INTRODUCTION
Weed competition has been reported to reduce cereal crop yields in Canada, with documented losses of up to 29% in barley (O'Donovan et al. Reference O'Donovan, Harker, Clayton and Hall2000) and up to 63% in wheat (Kirkland & Hunter Reference Kirkland and Hunter1991; Hucl Reference Hucl1998). Many options exist for weed control, perhaps the most common being the use of agrochemicals. Increased herbicide resistance, rising costs of production and an increased interest in environmental protection (through the adoption of sustainable and organic management systems) are creating the need for researchers to explore non-chemical methods of weed control (Jordan Reference Jordan1993; Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996). Such methods include the use of various tillage regimes (Barberi et al. Reference Barberi, Silvestri, Peruzzi and Raffaelli2000), crop rotations and intercropping (Hartl Reference Hartl1989), crop seeding density (Korres & Froud-Williams Reference Korres and Froud-Williams2002) and the use of competitive cultivars (Huel & Hucl Reference Huel and Hucl1996; Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996).
There are two ways to consider the competitive ability of a crop or a plant cultivar: (a) the ability of a crop to tolerate weed pressure by maintaining grain yield and (b) the ability of a crop to suppress weed growth and seed production (Coleman et al. Reference Coleman, Gill and Rebetzke2001). Both are important, since yield stability and the prevention of weed seed production, and subsequent seed bank build-up, are desirable in crops growing in association with weeds (Jordan Reference Jordan1993). Lemerle et al. (Reference Lemerle, Gill, Murphy, Walker, Cousens, Mokhtari, Peltzer, Coleman and Luckett2001a) suggested that weed tolerance and weed suppression be considered separately, as they may or may not occur together.
Morphological, physiological and biochemical traits are thought to control plant competitiveness (Lemerle et al. Reference Lemerle, Gill, Murphy, Walker, Cousens, Mokhtari, Peltzer, Coleman and Luckett2001a) and many studies have been conducted to determine which characters confer competitive ability in wheat. Plant height plays a role in the competitive ability of wheat (Wicks et al. Reference Wicks, Ramsel, Nordquist, Schmidt and Challaiah1986; Huel & Hucl Reference Huel and Hucl1996; Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996; Cosser et al. Reference Cosser, Gooding, Thompson and Froud-Williams1997; Champion et al. Reference Champion, Froud-Williams and Holland1998; Hucl Reference Hucl1998; Korres & Froud-Williams Reference Korres and Froud-Williams2002). In a study of Canadian spring wheat cultivars, crop height appeared to have the greatest impact on competitive ability, with the shortest wheat cultivars experiencing the largest yield reductions and allowing the greatest weed growth (Huel & Hucl Reference Huel and Hucl1996). Wicks et al. (Reference Wicks, Ramsel, Nordquist, Schmidt and Challaiah1986), however, suggested that height alone does not explain competitive ability, since some shorter cultivars have been found to be good competitors. In a comparison of tall and short winter wheat cultivars, the taller cultivar intercepted more photosynthetically active radiation (PAR), accumulated more early dry matter, and accumulated the most nitrogen early in the season. However, the taller cultivar was variable in its ability to suppress weeds and was lower yielding than the shorter cultivars (Cosser et al. Reference Cosser, Gooding, Thompson and Froud-Williams1997). The association of plant height with other competitive traits further implies that height is not the only factor responsible for competitive ability in wheat.
Canopy structure may also have an influence on competitive ability. Champion et al. (Reference Champion, Froud-Williams and Holland1998) found that a tall cultivar that intercepted a greater proportion of PAR was more effective at suppressing weed growth than a short cultivar with low light interception capabilities. Interception of PAR at both early (Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996) and late (Wicks et al. Reference Wicks, Ramsel, Nordquist, Schmidt and Challaiah1986) growth stages was associated with wheat grain yield maintenance under competition from weeds. Huel & Hucl (Reference Huel and Hucl1996) found leaf area index to be negatively correlated with weed seed yield. Leaf area index was not, however, associated with wheat yield reduction resulting from competition with weeds (Huel & Hucl Reference Huel and Hucl1996). Flag leaf length was strongly negatively correlated with wheat yield loss (Huel & Hucl Reference Huel and Hucl1996; Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996) and weed dry matter yield (Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996), while flag leaf angle was positively correlated with wheat yield reduction (Huel & Hucl Reference Huel and Hucl1996). Evidence that early season ground cover also reduces subsequent weed biomass has been reported by Richards & Whytock (Reference Richards and Whytock1993) and Huel & Hucl (Reference Huel and Hucl1996). In the Lemerle et al. (Reference Lemerle, Verbeek, Cousens and Coombes1996) study, elevated PAR interception, resulting in high early biomass accumulation, was found in the most competitive wheat genotypes.
In addition to height and canopy structure, tillering capacity (measured as the number of fertile tillers/unit area) has often been reported to confer greater competitive ability in wheat (Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996; Hucl Reference Hucl1998; Korres & Froud-Williams Reference Korres and Froud-Williams2002). Among other traits, high tiller numbers were found in the most competitive wheat (mainly Australian) from around the world (Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996). On the other hand, tiller number has been found to be weakly correlated with weed suppression and grain yield in other studies (Wicks et al. Reference Wicks, Ramsel, Nordquist, Schmidt and Challaiah1986; Champion et al. Reference Champion, Froud-Williams and Holland1998). Various other characters have been found to contribute to competitiveness, though they are not as commonly reported. In a study of Canadian spring wheat, time of spike emergence was positively correlated with wheat yield reduction and early maturity was associated with competitiveness (Huel & Hucl Reference Huel and Hucl1996). In a later study, however, Hucl (Reference Hucl1998) found no association between maturity and competitiveness.
A better understanding of the mechanisms by which a crop cultivar becomes competitive would not only serve to assist plant breeders in developing competitive cultivars more quickly and effectively, but would also justify the use of plant breeding to increase crop competitive ability (Lemerle et al. Reference Lemerle, Gill, Murphy, Walker, Cousens, Mokhtari, Peltzer, Coleman and Luckett2001b). For producers, knowledge about the competitive ability of cultivars would be useful for choosing cultivars suited to their environment (Lemerle et al. Reference Lemerle, Gill, Murphy, Walker, Cousens, Mokhtari, Peltzer, Coleman and Luckett2001b). Yield gains of 7–9% have been identified in ‘competitive’ wheat cultivars when compared with ‘non-competitive’ cultivars (Hucl Reference Hucl1998).
The objectives of the present study were to identify competitive traits in wheat cultivars grown in differing levels of natural weed environments and to determine whether traits associated with competitive ability differ under increasing weed pressure. Additionally, identification of differences among cultivar stability in and adaptation to environments differing in yield potential and weed competition was sought.
MATERIALS AND METHODS
Nine spring wheat cultivars were chosen for this experiment on the basis of height, tillering potential and maturity characters (Table 1). The cultivars were planted at 300 seeds/m2, the upper end of the recommended hard red wheat seeding range for the growing region (Miller & McLelland Reference Miller and McLelland2001). Field trials were conducted at one conventional and two organic locations in both 2003 and 2004. The conventionally managed site and one of the organically managed sites were located at the Edmonton Research Station (ERS), Edmonton, Alberta (53°34′N, 113°31′W), approximately 1 km apart, with the other organic site located at a certified organic farm near New Norway, Alberta (52°52′N, 112°56′W). Soils at New Norway sites were Eluviated Black Chernozemics, while soils at Edmonton sites were classified as Orthic Black Chernozemics, typical of central Alberta (Alberta Agriculture Food and Rural Development 1995). Tillering potential of cultivars was determined from data generated in various trials conducted at the ERS on conventional land between 1999 and 2002.
Table 1. Cultivar descriptions for spring wheat cultivars and breeding lines grown in trials conducted in 2003 and 2004 in Edmonton and New Norway, Alberta, Canada

* CWRS-Canadian Western Red Spring.
† Denotes unknown character prior to start of experiment.
‡ The authors gratefully acknowledge Dr R. DePauw for allowing us to use this line for experimental purposes.
The experiment was designed as a strip-plot with three replicates, where the horizontal factor was competition with common oats (Avena sativa L.) and the vertical factor was cultivar. There were thus nine wheat cultivars×three replicates×two common oat competition levels (54 plots) per trial. In 2003, plot dimensions were 4·5×0·9 m consisting of four rows spaced approximately 230 mm apart. Plots were seeded using a four row, double disk drill (Fabro Enterprises Ltd., Swift Current, SK, Canada). In 2004, plot dimensions were 4×1·38 m consisting of six rows spaced 230 mm apart. Plots were seeded using a six row, no-till double disk drill (Fabro Enterprises Ltd., Swift Current, SK, Canada). In both years, plots receiving the common oat treatment were cross-seeded immediately after crop seeding at a rate of 60 common oat seeds/m2. Seed used in the 2003 trial was either certified seed or was increased at the ERS in 2002, and seed used in the 2004 trials was increased on an organically managed site at ERS in 2003. The trials were not irrigated. All trials were planted in mid- to late May and harvested in early to mid-September. Conventionally managed sites received mineral fertilizer applications according to soil test recommendations and also received recommended rates of MCPA Amine 500 to control broadleaf weeds (Alberta Agriculture Food and Rural Development 2006). Organically managed sites were managed according to Organic Crop Improvement Association recommendations (Organic Crop Improvement Association 2000) and did not receive any applications of chemical fertilizers and herbicides. At ERS-Organic, composted dairy manure at c. 60 t/ha was applied annually. At the certified organic farm, experimental trials followed cereal-legume plough-downs without crop removal in the year prior to planting.
Data collection
Spike emergence (heading) was recorded as the day when three quarters of the spikes in the plot emerged with visible peduncles. After stem elongation was complete, plant height (representing the distance from the soil surface to the tip of the spike, excluding awns in awned cultivars) was recorded on a per plot basis. Maturity was recorded as the day when three quarters of the spikes and peduncles in the plot were tan brown in colour; approximately 300 mg water/g seed. Plot lodging was rated on a scale from 1=no lodging to 9=highest degree of lodging. Lodging ratings were conducted at various sites after specific lodging events (heavy rains, snowfall) and at all sites just prior to harvest. At maturity, all spikes in either a 1 m2 (2003) or 1 m row (2004) section of each plot were counted and used to calculate spikes/m2.
In 2003, weed biomass/m2 in each plot was determined by weighing the above ground portion of the weeds from the harvested 1 m2. In 2004, weed biomass/m2 in each plot was determined by harvesting the above ground portion of weeds from within two randomly placed 0·0625 m2 quadrats (0·25×0·25 m) at plot maturity. Weeds present in both 2003 and 2004 at the ERS included stinkweed (Thlaspi arvense L.), common lambsquarters (Chenopodium album L.), wild buckwheat (Polygonum convolvulus L.), shepherd's purse (Capsella bursa-pastoris L.) and Canada thistle (Cirsium arvense L.), while weeds in both years at the New Norway site were mainly wild oats (Avena fatua L.) and common lambsquarters.
In both years, common oats (A. sativa, hereafter referred to as oats) were harvested from plots just prior to crop harvest. In 2003, oats were harvested from a 1 m2 area and were counted, dried, weighed to obtain oat biomass and threshed to calculate grain weight. In 2004, oats were harvested from within two randomly placed 0·0625 m2 quadrats and were dried, weighed for oat biomass and threshed to calculate grain weight.
Prior to harvest, ten randomly chosen spikes in each plot were collected and used to determine kernels/spike and thousand kernel weight. Plots were harvested using a Wintersteiger plot combine following maturity. Grain yield was recorded on a dry weight basis. Harvested grain samples were dried at 60°C for c. 24 h and weed seeds were removed using a 2 mm mesh sieve (Canadian Standard Sieve Series No. 10). The weed-seed free grain samples were weighed and plot yields were recorded for those plots without oats. For plots with oats, the weed-seed free grain sample was weighed and then a 100 g sample of grain was removed and oats and wheat were separated and weighed. Grain yields for plots with oats were based on multiplication of the weed-seed free plot grain yield by the ratio of grain:oats from the 100 g sample.
Data analysis
Weed pressure levels
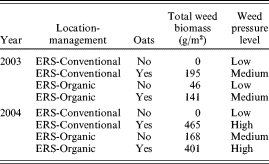
For all analyses using high, medium and low weed pressure levels (i.e. analysis of variance, correlation, principal component analysis (PCA) and multiple regression), data from the certified organic farm were not used, due to missing values for days to heading and maturity. These observations were not recorded at the certified organic farm because it was far away from the ERS. Data from the two years of trials on conventional and organic land at the ERS were divided into eight groups, based on year, location-management and oat competition. These eight environments were grouped into low, medium and high weed pressure levels based on the average total weed biomass (oats plus natural weeds) of each environment (Table 2). An analysis of variance was performed using the PROC MIXED procedure of SAS (SAS Institute 2003), where weed pressure level, cultivar and weed pressure level×cultivar were considered fixed, while environment within weed pressure level, replication and associated interactions were considered random effects. For all analyses, a variance-stabilizing square root transformation ((Y+0·5)1/2) was used for total weed biomass data (Gomez & Gomez Reference Gomez and Gomez1984).
Table 2. Growing year, location, management, oat competition and total weed biomass data for weed pressure levels of four experimental sites in 2003 and 2004
Raw data were then analysed by weed pressure level using the PROC CORR and PROC PRINCOMP procedures of SAS (SAS Institute 2003). Whereas simple linear correlation analysis allows one to measure the degree of linear association between two variables (Gomez & Gomez Reference Gomez and Gomez1984), PCA allows one to analyse relationships among a wide range of variables (Timm Reference Timm2002). The PCA removes intercorrelations that may exist between variables by transforming the original variables into smaller, hypothetical components (PCs) (Smith Reference Smith1991; Timm Reference Timm2002). The new PCs are orthogonal to one another, so that the data expressed in each PC is uncorrelated with all other PCs (Smith Reference Smith1991). A total of nine variables were analysed: height, days to heading and maturity, lodging, grain yield, spikes/m2, kernel weight, kernels/spike and total weed biomass. The PCA was done on the correlation matrix, since original measurements were made in different units (Jolliffe Reference Jolliffe2002). Interpretation of PCs can be a fairly subjective process, for which there are no general rules (Mallarino et al. Reference Mallarino, Oyarzabal and Hinz1999). Retention of PCs was based on those PCs with eigenvalues >1 (Jolliffe Reference Jolliffe2002). In the low and medium weed pressure levels, the first three PCs fit this criteria and for the high weed pressure level, the first four PCs had eigenvalues >1. For simplicity of interpretation, the first three PCs are presented for all levels. As a starting point, variables with absolute loadings (eigenvalues) greater than the mean of the absolute loading value were selected as variables in the PCs. Subsequently, the criteria used to interpret variables within each PC included both the loading value and its relative difference from the other loading values in that PC. Loading plots for PC1 versus PC2 were constructed for each weed pressure level in order to visually evaluate variables that tend to be associated with one another, since two factors with high loadings in the same PC tend to vary together within a particular environment (Mallarino et al. Reference Mallarino, Oyarzabal and Hinz1999). Values for the loading plots were determined by multiplying the loading value for each variable by the square root of the eigenvalue for the respective PC, which also shows the correlation between the variables and PCs (Smith Reference Smith1991). The length of a vector (the line from the origin to the point) shows the strength of the correlation of a variable with PC1 or PC2. For example, a long vector (approaching a length of 1) in the direction of PC1 indicates a strong relationship between that variable and PC1, while a short vector indicates that the variable has little to do with PC1 or PC2 (Smith Reference Smith1991).
Stability analysis
The two growing years, three locations (ERS-Conventional, ERS-Organic and the certified organic farm) and two competition levels (with or without oats) used in the study produced 12 different environments in which wheat cultivars were grown. Cultivar (genotypic) response to the 12 environments (also referred to as ‘sites’) were described using an adaptation of the Finlay–Wilkinson analysis (Finlay & Wilkinson Reference Finlay and Wilkinson1963). In the Finlay–Wilkinson analysis, site mean yield is used to describe each environment (e.g. low or high yielding) and a linear regression of individual cultivar yield on site mean yield for each site is calculated. The regression coefficient (b) of each cultivar describes its stability across sites, which is used to characterize its adaptability to specific environments. A visual representation of cultivar adaptation is achieved by plotting the regression coefficient of each cultivar against the cultivar mean yield across all environments. In the current study, both grain yield and total weed biomass, considered here to be indicators of competitive ability, were used to describe environments.
Regression analyses were conducted using the PROC REG procedure of SAS (SAS Institute 2003). Grain yield and total weed biomass data were log transformed prior to analysis (Finlay & Wilkinson Reference Finlay and Wilkinson1963). Ten environments were used for the total weed biomass analysis due to the absence of weeds at two of the 12 sites.
RESULTS
Weed pressure levels
The three natural weed pressure levels differed (P<0·01) for total weed biomass, with 15, 168 and 433 g/m under low, medium and high weed biomass, respectively (Table 3). Grain yield differed between weed pressure levels (P<0·06), with yield reductions of 37 and 31% under high weeds compared with the low and medium weed pressure environments, respectively (Table 3). Grain yield did not differ between low and medium weed pressure levels. Mean values for the three weed pressure levels did not differ for the remaining seven traits (P>0·10). Wheat cultivars differed (P<0·01) for all traits with the exception of lodging, which exhibited a high degree of variability (Table 3).
Table 3. Analysis of variance and least squares means for the effects of weed pressure, wheat cultivar and their interaction on weed biomass, plant height, days to heading and maturity, lodging, grain yield and the yield components from eight environments grown at Edmonton, Alberta, Canada in 2003 and 2004

ns=not significant.
† Standard error of the difference (s.e.d.) for total weed biomass has been back transformed.
The fewest significant correlations occurred at high weed pressure, where grain yield was positively correlated with height and negatively correlated with days to heading and maturity (Table 4). Weed biomass and spikes/m2 were negatively correlated (Table 4). Under medium weed pressure, grain yield was positively correlated with days to maturity, lodging, spikes/m2 and kernel weight, and was negatively correlated with kernels/spike and weed biomass (Table 4). Weed biomass was negatively associated with height and positively associated with heading time. Under low weed pressure, grain yield was negatively associated with time to heading and was positively associated with spikes/m2 and kernel weight (Table 4). Days to maturity, lodging, yield and spikes/m2 were negatively correlated with weed biomass. In all three weed pressure levels, days to heading and maturity were positively correlated, which was expected, as the two variables are measures of development rate (Table 4).
Table 4. Correlations among plant height, time to heading and maturity, lodging, grain yield, and yield components under high, medium and low weed pressure for wheat cultivars grown in eight environments at Edmonton, Alberta, Canada in 2003 and 2004Footnote *,Footnote †

* HT, height; HDG, days to heading; MAT, days to maturity; LDG, lodging; YLD, grain yield; SM2, spikes/m2; KPS, kernels/spike; TKW, kernel weight.
† r values below 0·36 were significant at P<0·05 and those above were significant at P<0·01. Exceptions were correlations between MAT and HT under medium weed biomass, and KPS and HT, TKW and HDG, TKW and LDG, and WBS and LDG under low weed biomass, due to missing values for one of the variables.
Under high weed biomass, the first three components of the PCA described 0·26, 0·22 and 0·15 of the variation, respectively (Table 5). PC1 had equally high absolute loadings for days to heading and maturity, lodging, weed biomass, grain yield and height. PC2 had the highest loadings for the yield components kernels/spike and spikes/m2. PC3 exhibited high loadings for height, and days to heading and maturity. The loading plot of PCs 1 and 2 for the high weed pressure level reveal that height and yield are positively related and that there is a positive association between weed biomass, days to heading and maturity, and lodging (Fig. 1a). Under medium weed pressure, the first three PCs accounted for 0·37, 0·20 and 0·16 of the variation, respectively (Table 5). Days to maturity, lodging, grain yield and spikes/m2 had relatively high positive loadings in PC1. PC2 had the highest positive loadings for days to heading and weed biomass. In PC3, kernel weight had the highest loading values, followed by height and weed biomass. The loading plot shows that spikes/m2 and grain yield are most closely associated at this weed level (Fig. 1b). At the low weed level, PCs 1, 2 and 3 accounted for 0·30, 0·23 and 0·16 of the variation, respectively (Table 5). PC1 had equally high positive loadings for days to maturity, lodging and spikes/m2. PC2 had high loadings for days to heading and kernels/spike, and to a lesser extent, grain yield and kernel weight. PC3 had high loadings for height and kernel weight. The loading plot of PCs 1 and 2 for the low weed pressure level shows comparatively less association between plant traits than the two weedy environments, with the exception of maturity, lodging and spikes/m2 (Fig. 1c).

Fig. 1. Loading plot describing the relationship among HT, height; HDG, days to heading; MAT, days to maturity; LDG, lodging; YLD, grain yield; SM2, spikes/m2; KPS, kernels/spike and TKW, kernel weight in high (A), medium (B) and low (C) weed pressure levels, using PCs 1 and 2, for wheat cultivars grown in eight environments at Edmonton, Alberta, Canada in 2003 and 2004.
Table 5. Factor loadings, eigenvalues, and proportions of total and cumulative variance for the first three principal components in each level of weed pressure for wheat cultivars grown in eight environments at Edmonton, Alberta, Canada in 2003 and 2004Footnote *,Footnote †

* HT, height; HDG, days to heading; MAT, days to maturity; LDG, lodging; YLD, grain yield; SM2, spikes/m2; KPS, kernels/spike; TKW, kernel weight.
† Bold font indicates variables selected to create each principal component, based on both absolute loading values greater than the mean absolute loading value and relative loading values within each component.
Multiple regression analyses were carried out on the raw data to determine which of the traits most determined yield and weed biomass (Table 6). Although all models were statistically significant, R 2 values ranged from 0·23 to 0·51, suggesting that factors not considered here may be responsible for variation in grain yield and weed biomass among wheat cultivars. Nevertheless, some trends were identified. Under high weed pressure, tall plants, early heading, high kernel weight and high kernels/spike determined grain yield, while shorter plants and reduced spikes/m2 led to increased weed biomass. Under medium weed pressure, shorter plants, high spikes/m2, high thousand kernel weight and low weed biomass increased yield while shorter height, later time to heading, reduced yield and high kernel weights led to increased weed biomass. With few weeds, early heading and low weeds contributed most to high grain yield, while longer times to heading, shorter times to maturity and reduced yield contributed to increased weed biomass.
Table 6. Multiple regression statistics and equations relating grain yield and weed biomass in high, medium and low weed pressure levels with the variables height, days to heading and maturity, lodging, grain yield, spikes/m2, kernels/spike, kernel weight and weed biomass for wheat cultivars grown in eight environments at Edmonton, Alberta, Canada in 2003 and 2004

Stability analysis
The regression lines of the five cultivars with the highest and lowest yield stability are presented in Fig. 2a. Cultivars exhibited differences in their response to variation in environmental yield potential. Park (b=0·84), a cultivar released in 1963, demonstrated above average stability, with small yield changes despite large changes in the yield potential of the environment. Park produced above average yields in low yielding environments and below average yields in high yielding environments. Sapphire (b=0·91) was relatively stable, but below average yielding in all environments. Katepwa, CDC Go and McKenzie were characterized by regression coefficients ⩾1·07 (Fig. 2a).

Fig. 2. Regression lines, showing the relationship between individual cultivar yield and environment mean yield (A) and between individual cultivar total weed biomass and environment mean total weed biomass (B) of wheat cultivars grown in 12 different environments at Edmonton and New Norway, Alberta, Canada in 2003 and 2004.
Park (b=0·69) and McKenzie (b=0·83) exhibited the least sensitivity to changes in weed pressure (Fig. 2b). Kohika (b=1·14), CDC Go (b=1·17) and Sapphire (b=1·21, not shown) became increasingly less competitive against weeds as weed pressure increased. Hard Red Calcutta (b=1·07) demonstrated average weed stability as weed pressure increased, yet consistently allowed below average weed growth at all sites.
DISCUSSION
Weed pressure levels
The correlation and principal components analyses grouped traits associated with grain yield in the three weed biomass environments similarly. Both analyses indicated that tallness, together with early heading and maturity, were related to grain yield in the high weed pressure environment. Height and time to maturity have been previously identified as competitive traits in wheat (Huel & Hucl Reference Huel and Hucl1996; Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996). In terms of maintaining grain yield, tallness may allow the crop to intercept more solar radiation above the weed layer, while early heading and maturity may allow the crop to escape increasingly heavy competition for soil moisture and nutrients. Time to heading has been less studied, although other measures of early plant development, such as rapid ground cover and early biomass accumulation, have been identified as competitive traits (Richards & Whytock Reference Richards and Whytock1993; Mason et al. Reference Mason, Navabi, Frick, O'Donovan and Spaner2007). Spikes/m2 was associated with grain yield under medium weed pressure, and to a lesser extent under low weed biomass, indicating that tillering capacity influences yielding ability in weedy environments. Other researchers have similarly reported tillering capacity to be associated with competitive ability (Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996; Hucl Reference Hucl1998).
In contrast to high weed biomass, grain yield under medium weed pressure was positively correlated with days to maturity. This would be expected under ideal growing conditions since later maturing cultivars often exhibit higher grain yield as a result of increased growing period (Baker & Townley-Smith Reference Baker, Townley-Smith, Slinkard and Fowler1986). Under medium and low weed pressure, associations among yield and yield components demonstrated the typical compensatory mechanisms that exist among spikes/m2, kernels/spike and kernel weight, and their influence on grain yield. When one of these factors increases, a decrease in another factor usually follows (Baker & Townley-Smith Reference Baker, Townley-Smith, Slinkard and Fowler1986). Although the yield components exhibited this same compensatory relationship under high weed biomass, none of the yield components were associated with yield itself, reflecting the complexity and variability of associations among plant traits in high weed environments.
The different associations between grain yield and competitive traits under high, medium and low weed biomass suggests that the level of weediness alters the importance of certain competitive traits. The absence of associations between height and grain yield in the low and medium weed biomass environments, along with the absence of a correlation between yield and spikes/m2 at the high weed level suggests that height and rapid early growth (as measured by heading and maturity) are stronger determinants of grain yield in extremely weedy environments.
Correlation analyses indicated that only spikes/m2 was associated with weed biomass under high weed pressure, but PC analyses suggested that reduced height and greater days to heading and maturity were also related to increased weed biomass. Taller plants that develop more quickly may be able to pre-empt weeds from capturing above- and below-ground resources, allowing more tiller initiation and survival, promoting further weed suppression. The PC and correlation analyses show that tallness and early heading were associated with reduced weed biomass in medium weed pressure environments. Tallness is reported to be associated with weed suppression (Wicks et al. Reference Wicks, Ramsel, Nordquist, Schmidt and Challaiah1986; Gooding et al. Reference Gooding, Thompson and Davies1993; Cosser et al. Reference Cosser, Gooding, Thompson and Froud-Williams1997; Korres & Froud-Williams Reference Korres and Froud-Williams2002), as is early growth (Richards & Whytock Reference Richards and Whytock1993; Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996; Champion et al. Reference Champion, Froud-Williams and Holland1998), and though these traits are common to both weed environments here, the roles of maturity and spikes/m2 in weed suppression are most apparent at the high weed pressure level. The present results show negative correlations between weed biomass and both maturity and spikes/m2 at the low weed biomass level, but the PC analysis showed that weed biomass was not strongly associated with any of the variables studied, which may be due to the very low weed presence in that environment overall.
The fact that the degree of weed pressure affects competitive traits differently may help to explain some of the discrepancies commonly found in this research area. Studies have reported, for example, tillering ability to be an important competitive trait (Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996; Hucl Reference Hucl1998), while others have reported tillering as less important (Wicks et al. Reference Wicks, Ramsel, Nordquist, Schmidt and Challaiah1986; Huel & Hucl Reference Huel and Hucl1996; Champion et al. Reference Champion, Froud-Williams and Holland1998). Levels of weed pressure, competing weed species and cultivars tested may all have an impact on which traits emerge as competitive, as do the varying criteria for measuring competitive ability.
The present results suggest that although plant height was important for grain yield under high weed pressure, it may play a more general and consistent role in weed suppression, especially over a range of environments. Although time to heading was related to both yield and weed suppression, its function was not consistent across weed pressure levels. As with other studies, the results of these analyses suggest that spikes/m2 influence competitive ability; however, their exact role in grain yield and weed suppression is unpredictable and may be influenced by other traits such as height (Champion et al. Reference Champion, Froud-Williams and Holland1998). While time to maturity did not figure prominently in the regression analyses, the PC analysis suggests that it may influence grain yield and weed biomass in high weed environments. These results suggest that a combination of competitive traits results in cultivars with differing weed-competitive abilities, as has been previously proposed (Lemerle et al. Reference Lemerle, Verbeek, Cousens and Coombes1996; Champion et al. Reference Champion, Froud-Williams and Holland1998).
Stability analysis
With its above average stability and high yields in low yielding environments, Park (released in 1963) is relatively well adapted to lower yielding environments, compared with the more modern and least yield-stable (b⩾1·07) cultivars Katepwa, CDC Go and McKenzie (Fig. 3a). Katepwa exhibited below average yields in low yielding environments, and above average yields in high yielding environments, thus is best adapted to high yielding environments. CDC Go displayed above average yield in all environments, which increased with environmental yield potential, suggesting that it is well adapted to all environments included in the present study. In contrast, McKenzie yielded below average at all sites, indicating that it is poorly adapted to all sites.

Fig. 3. The relationship between cultivar adaptation and cultivar mean yield for nine wheat cultivars grown in 12 environments (A), and between cultivar adaptation and cultivar mean total weed biomass for nine wheat cultivars grown in ten different environments (B). Solid lines indicate the mean values and dotted lines represent the 95% confidence limits.
Yield stability across environments did not appear to be conclusively associated with any of the plants traits measured in the present study. For example, the most stable cultivars differed in height, heading, maturity and tillering habit; Park was of medium height, high tillering and early heading and maturing, while Hard Red Calcutta was tall, low tillering and of medium heading and maturity. Three of the four most stable cultivars were developed between 1890 and 1963, while the least stable cultivars were released between 1981 and 2003, suggesting that older cultivars may exhibit greater yield stability across a wide range of productivity. Modern cultivars (typically described as those developed after the mid-1900s) are commonly reported to have decreased stability when compared with older cultivars, meaning that they are highly responsive to improved growing environments, which is often the result of increased inputs (Hucl & Baker Reference Hucl and Baker1987; Calderini & Slafer Reference Calderini and Slafer1999; Fufa et al. Reference Fufa, Baenziger, Beecher, Graybosch, Eskridge and Nelson2005). It has been proposed that stable cultivars may be desirable for low-input systems, while others argue that modern cultivars may still out-yield older ones in relatively poor environments despite their reduced stability (Calderini & Slafer Reference Calderini and Slafer1999). In the current study, the semidwarf CDC Go (released 2003) achieved above average grain yield at all sites, but was out-yielded by Park in the very lowest yielding environments, lending credence to the idea of cultivar yield stability as a desirable quality for low-input agriculture. The New Zealand cultivars Sapphire and Kohika differed in their stability; however both were relatively poor yielding overall, indicating that these cultivars are poorly adapted to northern Canadian growing environments. Overall yielding ability is similarly not explained by any of the competitive traits studied here. For example, the semidwarf CDC Go is of medium tillering, heading and maturity, Katepwa is medium height, medium heading, early maturing and high tillering, and McKenzie is of average height, early heading and maturity and high tillering ability.
Cultivars with high weed stability (or low sensitivity to weed pressure), Park and McKenzie, demonstrate superior weed suppressive abilities as weed competition increases, and are therefore notable competitors. Hard Red Calcutta, although not the most weed stable cultivar, similarly demonstrated high weed competitive ability by consistently allowing below average weed growth at all sites (Fig. 3b). Weed stability was not consistently explained by plant height, tillering or heading and maturity, although the three least weed stable cultivars were semidwarf in habit, suggesting that short stature or other semidwarf qualities may lead to diminishing competitive ability in increasingly weed environments. Weed suppression appeared to be influenced by height, as the tallest cultivars accumulated less weed biomass while the shorter cultivars allowed the most weed growth. Cousens et al. (Reference Cousens, Rebetzke and Barnett2003) reported greater yield loss due to weed competition and less weed suppression in semidwarf lines compared with conventional height isolines. Dwarfing genes may have pleiotropic effects on growth, resulting in semidwarf wheat cultivars with reduced cell size, contributing to smaller root systems, shorter coleoptile lengths and/or smaller leaf areas than conventional cultivars (Gale & Youssefian Reference Gale, Youssefian and Russell1985; Vandeleur & Gill Reference Vandeleur and Gill2004). This may affect future wheat production in Canada and the rest of the world, where the use of semidwarf wheat cultivars is increasing; in western Canada, the semidwarf Superb (released in 2003) was the most widely grown cultivar, representing close to one-fifth of the prairie wheat acreage only 3 years after its release (Canadian Wheat Board 2006).
A main conclusion of the present work is that cultivars differed in their competitive ability; therefore, selection for competitive cultivars is realistic. The high yielding ability of the cultivar Park, combined with its yield and weed stability, indicates that cultivars can both achieve high yield and be competitive against weeds. Park was characterized as well-adapted to low yielding or high weed environments. Identifying traits that consistently increase competitive ability is difficult. Plant height appears to be the trait most strongly associated with competitiveness in the wheat cultivars studied; though tillering and time to maturity are also related. Time to heading, which may be related to early growth/biomass accumulation, is also a competitive trait for wheat cultivars in Canada. Future studies should consider time to heading as a possible competitive indicator as it be may an easily identifiable character for plant breeders. Despite the identification of competitive traits, cultivars differing in their competitive ability could not be classified according to those traits, suggesting that cultivar competitive ability results from an interaction of those traits and/or traits not investigated in the current programme.











