using the following formula: 40 − [C t sample mean + (C t 28 s median − C t 28 s mean) × (gene slope/28 s slope)]. Each gene was analysed with line, diet, chicken age and interactions of line and diet, line and chicken age, diet and chicken age as fixed effects. When there was a significant interaction between factors, contrast analysis was applied to the treatment groups to determine the source of the significant interactions. Significance was set at P ⩽ 0.05 and a trend was accepted at P ⩽ 0.10.
Results
Chick growth performance
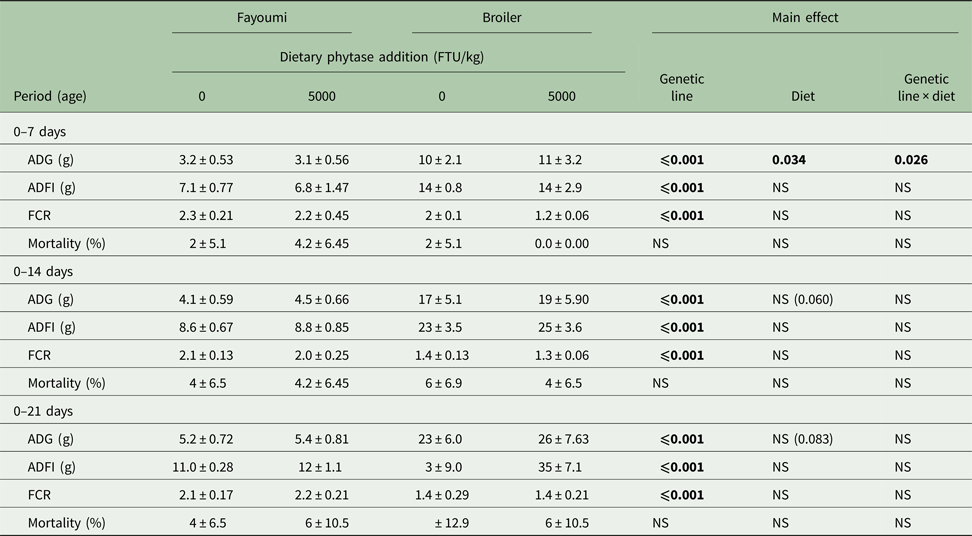
Chicken ADG, ADFI and FCR were affected significantly by genetic line during 0–7, 0–14 and 0–21 days (P < 0.05, Table 3). During the first period (0–7 days), there was a significant genetic line × diet interaction on ADG (P < 0.05). By contrast analysis, phytase supplementation increased broiler ADG significantly (P < 0.05), but had no effect on Fayoumi ADG. Diet main effects trends on chicken ADG during 0–14 days (P = 0.060) and 0–21 days (P = 0.083) were noted, as birds supplemented with 5000 FTU of phytase had increased ADG in comparison to birds without dietary phytase. There were no significant effects of diet and no significant interaction of genetic line × diet on ADFI, FCR or mortality.
Table 3. Effects of high phytase feeding and genetic line on growth performance of chicks from 0 to 21 days of agea
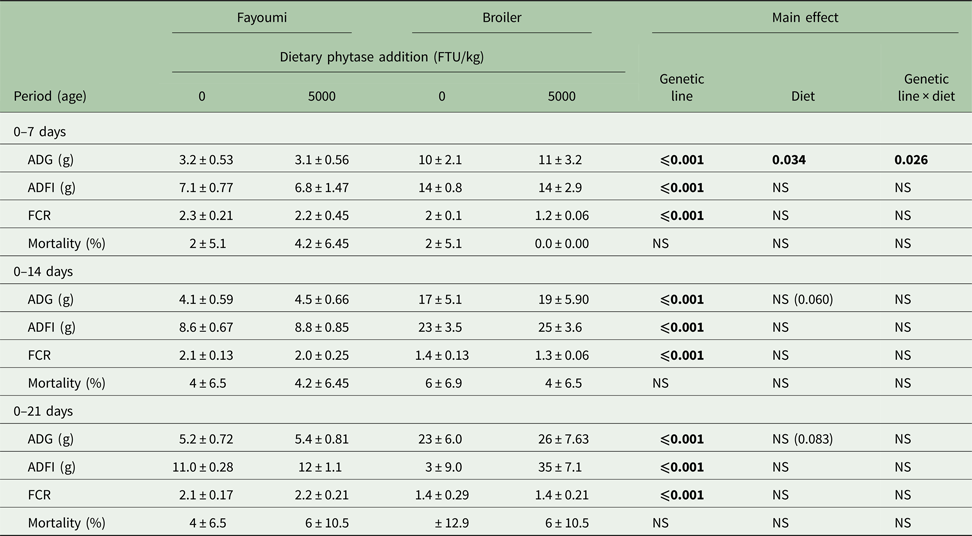
FTU, phytase activity unit; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio; NS, not significant.
a Values are means ± s.e.
Bold significance means P values.
Gene expression
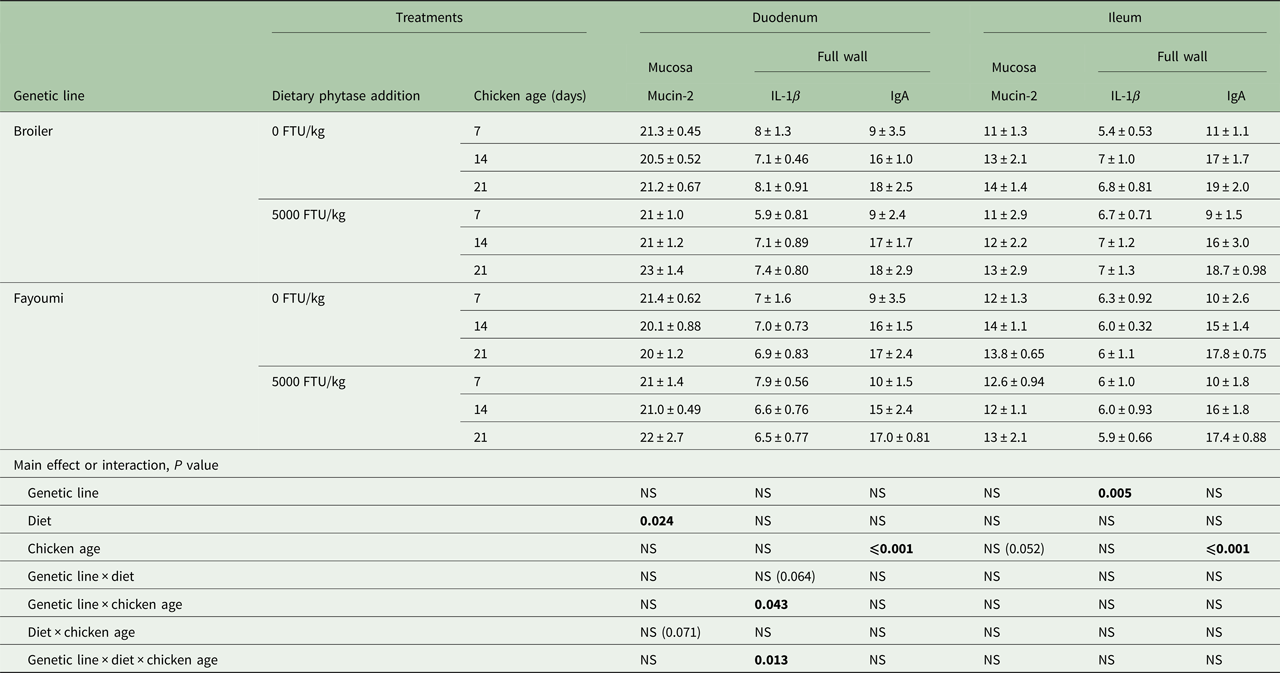
Diet affected mRNA expression of the mucin-2 gene in duodenal mucosa (P < 0.05; Table 4). There was a trend for an interaction between diet and chicken age (P = 0.071). Mucin-2 gene expression level in the ileal mucosa was not affected by any factor, although chicken age was very near significant (P = 0.052).
Table 4. Effect significances of genetic line, diet, chicken age and their interactions on mRNA expression of mucin-2 in the mucosa, and IL-1β and IgA in the tissue of chicken duodenum and ileuma
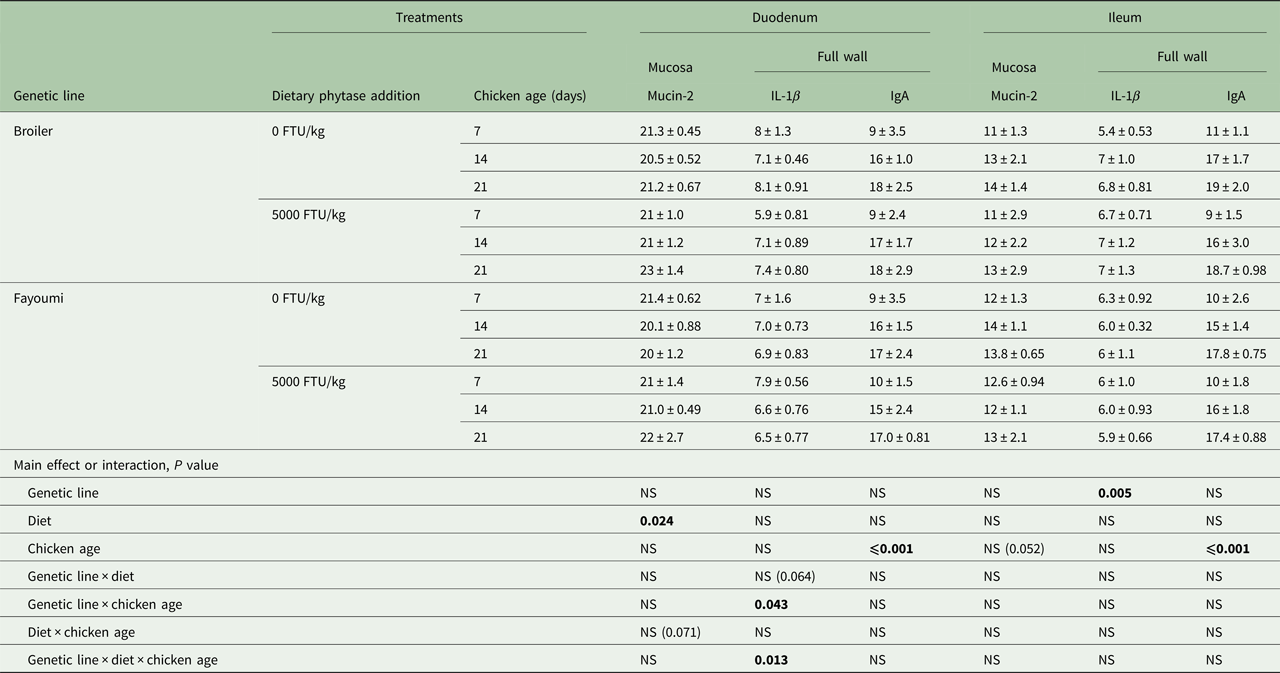
FTU, phytase activity unit; NS, not significant.
a Values are means ± s.e.
Bold significance means P values.
For expression of IL-1β mRNA in chicken duodenum, there were significant interactions between genetic line and age (P < 0.05), and genetic line and diet and age (P < 0.05), and a trend for the interaction between genetic line and diet (P = 0.064). In the ileum, genetic line had significant effects on the expression of IL-1β gene (P < 0.05).
Chicken age had significant effects on mRNA expression of IgA in both the duodenum and ileum (P < 0.05). Genetic line, diet and all interactions had no effect on IgA mRNA level in either tissue.
Fayoumi chickens fed phytase-free diets tended to express a lower level of IL-1β in the duodenum than broilers fed the same diet at 21 days (P = 0.064). Fayoumi chickens fed the phytase diet expressed a significantly higher level of IL-1β in the duodenum than broilers fed the same diet at 7 days (P < 0.05). In Fayoumi chickens fed the high-phytase diet, expression levels of IL-1β were significantly lower at both 14 and 21 days than at 7 days (P < 0.05), whereas in broilers, the levels were higher at 14 and 21 days (P < 0.05). High-concentration phytase addition decreased IL-1β expression in broilers (P < 0.05) and numerically increased expression in Fayoumi chickens (P = 0.06) at 7 days.
Discussion
Shirley & Edwards (Reference Shirley and Edwards2003) reported that phytase concentrations above 3000 FTU/kg resulted in over 75% disappearance of dietary phytate P and 12 000 FTU/kg phytase resulted in almost complete disappearance of dietary phytate P. The presence of high concentrations of phytase limits the interactions that phytate can have in the digestive tract, especially in the upper GIT before intestinal pH reduces the solubility of the phytate–Ca complex (Angel et al. Reference Angel, Tamim, Applegate, Dhandu and Ellestad2002). The results of the present experiment suggest that supplementation of diets adequate in non-phytate P with high concentration of phytase can improve growth performance of chickens. The response to high dietary phytase was strongest for the broiler chicks during the early growth period, 0–7 days, but the response persisted with near significance over both the 0–14 and 0–21-day periods for both broiler and Fayoumi chickens. The interaction between the two lines over the 0–7-day period suggests that this unselected Fayoumi line may be less susceptible to the anti-nutritional effects of phytate or at least less responsive to the high dietary concentrations of phytase. The current data are in agreement with previous reports that high phytase feeding (1500–7500 FTU/kg) resulted in improved performance as either increased weight gain or improved FCR and phytate degradation (Persia Reference Persia and Zimmermann2010; dos Santos et al. Reference dos Santos, Srinongkote, Bedford and Walk2013; Ribeiro et al. Reference Ribeiro, Salguero, Gomes, Barros, Silva, Barreto, Rostagno, Hannas and Albino2016; Truong et al. Reference Truong, Yu, Moss, Partridge, Liu and Selle2017). Pirgozliev & Bedford (Reference Pirgozliev and Bedford2013) showed that phytase supplementation increased net energy in a linear fashion with 2500 FTU/kg phytase resulting in a 153 kcal/kg increase in net energy over a control diet.
The protective mucus layer covers the entire surface of the GIT. Mucin serves a protective function by binding to pathogens, preventing their adhesion to the intestinal surface. Mucin-2 is one of several genes responsible for encoding mucin production (Cox et al. Reference Cox, Sumners, Kim, McElroy, Bedford and Dalloul2010). Cowieson et al. (Reference Cowieson, Acamovic and Bedford2004, Reference Cowieson, Acamovic and Bedford2006) found that 1000 FTU/kg phytase addition reduces secretion of mucin in excreta in broiler chickens and hypothesized that decreased mucin secretion would allow for increased productive energy and performance. In the current experiment, however, duodenum mucosal mucin-2 mRNA expression was increased by phytase addition, suggesting that phytase addition increased the potential of the birds to secrete at least one mucin in the duodenum. These results are contrary to previously measured indicators of mucin concentration in the intestinal tract and may be related to translational modifiers modifying protein production or production of mucin from other non-determined genes.
The cytokine IL-1β mediates the inflammatory response and increases antibody production in chicken (Leutz et al. Reference Leutz, Damm, Sterneck, Kowenz, Ness, Frank, Gausepohl, Pan, Smart, Hayman and Graf1989; Sterneck et al. Reference Sterneck, Blattner, Graf and Leutz1992). In the current experiment, duodenal IL-1β expression was decreased by phytase addition in broilers at an early age. This suggests that phytase supplementation may reduce inflammatory response in the duodenum of broilers. In the ileum, IL-1β expression was only affected by genetic line, not by phytase treatment. This result may be related to physical state of the phytate molecule as pH value in different gastrointestinal segments of poultry can affect the solubility of phytate–Ca complexes. Remaining dietary phytate not hydrolysed by endogenous or microbial enzymes in the duodenum would bind with dietary Ca and become insoluble after it passes out of the duodenum and reaches the higher pH conditions of the jejunum and ileum (Angel et al. Reference Angel, Tamim, Applegate, Dhandu and Ellestad2002). This effect would functionally remove phytate from the ileum and mask any high-phytase feeding effects on this tissue. It has been speculated that the degradation products of the action of phytase on phytate may regulate immunocyte activity (Vucenik & Shamsuddin Reference Vucenik and Shamsuddin2006; Bozsik et al. Reference Bozsik, Kokeny and Olah2007). It is unclear in the current experiment if it is the breakdown products of phytate or the lack of phytate that is increasing growth responses. There was different IL-1β expression in the duodenum and ileum tissues between Fayoumi and broilers lines. The divergence in IL-1β expression between these lines probably reflects a difference in the dominant immune mechanisms used by broiler and Fayoumi birds. Other experiments have demonstrated different immune-related gene expression among diverse chicken lines (Cheeseman et al. Reference Cheeseman, Kaiser and Lamont2004; Ye et al. Reference Ye, Avendano, Dekkers and Lamont2006; Redmond et al. Reference Redmond, Tell, Coble, Mueller, Palić, Andreasen and Lamont2010).
Secretory immunoglobulins form a first line of immune defence at mucosal surfaces. Several experiments suggest a role for chicken IgA in the protection of mucosal surfaces, similar to mammalian IgA (Klipper et al. Reference Klipper, Sklan and Friedman2000; Muir et al. Reference Muir, Bryden and Husband2000; Wieland et al. Reference Wieland, Orzàez, Lammers, Parmentier, Verstegen and Schots2004). In the current experiment, mRNA expression levels of IgA in duodenum and ileum were only affected by chicken age, and not by phytase supplementation and genetic line. Liu et al. (Reference Liu, Ru, Cowieson, Li and Cheng2008) found that the production of jejunal mucosal secretory IgA was improved with phytase addition in Cobb 500 female broilers, and the differences observed in the present experiment suggest that high-concentration phytase addition in the diet might not alter mRNA expression although secretion of mucosal IgA protein was not directly measured. The current data indicate that IgA expression was increased with age and agrees with previous literature (Lammers et al. Reference Lammers, Wieland, Kruijt, Jansma, Straetemans, Schots, den Hartog and Parmentier2010).
In summary, the results of the current experiment demonstrate that the addition of a high concentration of phytase to broilers fed adequate concentrations of non-phytate P resulted in improved growth performance early in life and that this response was reduced as the birds aged. Intestinal SIgA mRNA expression was not altered with high-concentration phytase feeding, but mucosal mucin-2 expression was increased with high-concentration phytase feeding across both lines, and IL-1β mRNA expression was reduced in the duodenum of broilers fed high concentrations of phytase. The increased growth performance and reduced IL-1β mRNA expression suggest that high-concentration phytase feeding can improve the growth performance of chickens, regardless of dietary P status, most likely mediated through decreased inflammation responses in the proximal intestinal tract.
Acknowledgements
The authors are grateful to W. Larson, W. Rogers, J. Tjelta and R. Holbrook of the Iowa State University Poultry Research and Teaching Unit for bird care and diet preparation, and to M. Kaiser, J. Green, L. Lian, D. Coble, E. Sandford, C. Ciraci, G. B. Chang, G. Murugesan, M. Higgins, N. Nachtrieb for assistance in sample collection. Appreciation is expressed to Feed Energy Corporation for donation of the animal/vegetable blended fat; ILC for donation of the limestone and Evonik, Inc. for donation of the lysine. This research was funded by the Iowa State University Agriculture and Home Economics Experiment Station.